Volatile Aroma Compounds of Brandy ‘Lozovača′ Produced from Muscat Table Grapevine Cultivars (Vitis vinifera L.)
Abstract
:1. Introduction
2. Results
2.1. Volatile Compounds Composition of Brandy Samples
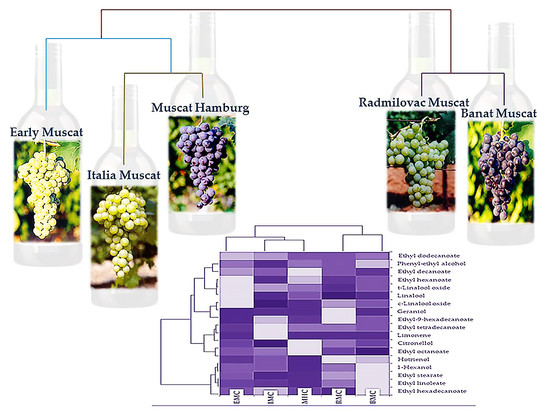
2.2. Principal Component Analysis and Hierarchical Component Analysis
3. Discussion
4. Materials and Methods
4.1. Cultivars Description
4.2. Chemical and Reagents
4.3. Grape Brandy Making Technology
4.4. Extraction and Analysis of Volatile Compounds
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tsakiris, A.; Kallithraka, S.; Kourkoutas, Y. Grape brandy production, composition and sensory evaluation. J. Sci. Food Agric. 2014, 94, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Mamede, E.O.M.; Cardello, M.A.B.H.; Pastore, M.G. Evaluation of an aroma similar to that of sparkling wine: Sensory and gas chromatography analyses of fermented grape musts. Food Chem. 2005, 89, 63–68. [Google Scholar] [CrossRef]
- Agosin, E.; Belancic, A.; Ibacache, A.; Baumes, R.; Bordeu, E.; Crawford, A.; Bayonove, C. Aromatic potential of certain Muscat grape varieties important for Pisco production in Chile. Am. J. Enol. Vitic. 2000, 51, 404–408. [Google Scholar]
- Lanaridis, P.; Salaha, M.-J.; Tzourou, I.; Tsoutsouras, E.; Karagiannis, S. Volatile compounds in grapes and wines from two Muscat varieties cultivated in Greek islands. J. Int. Sci. Vigne Vin. 2002, 36, 39–47. [Google Scholar] [CrossRef]
- Silva, M.L.; Malcata, F.X. Relationships between storage conditions of grape pomace and volatile composition of spirits obtained there from. Am. J. Enol. Vitic. 1998, 49, 56–64. [Google Scholar]
- Battilana, J.; Costantini, L.; Emanuelli, F.; Sevini, F.; Segala, C.; Moser, S. The 1-deoxy-d-xylulose 5-phosphate synthase gene co-localizes with amajor QTL affecting monoterpene content in grapevine. Appl. Genet. 2009, 118, 653–669. [Google Scholar] [CrossRef] [PubMed]
- Duchêne, E.; Legras, J.L.; Karst, F.; Merdinoglu, D.; Claudel, P.; Jaegli, N. Variation of linalool and geraniol content with in two pairs of aromatic and non-aromatic grapevine clones. Aust. J. Grape Wine Res. 2009, 15, 120–130. [Google Scholar] [CrossRef]
- Guillaumie, S.; Ilg, A.; Rety, S.; Brette, M.; Trossat-Magnin, C.; Decroocq, S. Genetic analysis of the biosynthesis of 2-methoxy-3-isobutylpyrazine, a major grape-derived aroma compound impacting wine quality. Plant Physiol. 2013, 162, 604–615. [Google Scholar] [CrossRef]
- Lacombe, T.; Audeguin, L.; Boselli, M.; Bucchetti, B.; Cabello, F.; Chatelet, P.; Crespan, M.; D’Onofrio, C.; Eiras Dias, J.; Ercisli, S.; et al. Grapevine European catalogue: Towards a comprehensive list. Vitis 2011, 50, 65–68. [Google Scholar]
- Maicas, S.; Mateo, J.J. Hydrolysis of terpenyl glycosides in grape juice and other fruit juices: A review. Appl. Microbiol. Biotechnol. 2005, 67, 322–335. [Google Scholar] [CrossRef]
- Mayr, M.C.; Parker, M.; Baldock, A.G.; Black, A.C.; Pardon, H.K.; Williamson, O.P.; Herderich, J.M.; Francis, I.L. Determination of the importance of in-mouth release of volatile phenol glycoconjugates to the flavor of smoke –tainted wines. J. Agric. Food Chem. 2014, 62, 2327–2336. [Google Scholar] [CrossRef] [PubMed]
- Radeka, S.; Herjavec, S.; Peršurić, Đ.; Lukić, I.; Sladonja, B. Effect of different maceration treatments on free and bound varietal aroma compounds in wine of Vitis vinifera L. cv. Malvazija istarska bijela. Food Technol. Biotehnol. 2008, 46, 86–92. [Google Scholar]
- Soufleros, E.H.; Mygdalia, S.A.; Natskoulis, P. Production process and characterization of the traditional Greek fruit distillate Koumaro by aromatic and mineral composition. J. Food Compos. Anal. 2005, 18, 699–716. [Google Scholar] [CrossRef]
- Cortés, S.; Gil, M.L.; Fernández, E. Volatile composition of traditional and industrial Orujo spirits. Food Control. 2005, 16, 383–388. [Google Scholar] [CrossRef]
- Ledauphin, J.; Saint-Clair, J.-F.; Lablanquie, O.; Guichard, H.; Founier, N.; Guichard, E.; Barillier, D. Identification of trace volatile compounds in freshly distilled calvados and Cognac using preparative separations coupled with gas chromatography-mass spectrometry. J. Agric. Food Chem. 2004, 52, 5124–5134. [Google Scholar] [CrossRef] [PubMed]
- Vujović, D.; Maletić, R.; Popović-Đorđević, J.; Pejin, B.; Ristić, R. Viticultural and chemical characteristics of Muscat Hamburg preselected clones grown for table grapes. J. Sci. Food Agr. 2016, 97, 587–594. [Google Scholar] [CrossRef]
- Matijasevic, S.; Todic, S.; Beslic, Z.; Rankovic Vasic, Z.; Zunic, D.; Atanackovic, Z.; Vukosavljevic, V.; Cirkovic, B. Volatile components of grape brandies produced from Muscat table grapevine (Vitis vinifera L.) cultivars. Bulg. J. Agric. Sci. 2013, 19, 783–791. [Google Scholar]
- Oliveira, J.M.; Faria, M.; Sá, F.; Barros, F.; Araújo, I.M. C6-alcohols as varietal markers for assessment of wine origin. Anal. Chim. Acta 2006, 563, 300–309. [Google Scholar] [CrossRef] [Green Version]
- Da Porto, C.; Cortella, G.; Freschet, G. Preliminary study on a cooling practice of grape pomace during storage on an industrial scale. Ital. J. Food Sci. 2004, 16, 87–95. [Google Scholar]
- Ribereau-Gayon, P.; Glories, Y.; Maujean, A.; Dubordieu, D. Handbook of Enology, The Chemistry of Wine: Stabilization and Treatments, 2nd ed.; John Wiley & Sons Ltd.: Chichester, UK, 2006; pp. 51–64. [Google Scholar]
- Lukić, I.; Miličević, B.; Banović, M.; Tomas, S.; Radeka, S.; Peršurić, Đ. Secondary Aroma compounds in fresh grape marc distillates as a result of variety and corresponding production technology. Food Technol. Biotechnol. 2011, 49, 214–227. [Google Scholar]
- Ferreira, V.; Ortin, N.; Escudero, A.; López, R.; Cacho, J. Chemical characterization of the aroma of Grenache Rosé wines: Aroma extract dilution analysis, quantitative determination and sensory reconstitution studies. J. Agric. Food Chem. 2002, 50, 4048–4054. [Google Scholar] [CrossRef] [PubMed]
- Peinado, R.A.; Mauricio, J.C.; Moreno, J. Aromatic series in sherry wines with gluconic acid subjected to different biological aging conditions by Saccharomyces cerevisiae var. capensis. Food Chem. 2006, 94, 232–239. [Google Scholar] [CrossRef]
- Mahattanatawee, K.; Perez-Cacho, P.R.; Davenport, T.; Rouseff, R. Comparison of three lychee cultivar odor profiles using gas chromatography-olfactometry and gas chromatography-sulfur detection. J. Agric. Food Chem. 2007, 55, 1939–1944. [Google Scholar] [CrossRef] [PubMed]
- Peinado, R.A.; Moreno, J.; Bueno, J.E.; Moreno, J.A.; Mauricio, J.C. Comparative study of aromatic compounds in two young white wines subjected to pre-fermentative cryomaceration. Food Chem. 2004, 84, 585–590. [Google Scholar] [CrossRef]
- Tešević, V.; Nikićević, N.; Jovanović, A.; Đoković, D.; Vujisić, L.; Vučković, I.; Bonić, M. Volatile components from old plum brandies. Food Technol. Biotechnol. 2005, 43, 367–372. [Google Scholar]
- Genovese, A.; Lamorte, S.A.; Gambuti, A.; Moio, L. Aroma of Aglianico and Uva di Troia grapes by aromatic series. Food Res. Int. 2013, 53, 15–23. [Google Scholar] [CrossRef]
- Rogerson, F.S.S.; De Freitas, V.A.P. Fortification spirit, a contributor to the aroma complexity of Port. J. Food Sci. 2002, 67, 1564–1569. [Google Scholar] [CrossRef]
- Garcia-Carpintero, E.G.; Sanchez-Palomo, E.; Gomez Gallego, M.A.; Gonzalez-Vinas, M.A. Effect of cofermentation of grape varieties on aroma profiles of La Mancha red wines. J. Food Sci. 2011, 76, C1169–C1180. [Google Scholar] [CrossRef]
- Ferrari, G.; Lablanquie, O.; Cantagrel, R.; Ledauphin, J.; Payot, T.; Fournier, N.; Guichard, E. Determination of key odorant compounds in freshly distilled cognac using GC-O, GC- MS and sensory evaluation. J. Agric. Food Chem. 2004, 52, 5670–5676. [Google Scholar] [CrossRef]
- Qian, M.C.; Wang, Y. Seasonal variation of volatile composition and odor activity value of ‘Marion’ (Rubus spp. hyb) and ‘Thornless Evergreen’ (R. laciniatus L.) blackberries. J. Food Sci. 2005, 70, C13–C20. [Google Scholar] [CrossRef]
- Hernández-Gómez, F.L.; Úbeda-Iranzo, J.; García-Romero, E.; Briones-Pérez, A. Comparative production of different melon distillates: Chemical and sensory analyses. Food Chem. 2005, 90, 115–125. [Google Scholar] [CrossRef]
- Guichard, H.; Lemesle, S.; Ledauphin, J.; Barillier, D.; Picoche, B. Chemical and sensorial aroma characterization of freshly distilled Calvados. 1. Evaluation of quality and defects on the basis of key odorants by olfactometry and sensory analysis. J. Agric. Food Chem. 2003, 51, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Park, S.K.; Morrison, J.C.; Adams, D.O.; Noble, A.C. Distribution of free and glycosidically bound monoterpenes in the skin and mesocarp of Muscat of Alexandria grapes during development. J. Agric. Food Chem. 1991, 39, 514–518. [Google Scholar] [CrossRef]
- Mateo, J.J.; Jimenez, M. Monoterpenes in grape juice and wines. J. Chromatogr. A 2000, 881, 557–567. [Google Scholar] [CrossRef]
- Battilana, J.; Emanuelli, F.; Gambino, G.; Gribaudo, I.; Gasperi, F.; Boss, P.K. Functional effect of grapevine 1-deoxy-D-xylulose5-phosphate synthase substitution K284 non Muscat flavor formation. J. Exp. Bot. 2011, 62, 5497–5508. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhu, B.; Tu, C.; Duan, C.; Pan, Q. Generation of volatile compounds in litchi wine during winemaking and short-term bottle storage. J. Agric. Food Chem. 2011, 59, 4923–4931. [Google Scholar] [CrossRef] [PubMed]
- Prosen, H.; Janeš, L.; Strlič, M.; Rusjan, D.; Kočar, D. Analysis of free and bound aroma compounds in grape berries using headspace solid-phase microextraction with GC-MS and a preliminary study of solid – phase extraction with LC-MS. Acta Chim. Slov 2007, 54, 25–32. [Google Scholar]
- Fang, L.; Mosandl, A.; Gubesch, M.; Wüst, M. Enantioselective analysis of monoterpens in different grape varieties during berry ripening using stir sorptive extraction—And solid phase extraction-enantioselective-multidimensional gas chromatography - mass spectrometry. J. Chromatogr. A 2006, 1112, 369–374. [Google Scholar] [CrossRef]
- García-Carpintero, E.G.; Sánchez-Palomo, E.; Gallego, M.A.G.; González-Viñas, M.A. Volatile and sensory characterization of red wines from cv. Moravia Agria minority grape variety cultivated in La Mancha region over five consecutive vintages. Food Res. Int. 2011, 44, 1549–1560. [Google Scholar] [CrossRef]
- Diéguez, C.S.; de la Peňa, M.L.G.; Gómez, F.E. Approaches to spirit aroma: Contribution of some aromatic compounds to the primary aroma in samples of Orujo spirits. J. Agric. Food Chem. 2003, 51, 7385–7390. [Google Scholar] [CrossRef]
- Žunić, D.; Garić, M. Ampelografija., 2nd ed.; Poljoprivredni fakultet Univerziteta u Prištini, Kosovska: Mitrovica, Serbia, 2017; pp. 350–370. (In Serbian) [Google Scholar]
- Organisation Internationale de la Vigne et du Vin (OIV). Code des caractères descriptifs des variétés et espèces de Vitis. A.; OIV: Dedon, Paris, 1983. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |




| Cultivar | Early Muscat (EM) | Radmilovac Muscat (RM) | Banat Muscat (BM) | Italia Muscat (IM) | Muscat Hamburg (MH) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatments | C | V1 | V2 | C | V1 | V2 | C | V1 | V2 | C | V1 | V2 | C | V1 | V2 |
| ALCOHOLS | |||||||||||||||
| 3-Ethoxy-1-propanol | /2 | / | 0.423 | 0.72 | / | / | / | / | / | / | / | / | 0.26 | / | 1.71 |
| 4.4-Dimethyl-3-hexanol | 1.51 | / | / | / | / | / | / | / | / | / | / | / | / | / | / |
| 1-Hexanol* | 4.93 | 5.64 | 6.01 | 1.9 | 9.12 | 8.18 | 6.74 | 3.41 | 4.2 | 3.51 | 3.61 | 0.81 | 6.17 | ||
| Phenyl–ethyl alcohol* | 9.62 | 12.22 | 18.09 | 0.42 | 12.22 | / | 11.75 | 21.78 | 10.89 | 5.6 | 1.54 | 3.51 | 9.97 | 25.43 | 18.68 |
| 3-Methyl-1-pentanol | / | / | / | / | / | / | / | / | / | / | / | / | / | / | 0.33 |
| 1-Heptanol | / | / | / | / | / | / | / | / | / | / | / | / | 0.2 | / | 0.22 |
| 5-Hepten-2-ol | / | / | / | / | / | / | / | / | / | / | / | / | / | / | 0.17 |
| ∑ | 16.06 | 30.08 | 18.51 | 6.43 | 14.15 | 9.12 | 19.93 | 28.52 | 14.3 | 5.6 | 5.74 | 7.02 | 14.04 | 26.24 | 27.28 |
| ACIDS | |||||||||||||||
| Octanoic acid | 0.32 | 0.63 | 0.88 | / | / | / | / | 0.1 | 1.25 | 5 | 2.59 | / | 0.11 | / | 1.95 |
| Decanoic acid | 1.25 | 4.91 | 6.01 | / | 1.58 | 0.27 | 2.91 | / | / | / | 0.5 | 8.41 | / | / | / |
| Dodecanoic acid | 5.2 | 6.61 | / | 1.01 | 1.8 | / | / | 2.19 | 2.23 | 3.83 | 0.11 | 3.18 | 1.24 | ||
| Tetradecanoic acid | / | / | / | 0.42 | / | / | / | / | / | / | / | / | 0.62 | / | / |
| Hexadecanoic acid | 0.51 | 0.68 | 3.11 | 3.41 | 0.62 | / | / | / | / | / | / | 0.33 | / | / | |
| Hexanoic acid | / | / | / | / | / | 1.12 | / | / | 0.93 | 1.39 | / | / | / | / | / |
| ∑ | 7.28 | 5.54 | 14.18 | 3.53 | 6.0 | 3.81 | 2.91 | 0.1 | 4.37 | 8.62 | 3.09 | 12.24 | 1.17 | 3.18 | 3.19 |
| ESTERS | |||||||||||||||
| Isoamyl acetate | / | / | 0.17 | / | / | / | / | / | / | / | / | / | / | / | / |
| Ethyl hexanoate* | 4.96 | 0.84 | 1.91 | 1.01 | 0.39 | 2.04 | 1.97 | 0.58 | 1.71 | 9.8 | 7.19 | 4.58 | 4.97 | 4.32 | |
| Linalyl acetate | / | 1.53 | / | / | / | / | / | / | / | / | / | / | / | / | / |
| Ethyl benzoate | / | 0.97 | / | / | / | / | / | / | / | / | / | / | / | / | / |
| Ethyl octanoate* | 2.82 | / | 2.78 | 4.35 | 5.89 | 8.53 | 12.33 | 15.17 | 10.68 | 16.96 | 13.04 | 27.56 | 24.83 | / | 31.45 |
| 2-Fenilethyl acetate | / | / | 0.92 | / | / | / | / | / | / | / | / | / | / | / | / |
| Citronellyl acetate | / | / | / | / | 0.17 | / | / | / | / | / | / | / | / | / | 0.4 |
| Neryl acetate | / | / | / | / | / | / | / | / | / | / | / | / | / | / | / |
| Ethyl-9-decanoate | / | / | / | / | / | / | / | / | / | 1.09 | / | / | / | / | / |
| Ethyl decanoate* | 26.62 | 8.12 | 12.48 | 4.09 | 6.29 | / | 12.63 | 7.14 | 19.71 | 22.25 | 34.28 | 11.00 | 29.87 | 15.82 | 6.16 |
| Isoamyl octanoate | / | / | / | / | / | / | / | / | / | / | / | / | 0.27 | / | 0.11 |
| Ethyl dodecanoate* | 11.64 | 0.53 | 9.59 | 3.6 | 3.03 | 5.54 | 9.67 | 3.24 | 10.54 | 10.89 | 8.14 | 5.57 | 3.53 | 5.82 | 3.28 |
| 3-Methyl butyldecanoate | / | / | / | / | / | / | / | / | / | / | / | / | 0.06 | 0.54 | / |
| Ethyl tetradecanoate* | 0.62 | 0.72 | 0.52 | 1.51 | 1.51 | 1.47 | / | 0.74 | 2.28 | 2.23 | 1.23 | 0.98 | 1.62 | 2.58 | 0.24 |
| Ethyl 9-hexadecanoate* | 0.51 | 0.32 | / | 1.35 | 0.75 | / | / | 2.18 | 4.48 | 1.14 | 1.55 | 0.46 | 0.23 | 1.75 | 0.24 |
| Ethyl hexadecanoate* | 1.54 | 3.65 | 3.88 | 6.73 | 10.78 | 14.43 | 15 | 11.39 | 14.26 | 12.33 | 6.98 | 7.49 | 2.3 | 10.57 | 2.12 |
| Ethyl linoleate* | 4.77 | 6.66 | 3.44 | 12.33 | 8.63 | 16.42 | 19.97 | 15.53 | 1.76 | 5.23 | 6.7 | 8.07 | 6.99 | 15.63 | 3.26 |
| Ethyl stearate* | / | 0.83 | / | 1.36 | 1.03 | 1.56 | 1.67 | 1.18 | 1.68 | 0.68 | / | 0.81 | 0.65 | 1.23 | / |
| Diethyil-dibutanoate | / | / | / | / | 0.21 | / | / | / | 0.25 | / | / | / | 0.28 | / | 0.54 |
| Ethyl-3-hydroxy butyrate | / | / | / | / | / | / | / | / | / | / | / | / | 0.19 | / | / |
| 3-Methylbutyl butanoate | / | / | / | / | / | / | / | / | / | / | / | / | / | 0.46 | / |
| ∑ | 53.48 | 24.17 | 35.69 | 36.33 | 38.68 | 49.99 | 71.27 | 58.54 | 66.22 | 75.4 | 59.37 | 52.12 | 74.51 | 81.72 | 69.13 |
| TERPENOIDS | |||||||||||||||
| α-Pinene | / | / | / | / | / | 0.64 | / | / | / | / | / | / | / | / | / |
| Limonene* | / | 2.14 | / | / | 1.02 | / | / | 7.31 | 0.96 | 0.62 | / | / | / | 1.42 | 1.47 |
| γ−Terpinene | / | / | / | / | 0.79 | / | / | / | / | / | / | / | / | 0.86 | 0.59 |
| c-Linalool oxide* | 2.10 | 2.78 | 3.21 | 1.66 | 2.25 | 1.93 | / | / | 0.38 | 1.14 | 0.54 | 0.4 | 0.74 | 1.13 | 1.15 |
| t-Linalool oxide* | 0.56 | 0.61 | 3.37 | 0.23 | 2.30 | 0.39 | / | / | / | 0.47 | 0.3 | / | 0.41 | 0.92 | 0.73 |
| α−Terpinolene | / | / | / | 5.31 | / | / | / | / | / | / | / | / | / | / | / |
| Linalool* | 11.74 | 17.31 | 13.1 | 5.58 | 15.41 | 19.22 | 3.36 | 2.14 | 1.73 | 4.5 | 7.21 | 9.46 | 0.88 | 4.28 | 5.10 |
| Hotrienol* | 0.71 | 5.98 | 3.41 | 1.55 | 5.92 | 7.17 | 1.44 | 2.53 | 1.00 | 0.49 | / | / | 0.88 | 1.62 | / |
| Rose oxide | / | / | / | / | 0.28 | / | / | / | / | / | / | / | / | / | / |
| Neroloxide | 0.85 | / | / | 0.67 | / | / | / | / | / | / | / | / | / | / | |
| α-Terpineol | / | 4.00 | / | 1.02 | / | / | / | / | / | / | / | / | / | / | / |
| Citronellol* | / | 17.05 | 2.46 | 2.63 | 4.76 | 4.72 | / | 0.49 | 5.50 | 1.72 | / | 1.21 | 4.75 | 0.3 | 7.95 |
| Geraniol* | 0.85 | 0.13 | / | 6.80 | 2.78 | / | / | / | 1.59 | / | / | / | / | 0.36 | 0.12 |
| Farnesol | 0.52 | / | / | / | / | / | / | / | / | 0.61 | / | / | / | / | / |
| β-Fenchene | / | / | / | / | / | 0.71 | / | / | / | / | / | / | / | / | / |
| Epoxy-linalool | / | / | / | / | / | / | / | / | / | / | / | / | 0.46 | / | / |
| ∑ | 16.48 | 50.85 | 25.55 | 24.78 | 36.18 | 34.78 | 4.8 | 12.47 | 11.16 | 8.12 | 10.89 | 17.11 | 9.55 | 8.05 | 11.07 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matijašević, S.; Popović-Djordjević, J.; Ristić, R.; Ćirković, D.; Ćirković, B.; Popović, T. Volatile Aroma Compounds of Brandy ‘Lozovača′ Produced from Muscat Table Grapevine Cultivars (Vitis vinifera L.). Molecules 2019, 24, 2485. https://doi.org/10.3390/molecules24132485
Matijašević S, Popović-Djordjević J, Ristić R, Ćirković D, Ćirković B, Popović T. Volatile Aroma Compounds of Brandy ‘Lozovača′ Produced from Muscat Table Grapevine Cultivars (Vitis vinifera L.). Molecules. 2019; 24(13):2485. https://doi.org/10.3390/molecules24132485
Chicago/Turabian StyleMatijašević, Saša, Jelena Popović-Djordjević, Renata Ristić, Dušica Ćirković, Bratislav Ćirković, and Tatjana Popović. 2019. "Volatile Aroma Compounds of Brandy ‘Lozovača′ Produced from Muscat Table Grapevine Cultivars (Vitis vinifera L.)" Molecules 24, no. 13: 2485. https://doi.org/10.3390/molecules24132485