Suitability Evaluation of LaNi5 as Hydrogen-Storage-Alloy Actuator by In-Situ Displacement Measurement during Hydrogen Pressure Change
Abstract
:1. Introduction
2. Results and Discussions
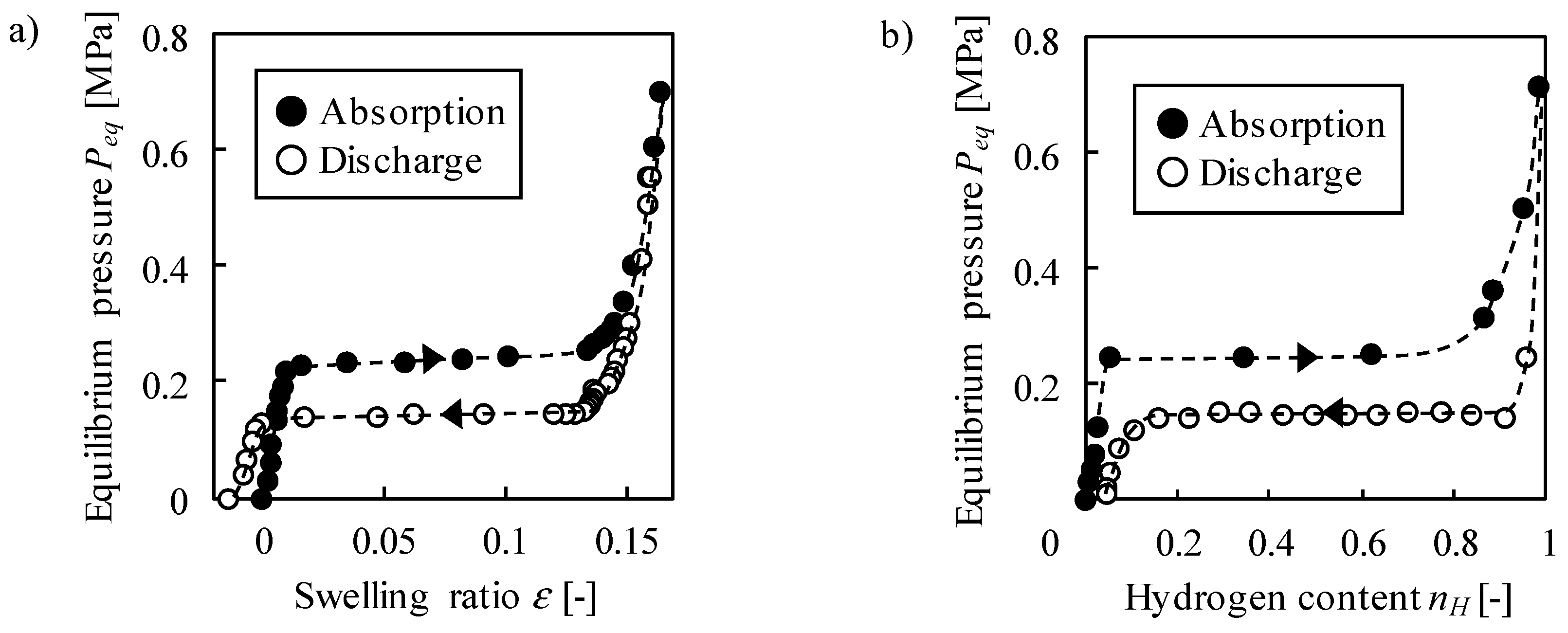
2.1. Static Properties—Equilibrium Swelling Ratio
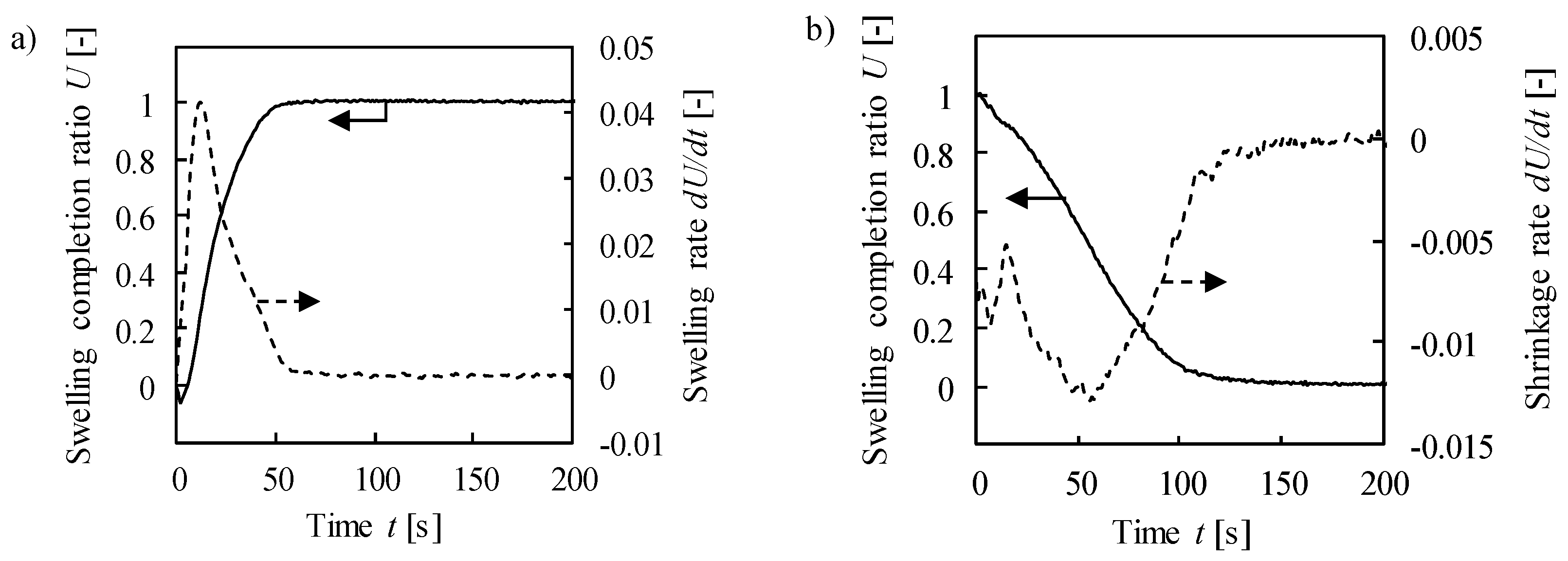
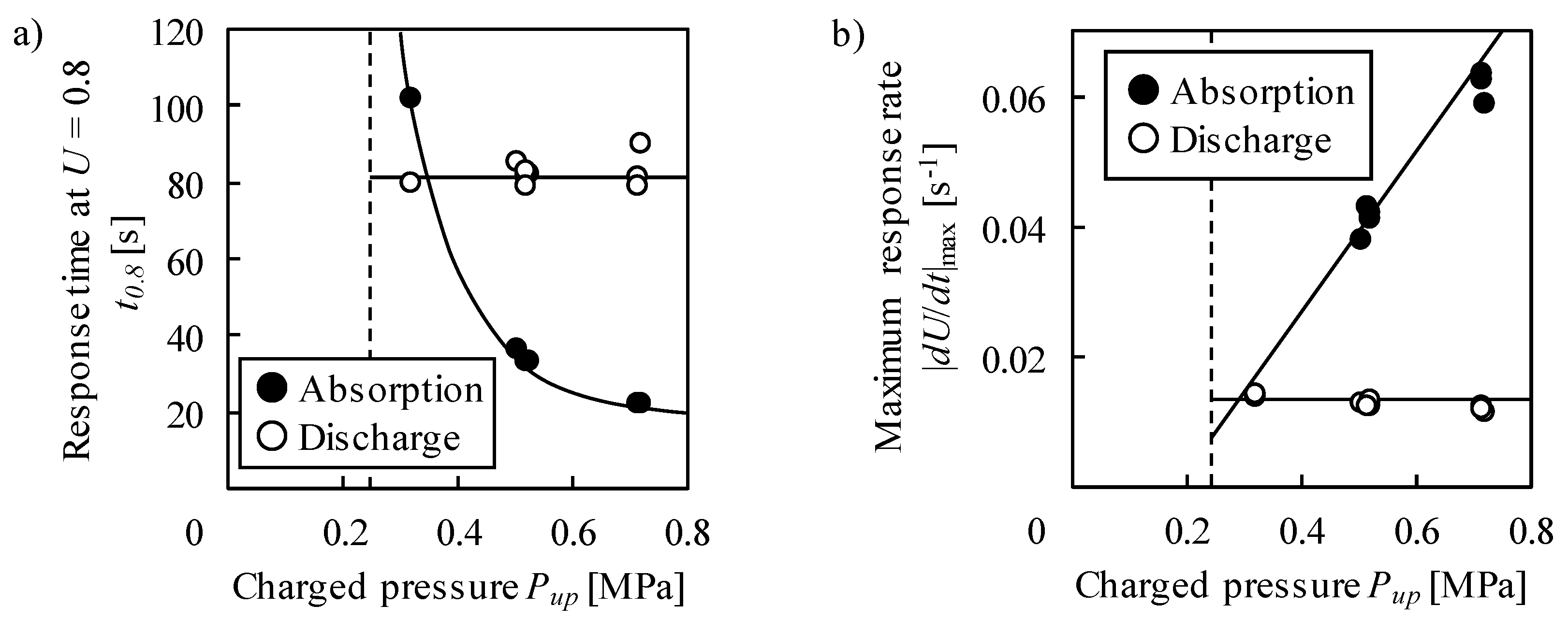
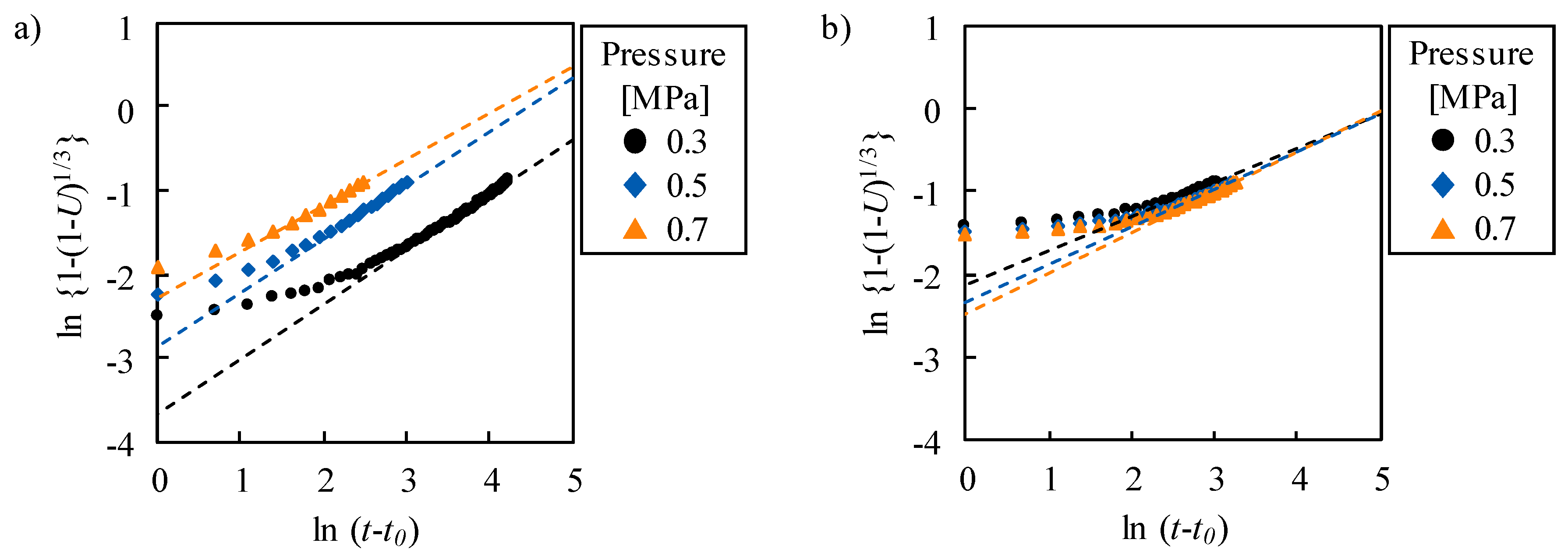
2.2. Kinetic Properties—Response Time
2.3. Suitability of LaNi5 as an Actuator Material
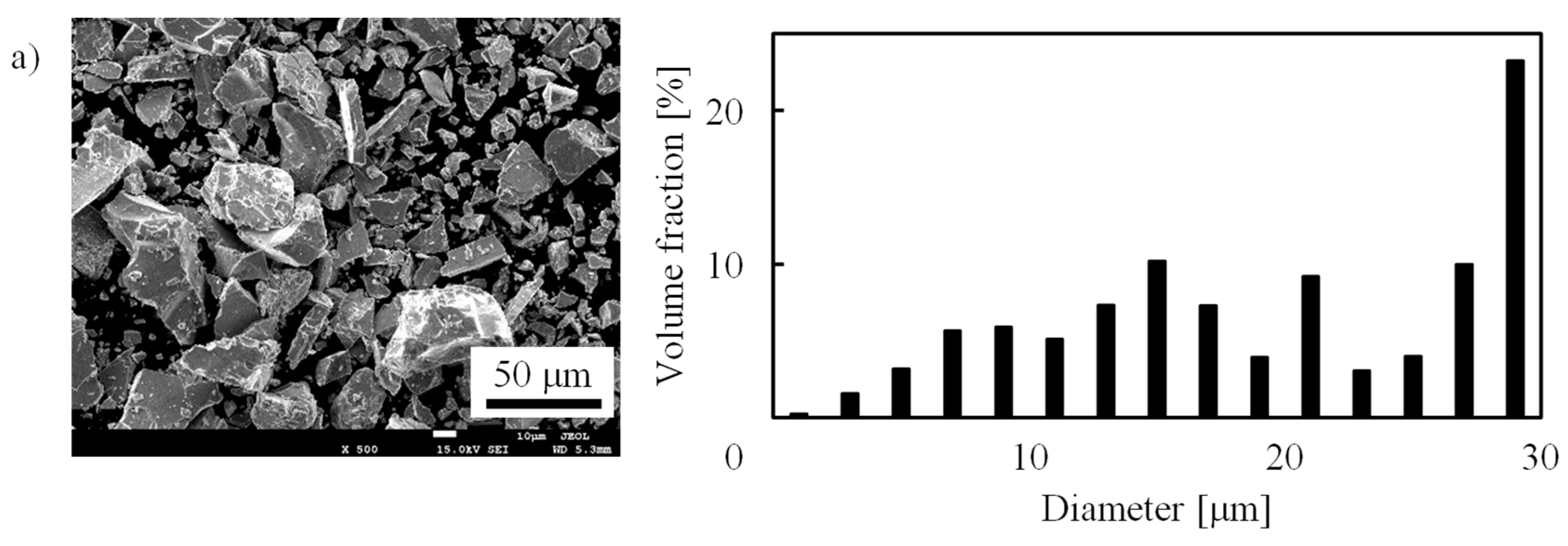
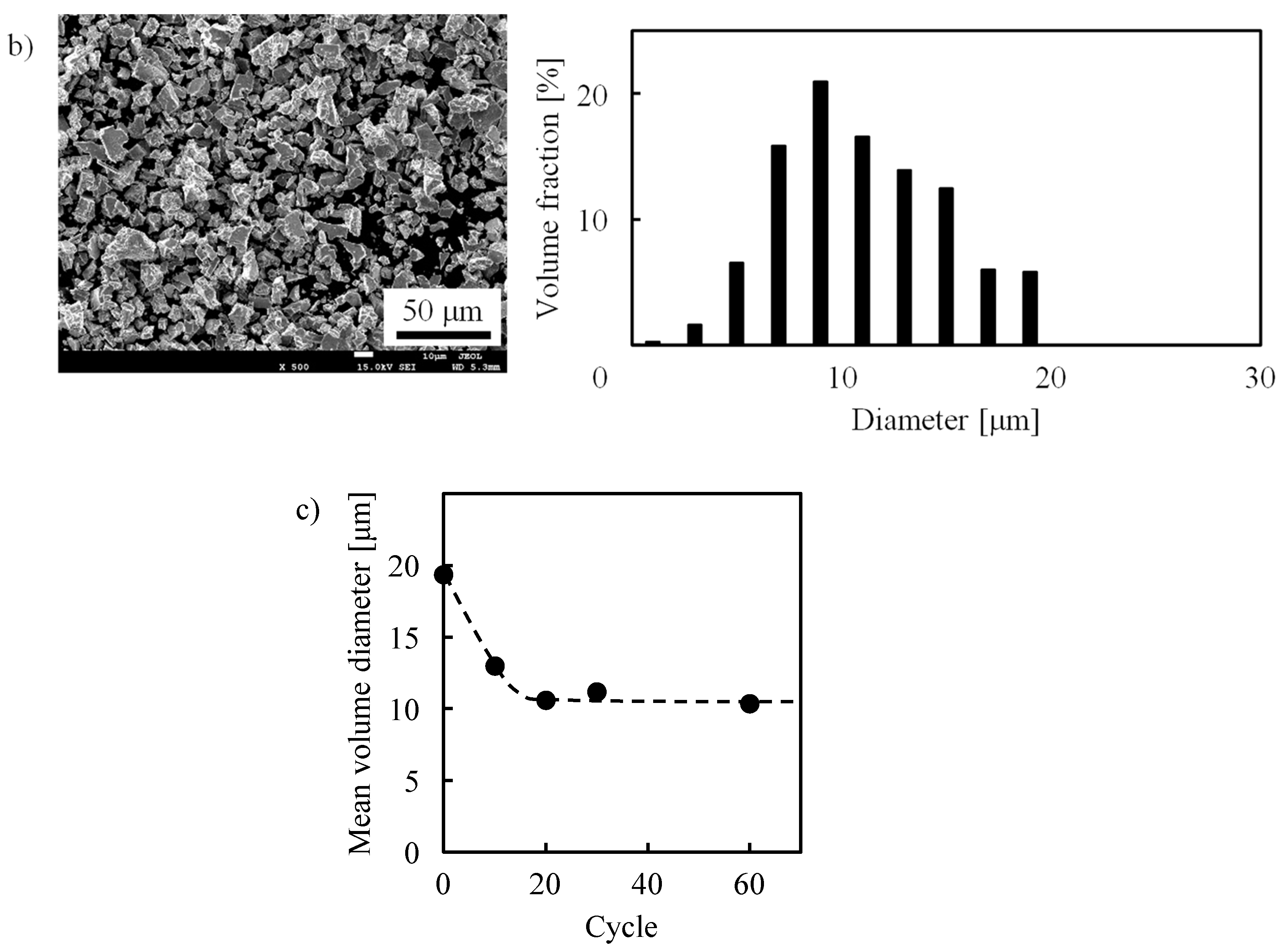
3. Experiment
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A. Dependence of Lattice Swelling Ratio on Hydrogen Content
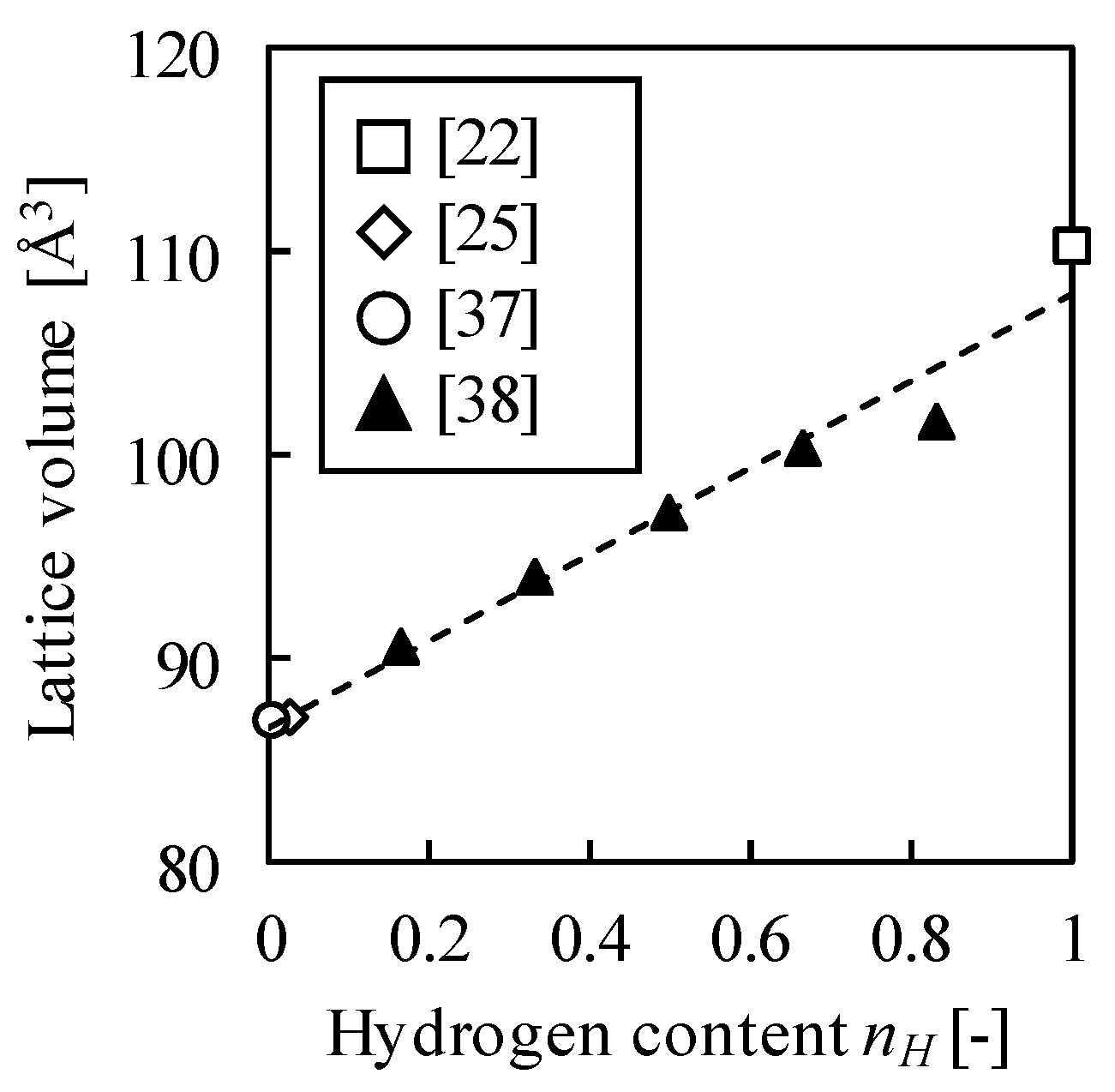
References
- Goshome, K.; Jain, A.; Miyaoka, H.; Yamamoto, H.; Kojima, Y.; Ichikawa, T. Eutectic phenomenon of LiNH2-KH composite in MH-NH3 hydrogen storage system. Molecules 2019, 24, 1348. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Pan, H.; Gao, M.; Wang, Q. Advanced hydrogen storage alloys for Ni/MH rechargeable batteries. J. Mater. Chem. 2011, 21, 4743–4755. [Google Scholar] [CrossRef]
- Jurczyk, M.; Nowak, M. Introduction to hydrogen based thermal energy conversion. In Hydrogen Storage Materials; Burzo, E., Ed.; Springer: Berlin, Germany, 2018. [Google Scholar]
- Nakai, A.; Mizumoto, M.; Kagawa, A. Bending and rotation movement control of a novel actuator utilizing hydrogen storage alloys. Adv. Mater. Res. 2011, 156–157, 1170–1175. [Google Scholar] [CrossRef]
- Mizumoto, M.; Ohgai, T.; Kagawa, A. Bending behavior of Cu-plated Pd-Ni alloys ribbon driven by hydrogenation. J. Alloy. Comp. 2009, 482, 416–419. [Google Scholar] [CrossRef]
- Kagawa, A.; Taniguchi, K.; Yamamoto, M. Designs for miniaturization of bending actuator utilizing hydrogen storage alloy. J. Alloy. Comp. 2013, 563, 203–206. [Google Scholar] [CrossRef] [Green Version]
- Mizumoto, M.; Ohgai, T.; Kagawa, A. Development of actuator utilizing hydrogen storage alloys. In Next-Generation Actuators Leading Breakthroughs; Higuchi, T., Suzumori, K., Tadokoro, S., Eds.; Springer: London, UK, 2010; pp. 337–349. [Google Scholar]
- Nishi, Y.; Uchida, H.; Honjo, T. High responsiveness induced by palladium deposition on thin film actuator of LaNi5 hydrogen storage alloy. Mater. Trans. 2005, 46, 126–129. [Google Scholar] [CrossRef]
- Okawa, J.; Kanda, M.; Yuse, K.; Uchida, H.; Guyomar, D.; Nishi, Y. Reversible bending motion of unimorph composites driven by combining LaNi5 alloy powders dispersed polyurethane and thin supporting copper sheet under partial hydrogen gas pressure. Mater. Trans. 2010, 51, 994–1001. [Google Scholar] [CrossRef]
- Nishi, Y.; Ohkawa, J.; Faudree, M.C.; Kanda, M.; Yuse, K.; Guyomar, D.; Uchida, H. An improved H2-gas pressure operated LaNi5 powder-dispersed polyurethane/titanium 2-layer actuator with reversible giant and rapid expansion by hydrogenation. Mater. Trans. 2018, 59, 129–135. [Google Scholar] [CrossRef]
- Goto, K.; Higuchi, T.; Fuchiwaki, O.; Nakao, W. Design of capsule-type micro actuator utilizing hydrogen storage alloys by finite element analysis. Trans. Mater. Res. Soc. Jpn. 2014, 39, 145–152. [Google Scholar] [CrossRef]
- Goto, K.; Hirata, T.; Higuchi, T.; Fuchiwaki, O.; Ozaki, S.; Nakao, W. Deformation mechanism of capsule-type hydrogen-storage-alloy actuator. Int. J. Hydrogen Energy. (in press). [CrossRef]
- Nasako, K.; Ito, Y.; Hiro, N.; Osumi, M. Stress on a reaction vessel by the swelling of a hydrogen absorbing alloy. J. Alloy. Comp. 1998, 264, 271–276. [Google Scholar] [CrossRef]
- Saito, T.; Suwa, K.; Kawamura, T. Influence of expansion of metal hydride during hydriding-dehydriding cycles. J. Alloy. Comp. 1997, 253–254, 682–685. [Google Scholar] [CrossRef]
- Lin, C.; Huang, S.; Jhang, Y. Effects of cyclic hydriding-dehydriding reactions of Mg2Ni alloy on the expansion deformation of a metal hydride storage vessel. J. Alloy. Comp. 2011, 509, 7162–7167. [Google Scholar] [CrossRef]
- Goto, K.; Hirata, T.; Yamamoto, I.; Nakao, W. Swelling response behavior of palladium during hydrogen absorption and discharge. Int. J. Hydrogen Energy 2018, 43, 11092–11099. [Google Scholar] [CrossRef]
- Matsushita, M.; Monde, M.; Mitsutake, Y. Experimental formula for estimating porosity in a metal hydride packed bed. Int. J. Hydrogen Energy 2013, 38, 7056–7064. [Google Scholar] [CrossRef]
- Ribeiro, E.S.; Gil, J.M. A novel capacitive device for the study of volumetric expansion of hydride powders. Int. J. Hydrogen Energy 2015, 40, 14900–14910. [Google Scholar] [CrossRef]
- Charlas, B.; Chaise, A.; Gillia, O.; Doremus, P.; Imbault, D. Investigation of hydride powder bed swelling and shrinking during hydrogen absorption/desorption cycles under different compressive stresses. J. Alloy. Comp. 2013, 580, S149–S152. [Google Scholar] [CrossRef]
- Mendelsohn, M.; Gruen, D.; Dwight, A. Group 3A and 4A substituted AB5 hydrides. Inorg. Chem. 1979, 18, 3343–3345. [Google Scholar] [CrossRef]
- Wallace, W.E.; Karlicek, R.F.; Imamura, H. Mechanism of hydrogen absorption by LaNi5. J. Phys. Chem. 1979, 83, 1708–1712. [Google Scholar] [CrossRef]
- Halstead, T.K. Proton NMR studies of lanthanum nickel hydride: Structure and diffusion. J. Solid State Chem. 1974, 11, 114–119. [Google Scholar] [CrossRef]
- Wicke, E.; Brodowsky, H.; Züchner, H. Hydrogen in palladium and palladium alloy. In Hydrogen in Metals; Alefeld, G., Völkl, J., Eds.; Springer: Berlin, Germany, 1978; pp. 73–155. [Google Scholar] [CrossRef]
- Bowman, R.C., Jr.; Gruen, D.M.; Mendelsohn, H. NMR studies of hydrogen diffusion in β-LaNi5-yAly hydrides. Solid State Commun. 1979, 32, 501–506. [Google Scholar] [CrossRef]
- Lynch, J.F.; Reilly, J.J. Behavior of H-LaNi5 solid solutions. J. Less Common Met. 1982, 87, 225–236. [Google Scholar] [CrossRef]
- Van Mal, H.H.; Buschow, K.H.J.; Miedema, A.R. Hydrogen absorption in LaNi5 and related compounds: Experimental observations and their explanation. J. Less Common Met. 1974, 35, 65–76. [Google Scholar] [CrossRef]
- Park, C.N.; Lee, J.Y. Kinetic properties of the hydrogenation of the FeTi intermetallic compound. J. Less Common Met. 1983, 91, 189–201. [Google Scholar] [CrossRef]
- Vigier, F.; Jurczakowski, R.; Lasia, A. Determination of hydrogen absorption isotherm and diffusion coefficient in Pd81Pt19 alloy. J. Electroanal. Chem. 2006, 588, 32–43. [Google Scholar] [CrossRef]
- Goto, K.; Ozaki, S.; Nakao, W. Effect of diffusion coefficient variation on interrelation between hydrogen diffusion and induced internal stress in hydrogen storage alloys. J. Alloy. Comp. 2017, 691, 705–712. [Google Scholar] [CrossRef]
- Zhang, W.S.; Hou, M.Q.; Wang, H.Y.; Fu, Y.B. Numerical simulation of diffusivity of hydrogen in thin tubular metallic membranes affected by self-stresses. Int. J. Hydrogen Energy 2004, 29, 1165–1172. [Google Scholar] [CrossRef]
- Kirchheim, R. Hydrogen solubility and diffusivity in defective and amorphous metals. Prog. Mater. Sci. 1988, 32, 261–325. [Google Scholar] [CrossRef]
- Deutges, M.; Barth, H.P.; Chen, Y.; Borchers, C.; Kirchheim, R. Hydrogen diffusivities as a measure of relative dislocation densities in palladium and increase of the density by plastic deformation in the presence of dissolved hydrogen. Acta Mater. 2015, 82, 266–274. [Google Scholar] [CrossRef]
- Kuji, T.; Uchida, H.; Sato, M.; Cui, W. Thermodynamic properties of hydrogen in fine Pd powders. J. Alloy. Comp. 1999, 293–295, 19–22. [Google Scholar] [CrossRef]
- Sandrock, G. A panoramic overview of hydrogen storage alloys from a gas reaction point of view. J. Alloy. Comp. 1999, 293–295, 877–888. [Google Scholar] [CrossRef]
- ImageJ User Guide. Available online: https://imagej.nih.gov/ij/docs/guide/index.html (accessed on 7 May 2019).
- Frieske, H.; Wicke, E. Magnetic susceptibility and equilibrium diagram of PdHn. Ber. Bunsenges. Phys. Chem. 1973, 77, 48–52. [Google Scholar] [CrossRef]
- Thompson, P.; Reilly, J.J.; Hastings, J.M. The accommodation of strain and particle size broadening in Rietveld refinement; its application to de-deuterided LaNi5 alloy. J. Less Common Met. 1987, 129, 105–114. [Google Scholar] [CrossRef]
- Zhang, C.; Gao, T.; Qi, X.; Zhang, Y.; Tang, L.; Zhou, J.; Chen, B. First-principles study of the micro-arrangement of hydrogen atoms and electronic properties of LaNi5Hx (x: 0.5–7). Physica B 2008, 403, 2372–2382. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds LaNi5 are available from the authors. |
| LaNi5 | Palladium | |
|---|---|---|
| Phase transition pressure [MPa] | ||
| During absorption | 0.20 [20] | 0.002 [16] |
| During discharge | 0.16 [20] | 0.0009 [16] |
| Lattice expansion ratio of hydride [−] | 0.27 [22] | 0.11 [23] |
| Diffusion coefficient of hydride at 300 K [m2/s] | 1.5 × 10−12 [24] | 1.3 × 10−11 [16] |
| LaNi5 | Palladium [16] | |
|---|---|---|
| Swelling ratio/packing fraction ε/fV [−] | 0.23 | 0.13 |
| Response time at U = 0.8 t80 [s] | ||
| During absorption | 33 | 3 |
| During discharge | 55 | 12,230 |
| Maximum response rate [s−1] (time [s]) | ||
| During absorption | 0.041 (12) | 0.46 (1.3) |
| During discharge | −0.013 (56) | −0.01 (2) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goto, K.; Hirata, T.; Yamamoto, I.; Nakao, W. Suitability Evaluation of LaNi5 as Hydrogen-Storage-Alloy Actuator by In-Situ Displacement Measurement during Hydrogen Pressure Change. Molecules 2019, 24, 2420. https://doi.org/10.3390/molecules24132420
Goto K, Hirata T, Yamamoto I, Nakao W. Suitability Evaluation of LaNi5 as Hydrogen-Storage-Alloy Actuator by In-Situ Displacement Measurement during Hydrogen Pressure Change. Molecules. 2019; 24(13):2420. https://doi.org/10.3390/molecules24132420
Chicago/Turabian StyleGoto, Kenta, Tomoyuki Hirata, Isao Yamamoto, and Wataru Nakao. 2019. "Suitability Evaluation of LaNi5 as Hydrogen-Storage-Alloy Actuator by In-Situ Displacement Measurement during Hydrogen Pressure Change" Molecules 24, no. 13: 2420. https://doi.org/10.3390/molecules24132420






