Fabrication and Characterization of Zein Composite Particles Coated by Caseinate-Pectin Electrostatic Complexes with Improved Structural Stability in Acidic Aqueous Environments
Abstract
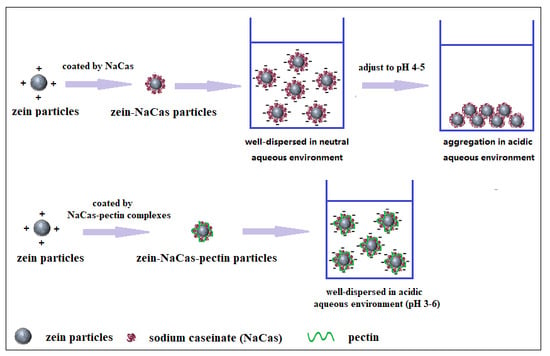
:1. Introduction
2. Results and Discussion
2.1. Preparation and Characterization of Zein-Caseinate-Pectin Particles
2.1.1. Influence of pH
2.1.2. Influence of Mass Ratio of Pectin to Caseinate
2.1.3. The Stability of Zein-Caseinate-Pectin Particles at Different Acidic pH Values
2.1.4. Re-Dispersibility
2.1.5. Storage Stability
2.2. Preparation and Characterization of Cur-Loaded Zein-Caseinate-Pectin Particles
2.3. Re-Dispersibility
2.4. TEM Study
2.5. Fluorescence Study
2.6. In-vitro Release Behavior
2.7. Application in Model Beverage and Evaluation of Antioxidant Activity
3. Materials and Methods
3.1. Materials
3.2. Preparation of Zein-Caseinate-Pectin Particles
3.3. Characterization of Zein-Caseinate-Pectin Particles
3.3.1. Particle Size, Size Distribution and Ζeta-Potential
3.3.2. Re-Dispersibility
3.3.3. Storage Stability
3.4. Preparation of Cur-Loaded Zein-Caseinate-Pectin Particles
3.5. Characterization of Cur-Loaded Zein-Caseinate-Pectin Particles
3.5.1. Encapsulation Efficiency (EE)
3.5.2. Re-Dispersibility
3.5.3. Transmission Electron Microscopy (TEM)
3.5.4. Fluorescence Spectroscopy
3.5.5. In-vitro Release Behavior
3.6. Application in Model Beverage and Evaluation of Antioxidant Activity
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wang, L.; Zhang, Y. Heat-induced self-assembly of zein nanoparticles: Fabrication, stabilization and potential application as oral drug delivery. Food Hydrocoll. 2019, 90, 403–412. [Google Scholar] [CrossRef]
- Luo, Y.C.; Wang, Q. Zein-based micro- and nano-particles for drug and nutrient delivery: A review. J. Appl. Polym. Sci. 2014, 131, 40696. [Google Scholar] [CrossRef]
- Soltani, S.; Madadlou, A. Gelation characteristics of the sugar beet pectin solution charged with fish oil-loaded zein nanoparticles. Food Hydrocoll. 2015, 43, 664–669. [Google Scholar] [CrossRef]
- Dai, L.; Sun, C.X.; Li, R.R.; Mao, L.K.; Liu, F.G.; Gao, Y.X. Structural characterization, formation mechanism and stability of Curcumin in zein-lecithin composite nanoparticles fabricated by antisolvent co-precipitation. Food Chem. 2017, 237, 1163–1171. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.C.; Zhang, B.C.; Whent, M.; Yu, L.L.; Wang, Q. Preparation and characterization of zein/chitosan complex for encapsulation of α-tocopherol, and its in vitro controlled release study. Colloids Surf. B 2011, 85, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Lai, L.F.; Guo, H.X. Preparation of new 5-fluorouracil-loaded zein nanoparticles for liver targeting. Int. J. Pharm. 2011, 404, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Hu, Y.C.; Tiwari, J.K.; Velikov, K.P. Synthesis and characterisation of zein–Curcumin colloidal particles. Soft Matter 2010, 6, 6192–6199. [Google Scholar] [CrossRef]
- Chen, J.J.; Zheng, J.K.; McClements, D.J.; Xiao, H. Tangeretin-loaded protein nanoparticles fabricated from zein/β-lactoglobulin: Preparation, characterization, and functional performance. Food Chem. 2014, 158, 466–472. [Google Scholar] [CrossRef]
- Li, H.; Xu, Y.; Sun, X.; Wang, S.H.; Wang, J.W.; Zhu, J.X.; Wang, D.F.; Zhao, L.L. Stability, bioactivity, and bioaccessibility of fucoxanthin in zein-caseinate composite nanoparticles fabricated at neutral pH by antisolvent precipitation. Food Hydrocoll. 2018, 84, 379–388. [Google Scholar] [CrossRef]
- Xue, J.L.; Zhang, Y.Q.; Huang, G.R.; Liu, J.; Slavin, M.; Yu, L.L. Zein-caseinate composite nanoparticles for bioactive delivery using Curcumin as a probe compound. Food Hydrocoll. 2018, 83, 25–35. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Niu, Y.G.; Luo, Y.C.; Ge, M.; Yang, T.; Yu, L.L.; Wang, Q. Fabrication, characterization and antimicrobial activities of thymol-loaded zein nanoparticles stabilized by sodium caseinate-chitosan hydrochloride double layers. Food Chem. 2014, 142, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; McClements, D.J. Fabrication of biopolymer nanoparticles by antisolvent precipitation and electrostatic deposition: Zein-alginate core/shell nanoparticles. Food Hydrocoll. 2015, 44, 101–108. [Google Scholar] [CrossRef]
- Yao, K.F.; Chen, W.J.; Song, F.L.; McClements, D.J.; Hu, K. Tailoring zein nanoparticle functionality using biopolymer coatings: Impact on Curcumin bioaccessibility and antioxidant capacity under simulated gastrointestinal conditions. Food Hydrocoll. 2017, 79, 262–272. [Google Scholar] [CrossRef]
- Chang, C.; Wang, T.R.; Hu, Q.B.; Zhou, M.Y.; Xue, J.Y.; Luo, Y.C. Pectin coating improves physicochemical properties of caseinate/zein nanoparticles as oral delivery vehicles for Curcumin. Food Hydrocoll. 2017, 70, 143–151. [Google Scholar] [CrossRef]
- Chang, C.; Wang, T.R.; Hu, Q.B.; Luo, Y.C. Zein/caseinate/pectin complex nanoparticles: Formation and characterization. Int. J. Biol. Macromol. 2017, 104, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Turgeon, S.L.; Schmitt, C.; Sanchez, C. Protein–polysaccharide complexes and coacervates. Curr. Opin. Colloid Interface Sci. 2007, 12, 166–178. [Google Scholar] [CrossRef]
- Wang, J.; Dumas, E.; Gharsallaoui, A. Low methoxyl pectin/sodium caseinate complexing behavior studied by isothermal titration calorimetry. Food Hydrocoll. 2018, 88, 163–169. [Google Scholar] [CrossRef]
- Jones, O.G.; Lesmes, U.; Dubin, P.; McClements, D.J. Effect of polysaccharide charge on formation and properties of biopolymer nanoparticles created by heat treatment of β-lactoglobulin–pectin complexes. Food Hydrocoll. 2010, 24, 374–383. [Google Scholar] [CrossRef]
- Sriamornsak, P.; Thirawong, N.; Weerapol, Y.; Nunthanid, J.; Sungthongjeen, S. Swelling and erosion of pectin matrix tablets and their impact on drug release behavior. Eur. J. Pharm. Biopharm. 2007, 67, 211–219. [Google Scholar] [CrossRef]
- Dickinson, E. Properties of emulsions stabilized with milk proteins: Overview of some recent developments. J. Dairy Sci. 1997, 80, 2607–2619. [Google Scholar] [CrossRef]
- Sung, A.-M.; Piirma, I. Electrosteric stabilization of polymer colloids. Langmuir 1994, 10, 1393–1398. [Google Scholar] [CrossRef]
- Koutsoulas, C.; Pippa, N.; Demetzos, C.; Zabka, M. The role of ζ-potential on the stability of nanocolloidal systems. Pharmakeftiki 2012, 24, 106–111. [Google Scholar]
- Naksuriya, O.; Okonogi, S.; Schiffelers, R.M.; Hennink, W.E. Curcumin nanoformulations: A review of pharmaceutical properties and preclinical studies and clinical data related to cancer treatment. Biomaterials 2014, 35, 3365–3383. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Huang, X.X.; Gao, Y.Q.; Huang, X.L.; Xiao, H.; Mcclements, D.J. Core–shell biopolymer nanoparticle delivery systems: Synthesis and characterization of Curcumin fortified zein–pectin nanoparticles. Food Chem. 2015, 182, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Barik, A.; Priyadarsini, K.I.; Mohan, H. Photophysical studies on binding of Curcumin to bovine serum albumin. Photochem. Photobiol. 2003, 77, 597–603. [Google Scholar] [CrossRef]
- Sahu, A.; Kasoju, N.; Bora, U. Fluorescence study of the Curcumin-casein micelle complexation and its application as a drug nanocarrier to cancer cells. Biomacromolecules 2008, 9, 2905–2912. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Jung, H.; Lee, H.J.; Kwak, H.K.; Hwang, K.T. Formation of electrostatic complexes using sodium caseinate with high-methoxyl pectin and carboxymethyl cellulose and their application in stabilisation of curcumin. Int. J. Food Sci. Technol. 2016, 51, 1655–1665. [Google Scholar] [CrossRef]
- Lange, D.C.; Kothari, R.; Patel, R.C.; Patel, S.C. Retinol and retinoic acid bind to a surface cleft in bovine beta-lactoglobulin: A method of binding site determination using fluorescence resonance energy transfer. Biophys. Chem. 1998, 74, 45–51. [Google Scholar] [CrossRef]
- Zhou, M.Y.; Wang, T.R.; Hu, Q.B.; Luo, Y.C. Low density lipoprotein/pectin complex nanogels as potential oral delivery vehicles for curcumin. Food Hydrocoll. 2016, 57, 20–29. [Google Scholar] [CrossRef] [Green Version]
- Barclay, L.R.C.; Vinqvist, M.R.; Mukai, K.; Goto, H.; Hashimoto, Y.; Tokunaga, A.; Uno, H. On the antioxidant mechanism of Curcumin: Classical methods are needed to determine antioxidant mechanism and activity. Org. Lett. 2000, 2, 2841–2843. [Google Scholar] [CrossRef]
- Mohammadian, M.; Salami, M.; Momen, S.; Alavi, F.; Emam-Djomeh, Z. Fabrication of Curcumin-loaded whey protein microgels: Structural properties, antioxidant activity, and in vitro release behavior. LWT--Food Sci. Technol. 2019, 103, 94–100. [Google Scholar] [CrossRef]
- Liu, F.G.; Ma, C.C.; McClements, D.J.; Gao, Y.X. A comparative study of covalent and non-covalent interactions between zein and polyphenols in ethanol-water solution. Food Hydrocoll. 2017, 63, 625–634. [Google Scholar] [CrossRef]
- Ro, J.; Kim, Y.; Kim, H.; Jang, S.B.; Lee, H.J.; Chakma, S.; Jeong, J.H.; Lee, J. Anti-oxidative activity of pectin and its stabilizing effect on retinyl palmitate. Korean J. Physiol. Pharmacol. 2013, 17, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Levine, R.L.; Mosoni, L.; Berlett, B.S.; Stadtman, E.R. Methionine residues as endogenous antioxidants in proteins. Proc. Natl. Acad. Sci. USA 1996, 93, 15036–15040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, T.R.; Ma, X.Y.; Lei, Y.; Luo, Y.C. Solid lipid nanoparticles coated with cross-linked polymeric double layer for oral delivery of Curcumin. Colloids Surf. B 2016, 148, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.S.; Xu, L.H.; Liu, C.T.; Zhang, A.F.; Wang, S.Q.; Deng, Z.N.; Lou, W.W.; Xu, H.H.; Bai, Q.; Ma, J.F. Preparation and characterization of cationic curcumin nanoparticles for improvement of cellular uptake. Carbohydr. Polym. 2012, 90, 16–22. [Google Scholar] [CrossRef]
- Cho, H.; Lee, H.J.; Yu, K.S.; Choi, Y.M.; Hwang, K.T. Characterisation and food application of Curcumin bound to sodium caseinate–polysaccharide electrostatic complexes. Int. J. Food Sci. Technol. 2017, 52, 1770–1776. [Google Scholar] [CrossRef]
- Moore, J.; Cheng, Z.H.; Su, L.; Yu, L.L. Effects of solid-state enzymatic treatments on the antioxidant properties of wheat bran. J. Agric. Food Chem. 2006, 54, 9032–9045. [Google Scholar] [CrossRef]
Sample Availability: Samples of blank and Cur-loaded zein-caseinate-pectin particles are available from the authors. |








| pH of the Dispersion | Z-Average (nm) | Polydispersity Index (PDI) | ζ-Potential (mV) | |||
|---|---|---|---|---|---|---|
| Freshly-Prepared | Re-Dispersion | Freshly-Prepared | Re-Dispersion | Freshly-Prepared | Re-Dispersion | |
| 3 | 291.6 a ± 4.0 | 385.0 a ± 4.7 | 0.2 a ± 0.0 | 0.2 a ± 0.0 | −9.8 a ± 0.3 | −23.4 a ± 0.6 |
| 3.5 | 344.2 b ± 3.9 | 407.2 a ± 6.0 | 0.2 a ± 0.0 | 0.2 a ± 0.0 | −20.5 b ± 0.5 | −26.3 b ± 0.8 |
| 4 | 358.3 b ± 3.5 | 513.9 b ± 13.5 | 0.2 a ± 0.0 | 0.1 a ± 0.0 | −26.2 c ± 0.7 | −32.9 c ± 0.8 |
| 4.5 | 359.7 b ± 7.8 | 510.3 b ± 10.5 | 0.2 a ± 0.0 | 0.2 a ± 0.0 | −29.2 d ± 0.4 | −31.4 c ± 0.7 |
| 5 | 386.9 c ± 8.6 | 542.1 c ± 3.1 | 0.2 a ± 0.0 | 0.2 a ± 0.0 | −33.3 e ± 1.4 | −37.0 d ± 0.5 |
| 6 | 405.7 d ± 8.1 | 573.1 d ± 6.3 | 0.2 a ± 0.0 | 0.2 a ± 0.0 | −37.6 f ± 0.8 | −41.6 e ± 0.9 |
| Zein:Cur (w/w) | Z-Average (nm) | Polydispersity Index (PDI) | ζ-Potential (mV) | Encapsulation Efficiency (%) | |||
|---|---|---|---|---|---|---|---|
| Freshly-Prepared | Re-Dispersion | Freshly-Prepared | Re-Dispersion | Freshly-Prepared | Re-Dispersion | ||
| 10:1 | 365.7 a ± 3.3 | 489.7 a ± 8.6 | 0.2 a ± 0.0 | 0.2 a ± 0.0 | −19.4 a ± 0.7 | −27.4 a ± 0.3 | 81.3 a ± 0.4 |
| 20:1 | 358.4 a ± 6.9 | 509.5 a ± 4.4 | 0.2 a ± 0.0 | 0.2 a ± 0.0 | −19.0 a ± 0.3 | −28.0 a ± 0.3 | 86.0 b ± 0.2 |
| 30:1 | 366.2 a ± 4.8 | 497.4 a ± 10.1 | 0.2 a ± 0.0 | 0.2 a ± 0.0 | −19.5 a ± 0.4 | −27.8 a ± 0.3 | 89.8 c ± 0.3 |
| 40:1 | 369.2 a ± 1.4 | 496.0 a ± 8.3 | 0.2 a ± 0.0 | 0.2 a ± 0.0 | −18.9 a ± 0.1 | −28.1 a ± 0.4 | 94.0 d ± 0.8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Wang, B.; Wu, Y.; Gao, B.; Yu, L. Fabrication and Characterization of Zein Composite Particles Coated by Caseinate-Pectin Electrostatic Complexes with Improved Structural Stability in Acidic Aqueous Environments. Molecules 2019, 24, 2535. https://doi.org/10.3390/molecules24142535
Zhang Y, Wang B, Wu Y, Gao B, Yu L. Fabrication and Characterization of Zein Composite Particles Coated by Caseinate-Pectin Electrostatic Complexes with Improved Structural Stability in Acidic Aqueous Environments. Molecules. 2019; 24(14):2535. https://doi.org/10.3390/molecules24142535
Chicago/Turabian StyleZhang, Yaqiong, Bo Wang, Yan Wu, Boyan Gao, and Liangli (Lucy) Yu. 2019. "Fabrication and Characterization of Zein Composite Particles Coated by Caseinate-Pectin Electrostatic Complexes with Improved Structural Stability in Acidic Aqueous Environments" Molecules 24, no. 14: 2535. https://doi.org/10.3390/molecules24142535