Solubility and Antioxidant Potential of a Pyrogallol Derivative for Biodiesel Additive
Abstract
:1. Introduction
2. Result and Discussion
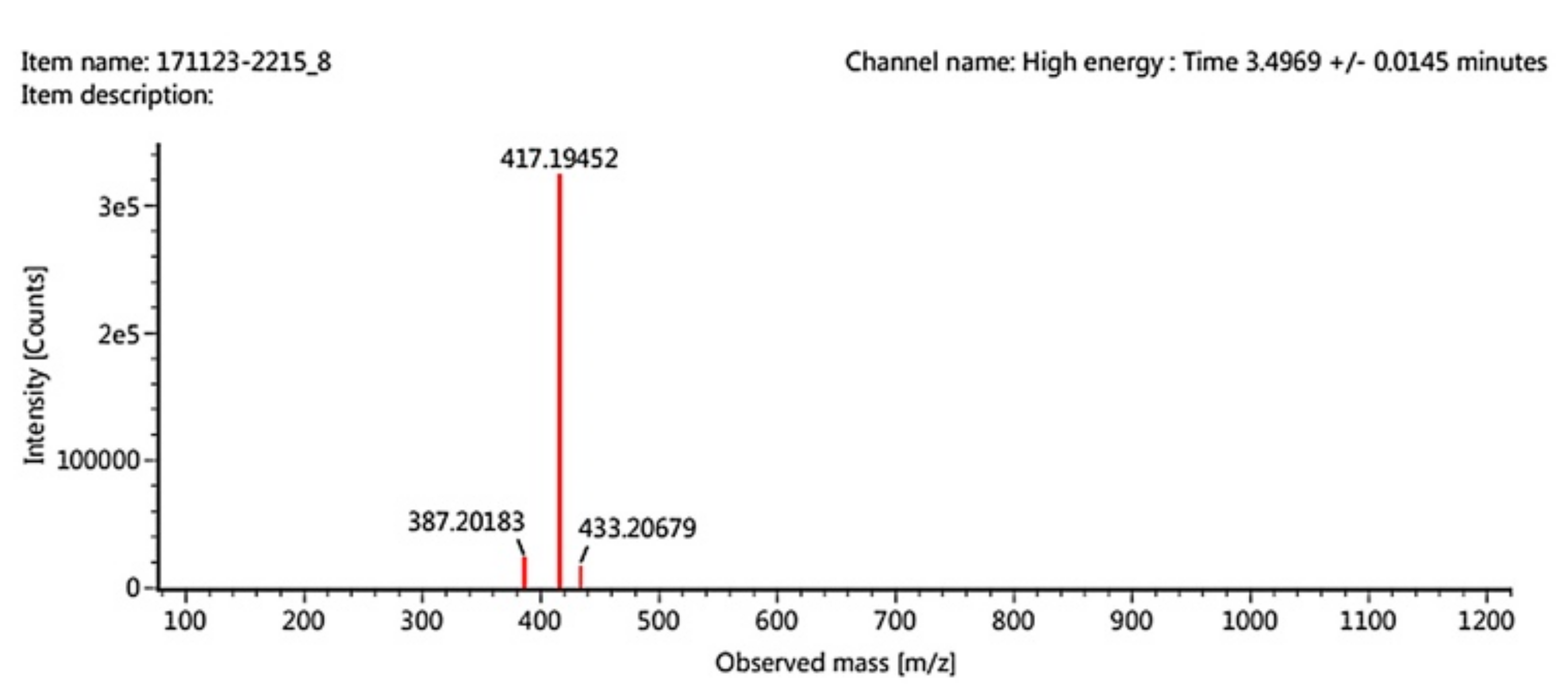
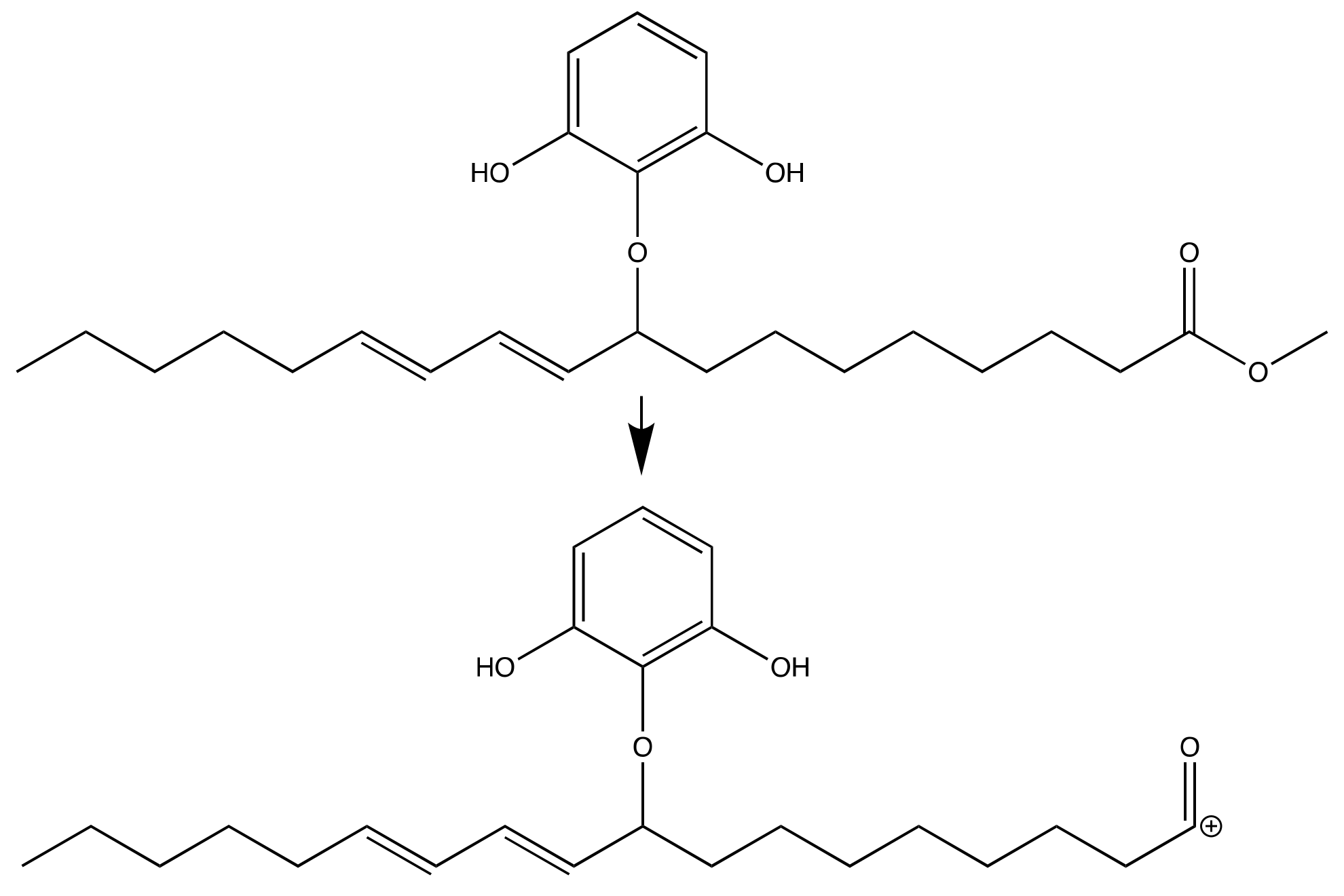
2.1. Synthesis Reaction
2.2. Application in Biodiesel
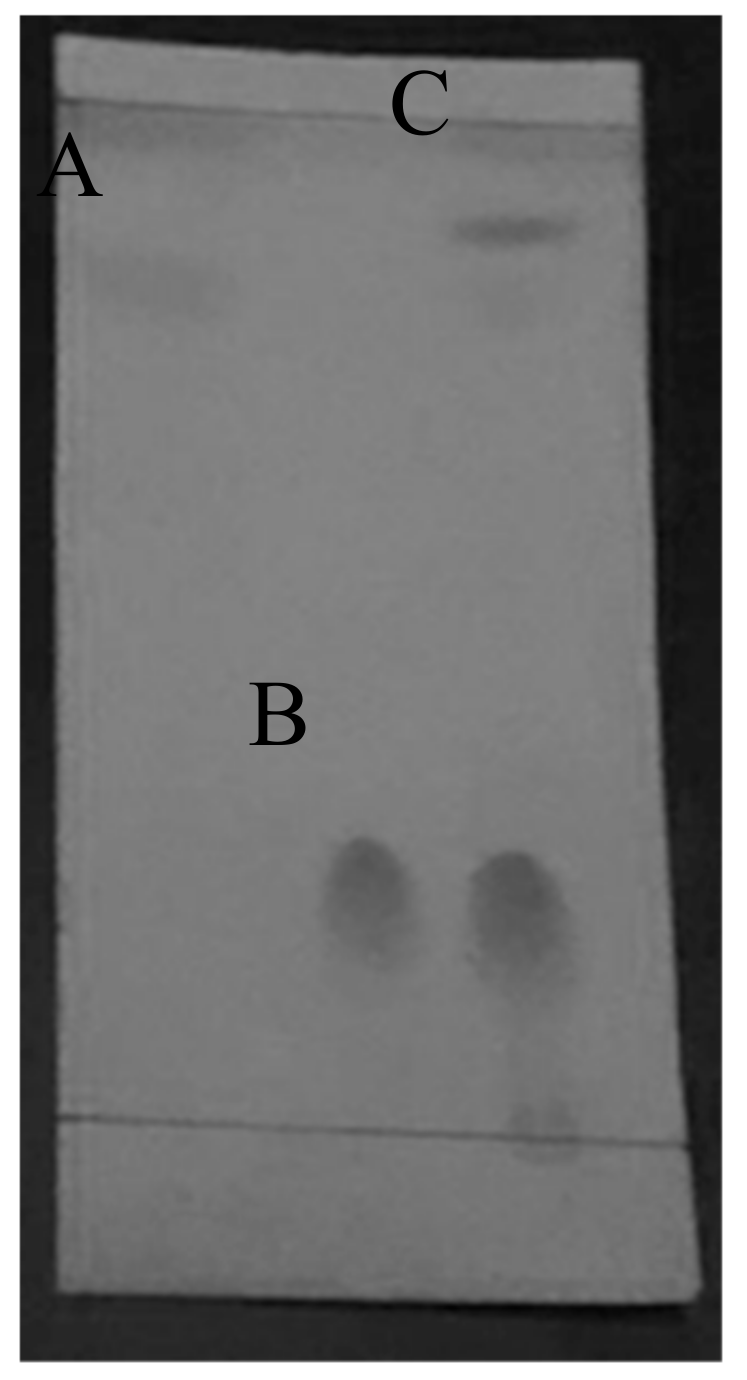
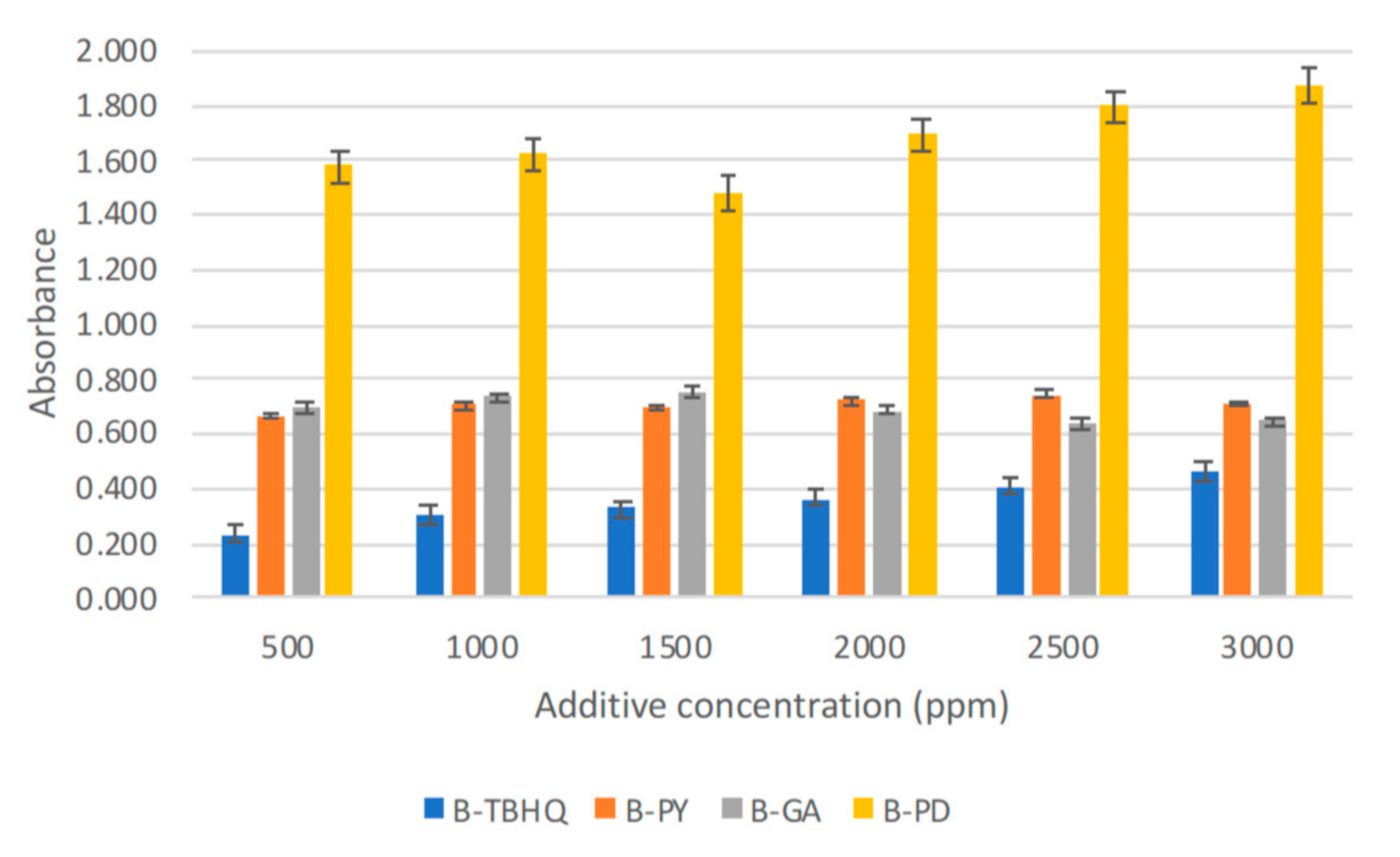
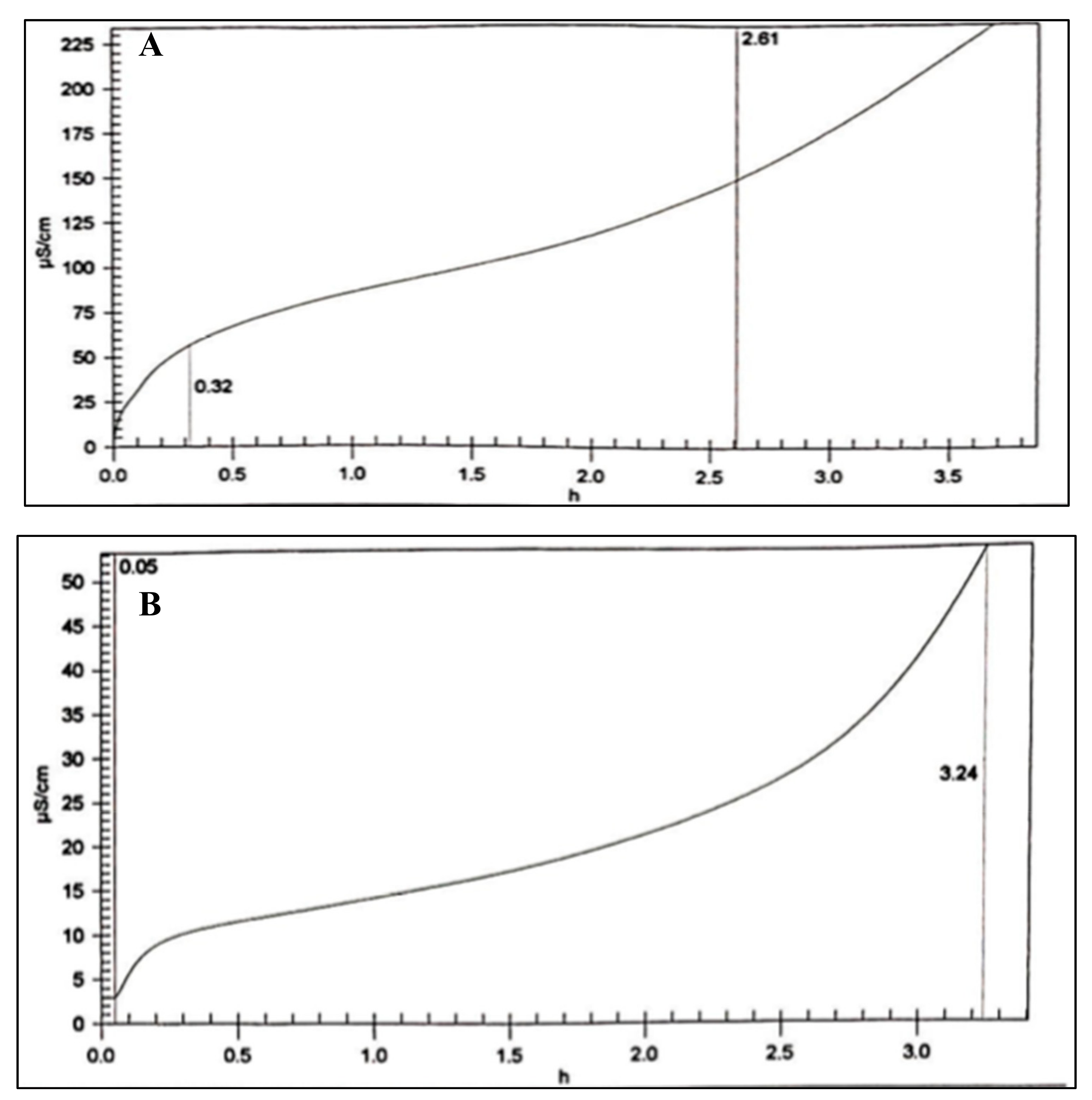
2.2.1. Solubility Test
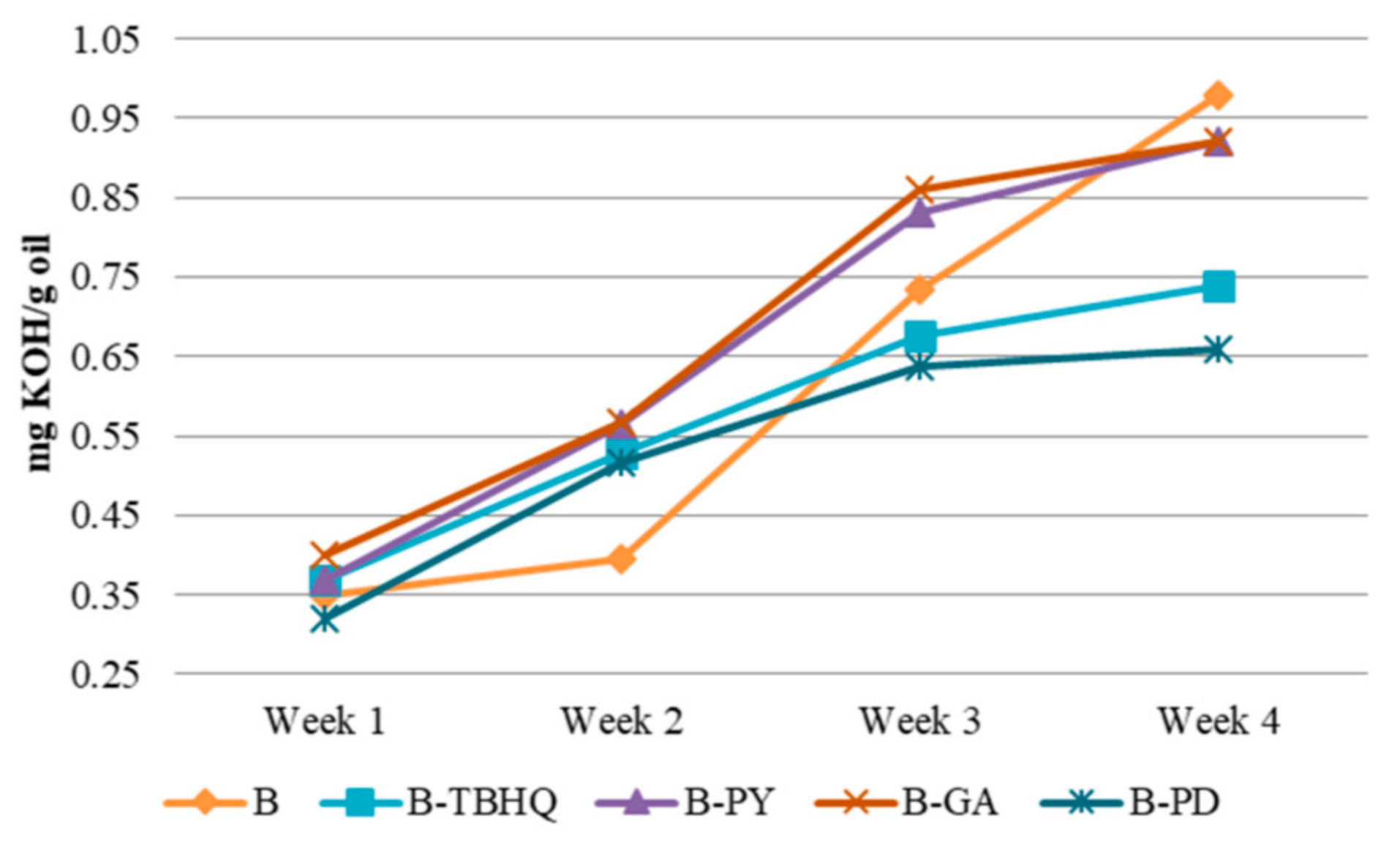
2.2.2. Acid Number
2.2.3. Viscosity
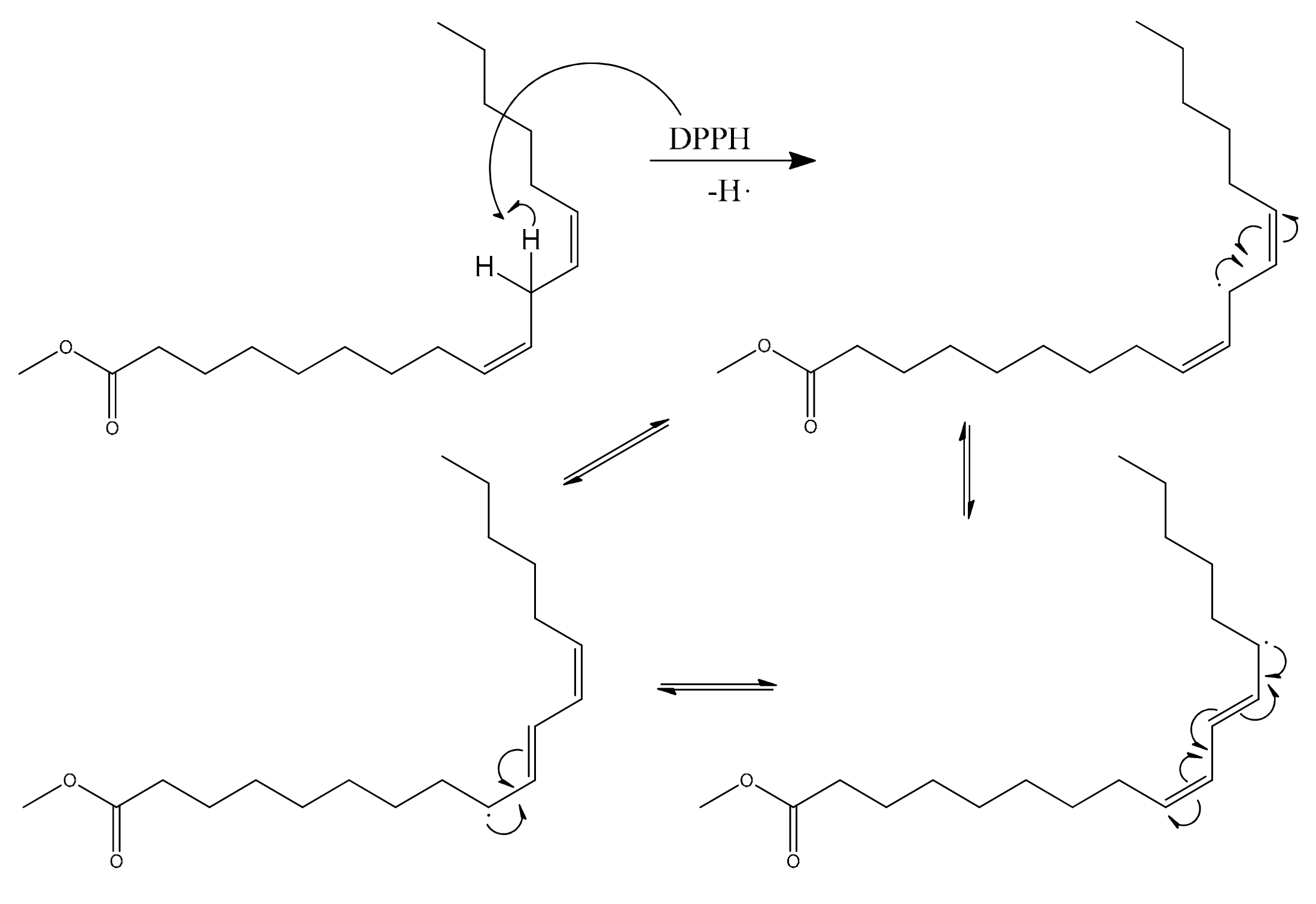
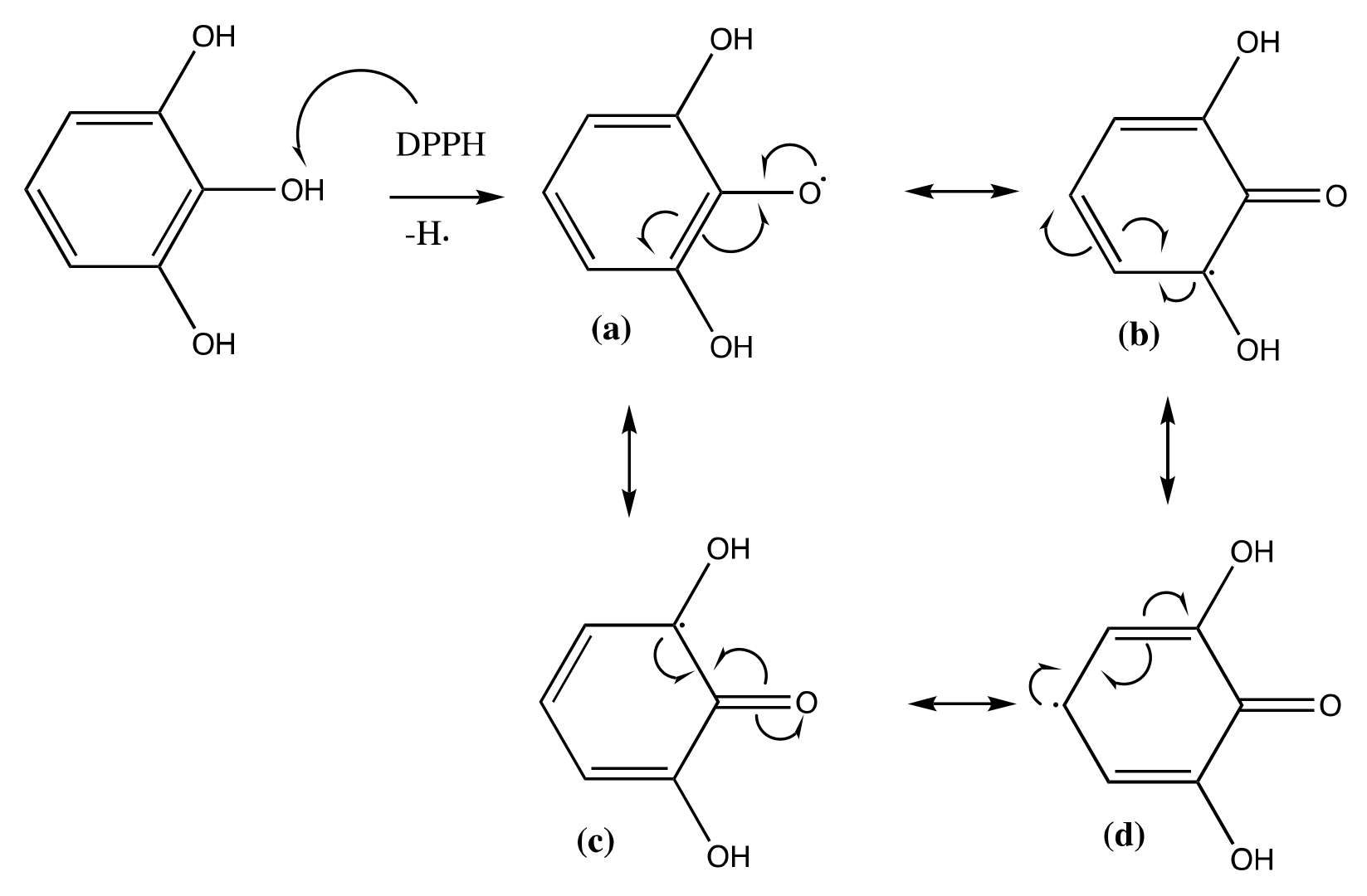
2.2.4. Oxidation Stability Test (Rancimat Test)
3. Methodology
3.1. Materials and Equipment
3.2. Synthesis Reaction
3.3. Application to Biodiesel
3.3.1. Solubility Test
3.3.2. Acid Number
3.3.3. Viscosity
3.3.4. Oxidation Stability Test (Rancimat Test)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Yaakob, Z.; Narayanan, B.N.; Padikkaparambil, S.; Unni K., S.; Akbar P., M. A review on the oxidation stability of biodiesel. Renew. Sustain. Energy Rev. 2014, 35, 136–153. [Google Scholar] [CrossRef]
- Waynick, J.A. Characterization of biodiesel oxidation and oxidation products (CRC Project No. AVFL-2b) SwRI® Project No. 08-10721. Natl. Renew. Energy Lab. 2005, 1–51. [Google Scholar]
- Agarwal, A.K.; Khurana, D. Long-term storage oxidation stability of Karanja biodiesel with the use of antioxidants Initiation. Fuel Process. Technol. 2013, 106, 447–452. [Google Scholar] [CrossRef]
- Sorate, K.A.; Bhale, P.V. Biodiesel properties and automotive system compatibility issues. Renew. Sustain. Energy Rev. 2015, 41, 777–798. [Google Scholar] [CrossRef]
- Brown, W.; Poon, T. Benzene and Its Derivatives. In Introduction to Organic Chemistry, 4th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; pp. 316–322; 334–341. [Google Scholar]
- Saluja, R.K.; Kumar, V.; Sham, R. Stability of biodiesel—A review. Renew. Sustain. Energy Rev. 2016, 62, 866–881. [Google Scholar] [CrossRef]
- Avase, S.A.; Srivastava, S.; Vishal, K.; Ashok, H.V.; Varghese, G. Effect of Pyrogallol as an Antioxidant on the Performance and Emission Characteristics of Biodiesel Derived from Waste Cooking Oil. Procedia Earth Planet. Sci. 2015, 11, 437–444. [Google Scholar] [CrossRef] [Green Version]
- Karavalakis, G.; Hilari, D.; Givalou, L.; Karonis, D.; Stournas, S. Storage stability and ageing effect of biodiesel blends treated with different antioxidants. Energy 2011, 36, 369–374. [Google Scholar] [CrossRef]
- Mittelbach, M.; Schober, S. The influence of antioxidants on the oxidation stability of biodiesel. J. Am. Oil Chem. Soc. 2003, 80, 817–823. [Google Scholar] [CrossRef]
- Varatharajan, K.; Pushparani, D.S. Screening of antioxidant additives for biodiesel fuels. Renew. Sustain. Energy Rev. 2018, 83, 2017–2028. [Google Scholar] [CrossRef]
- O’Neil, M.J.; Heckelman, P.E.; Koch, C.B.; Roman, K.J. The Merck Index, 14th ed.; Merck: Whitehouse Station, NJ, USA, 2006; p. 811. [Google Scholar]
- Sutanto, H.; Susanto, B.H.; Nasikin, M. The Effect of Surfactant Addition towards Dispersion and Antioxidant Activity of tert-butylhydroquinone in Biodiesel. Int. J. Renew. Energy Res. 2018, 8, 1974–1979. [Google Scholar]
- Subroto, E.; Manurung, R.; Heeres, H.J.; Broekhuis, A.A. Screening of antioxidants as stabilisers forJatropha curcasL. oil. Eur. J. Lipid Sci. Technol. 2013, 115, 909–920. [Google Scholar] [CrossRef]
- Santos, N.A.; Cordeiro, A.M.; Damasceno, S.S.; Aguiar, R.T.; Rosenhaim, R.; Filho, J.R.C.; Santos, I.M.; Maia, A.S.; Souza, A.G. Commercial antioxidants and thermal stability evaluations. Fuel 2012, 97, 638–643. [Google Scholar] [CrossRef]
- Jiang, T.; Fang, M.; Li, Y.; Zhao, Q.; Dai, L. Alkylation of phenol/tert‑butyl alcohol on ionic liquid-immobilized SBA-15 with different pore sizes. J. Taiwan Inst. Chem. Eng. 2017, 80, 1031–1040. [Google Scholar] [CrossRef]
- Wilson, C.P., Jr. Manufacture of Oil-Soluble Polyhydric Phenols. U.S. Patent 2063212, 8 December 1936. [Google Scholar]
- DeBlase, F.J.; Fox, B.E.; Migdal, C.A. Antioxidant Additive for Biodiesel Fuels. US Patents No. US20110209390A1, 20 November 2012. [Google Scholar]
- Freire, M.A.; Mendes, D.T.; Freitas, L.S.; Beerthuis, R.; Amarante, S.F.; Ramos, A.L. Acid-catalyzed liquid-phase alkylation of phenol with branched and linear olefin isomers. Catal. Today 2017, 289, 192–203. [Google Scholar] [CrossRef]
- Muniz-Wypych, A.S.; Da Costa, M.M.; Oliveira, A.R.S.; Neu, P.M.; Schober, S.; Mittelbach, M.; Ramos, L.P.; César-Oliveira, M.A.F.; Muniz-Wypych, A.S.; César-Oliveira, M.A.F. Phenolic compounds obtained from alkyl oleates as additives to improve the oxidative stability of methyl rapeseed biodiesel. Eur. J. Lipid Sci. Technol. 2017, 119, 1700179. [Google Scholar] [CrossRef]
- Leo, A.; Hansch, C.; Elkins, D. Partition coefficients and their uses. Chem. Rev. 1971, 71, 525–616. [Google Scholar] [CrossRef]
- Pullen, J.; Saeed, K. An overview of biodiesel oxidation stability. Renew. Sustain. Energy Rev. 2012, 16, 5924–5950. [Google Scholar] [CrossRef]
- Li, X. Improved Pyrogallol Autoxidation Method: A Reliable and Cheap Superoxide-Scavenging Assay Suitable for All Antioxidants. J. Agric. Food Chem. 2012, 60, 6418–6424. [Google Scholar] [CrossRef]
- Pines, E. The Chemistry of Phenols; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Park, S.-Y.; Shin, S.-R.; Shin, J.-I.; An, K.-L.; Jun, K. Synthesis of Antioxidant and Evaluation of Its Oxidation Stability for Biodiesel. J. Korean Soc. Tribol. Lubr. Eng. 2013, 29, 392–396. [Google Scholar] [CrossRef] [Green Version]
- Osawa, W.O.; Sahoo, P.K.; Onyari, J.M.; Mulaa, F.J. Effects of antioxidants on oxidation and storage stability of Croton megalocarpus biodiesel. Int. J. Energy Environ. Eng. 2016, 7, 85–91. [Google Scholar] [CrossRef]
- Sutanto, H.; Ainny, L.; Lukman; Susanto, B.H.; Nasikin, M. Reaction product of pyrogallol with methyl linoleate and its antioxidant potential for biodiesel. IOP Conf. Ser. Mater. Sci. Eng. 2018, 316, 012019. [Google Scholar] [CrossRef]
- Zhu, N.; Wang, M.; Wei, G.-J.; Lin, J.-K.; Yang, C.S.; Ho, C.-T. Identification of reaction products of (−)-epigallocatechin, (−)-epigallocatechin gallate and pyrogallol with 2,2-diphenyl-1-picrylhydrazyl radical. Food Chem. 2001, 73, 345–349. [Google Scholar] [CrossRef]
- Yusri, S.; Sutanto, H.; Nasikin, M. The optimization of pyrogallol and methyl linoleate coupling reaction The Optimization of Pyrogallol and Methyl Linoleate Coupling Reaction. AIP Conf. Proc. 2018, 020006, 1–7. [Google Scholar]
- Prevc, T.; Šegatin, N.; Ulrih, N.P.; Cigić, B. DPPH assay of vegetable oils and model antioxidants in protic and aprotic solvents. Talanta 2013, 109, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Jahan, M.S. Peroxy Radical Learn more about Peroxy Radical ESR Insights into Macroradicals in UHMWPE; William Andrew Publishing: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Yusri, S.; Sutanto, H.; Nasikin, M. Size-Selective Adsorption in Separation of Products from Pyrogallol and Methyl Linoleate Oxidative Coupling Reaction. Presented at 2018 2nd International Conference on Green Energy and Applications (ICGEA), ICGEA Committees, Singapore, 24–26 March 2018. [Google Scholar]
- Indonesia, B.S.N. SNI 01-3555-1998: Cara Uji Lemak dan Minyak; Badan Standarisasi Nas.: Jakarta, Indonesia, 1998. [Google Scholar]
- Basumatary, T.; Chetia, D.; Singh, H.; Bezboruah, T. Fiber Optic Viscometer Based on Sliding of Liquid Drop Under Gravity on Inclined Flow Channel. IEEE Trans. Instrum. Meas. 2016, 65, 1–9. [Google Scholar] [CrossRef]
- Zulkifli, N.W.M.; Rashedul, H.K.; Rashed, M.M.; Imdadul, H.K.; Mosarof, M.H.; Monirul, I.M.; Masjuki, H.H.; Kalam, M.A. A comprehensive review on biodiesel cold flow properties and oxidation stability along with their improvement processes. RSC Adv. 2015, 5, 86631–86655. [Google Scholar]
- Loyall, U.; Zumbrägel, B.; Kalcher, M. Determination of the oxidative stability of biodiesel (fatty acid methyl esters, FAME). Herisau. Metrohm. AG 2011, 8, 17. [Google Scholar]
Sample Availability: Samples of the compounds pyrogallol derivative are not available from the authors. |
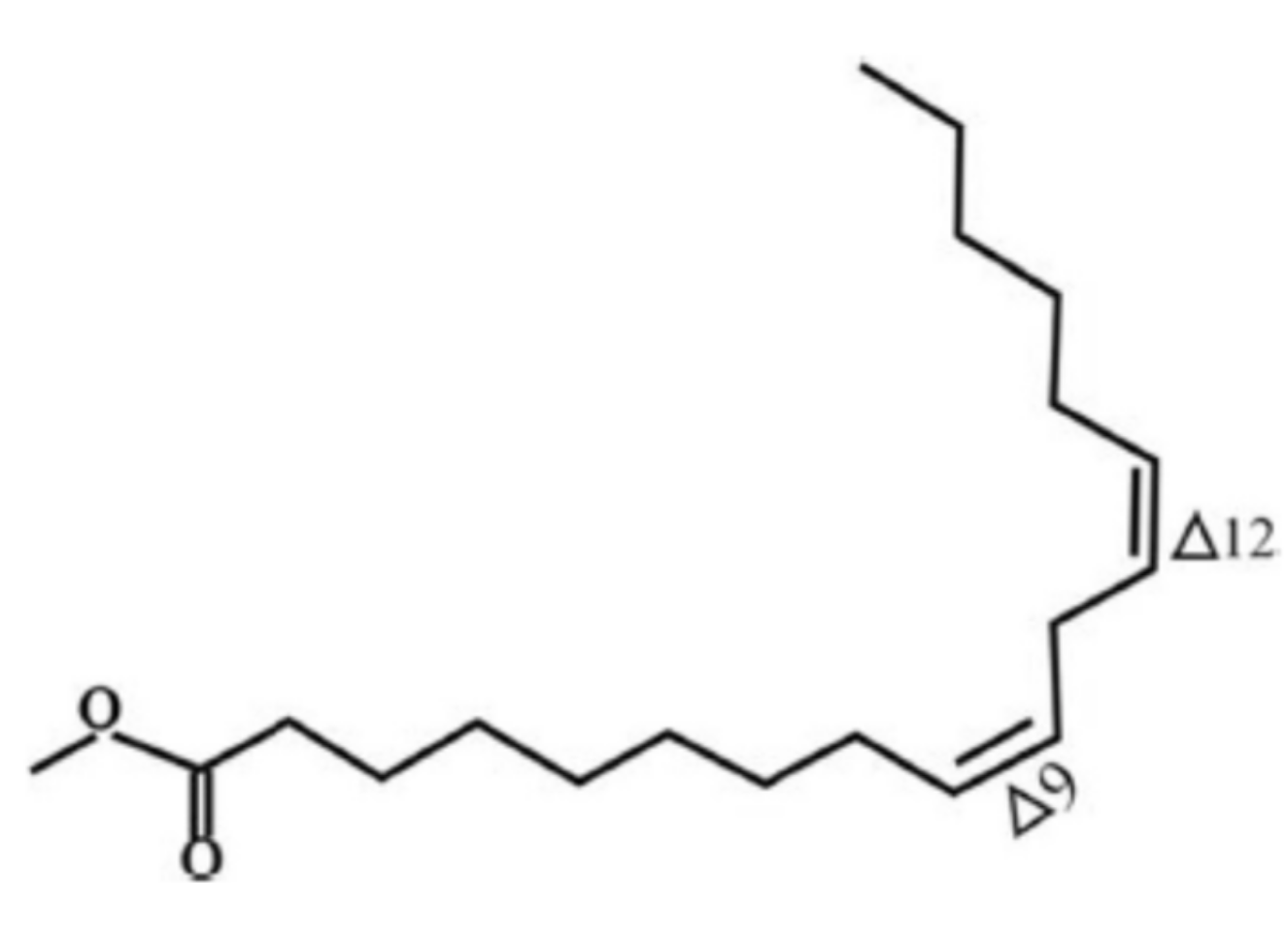







| Carbon | Chemical Shift (ppm) | 1H-NMR Signal | |
|---|---|---|---|
| 13C-NMR | 1H-NMR | ||
| a | 14.271 | 0.873 | t, 3H |
| b | 22.766 | 1.225–1.307 | m, 2H |
| c | 25.809 | m, 2H | |
| d | 29.303 | m, 2H | |
| l | 31.712 | m, 2H | |
| m | 29.773 | m, 2H | |
| n | 29.533 | m, 2H | |
| o | 27.373 | m, 2H | |
| p | 25.127 | m, 2H | |
| e | 27.373 | 2.025 | q, 2H |
| k | 27.373 | q, 2H | |
| q | 34.303 | 2.290 | t, 2H |
| f | 128.092 | 5.288–5.383 | m, 1H |
| g | 128.227 | m, 1H | |
| h | 130.242 | m, 1H | |
| i | 130.415 | m, 1H | |
| r | 174.656 | No proton | - |
| s | 51.696 | 3.653 | s, 3H |
| u | 108.175 | 6.435 | d, 1H |
| w | d, 1H | ||
| y | 144.362 | No proton | - |
| v | 120.308 | 6.634 | t, 1H |
| j | 58.742 | 3.682–3.724 | q, 1H |
| x | Not detected | Not detected | s, OH |
| t | s, OH | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sutanto, H.; Susanto, B.H.; Nasikin, M. Solubility and Antioxidant Potential of a Pyrogallol Derivative for Biodiesel Additive. Molecules 2019, 24, 2439. https://doi.org/10.3390/molecules24132439
Sutanto H, Susanto BH, Nasikin M. Solubility and Antioxidant Potential of a Pyrogallol Derivative for Biodiesel Additive. Molecules. 2019; 24(13):2439. https://doi.org/10.3390/molecules24132439
Chicago/Turabian StyleSutanto, Hery, Bambang Heru Susanto, and Mohammad Nasikin. 2019. "Solubility and Antioxidant Potential of a Pyrogallol Derivative for Biodiesel Additive" Molecules 24, no. 13: 2439. https://doi.org/10.3390/molecules24132439





