Purification, Characterization, Prebiotic Preparations and Antioxidant Activity of Oligosaccharides from Mulberries
Abstract
:1. Introduction
2. Results and Discussion
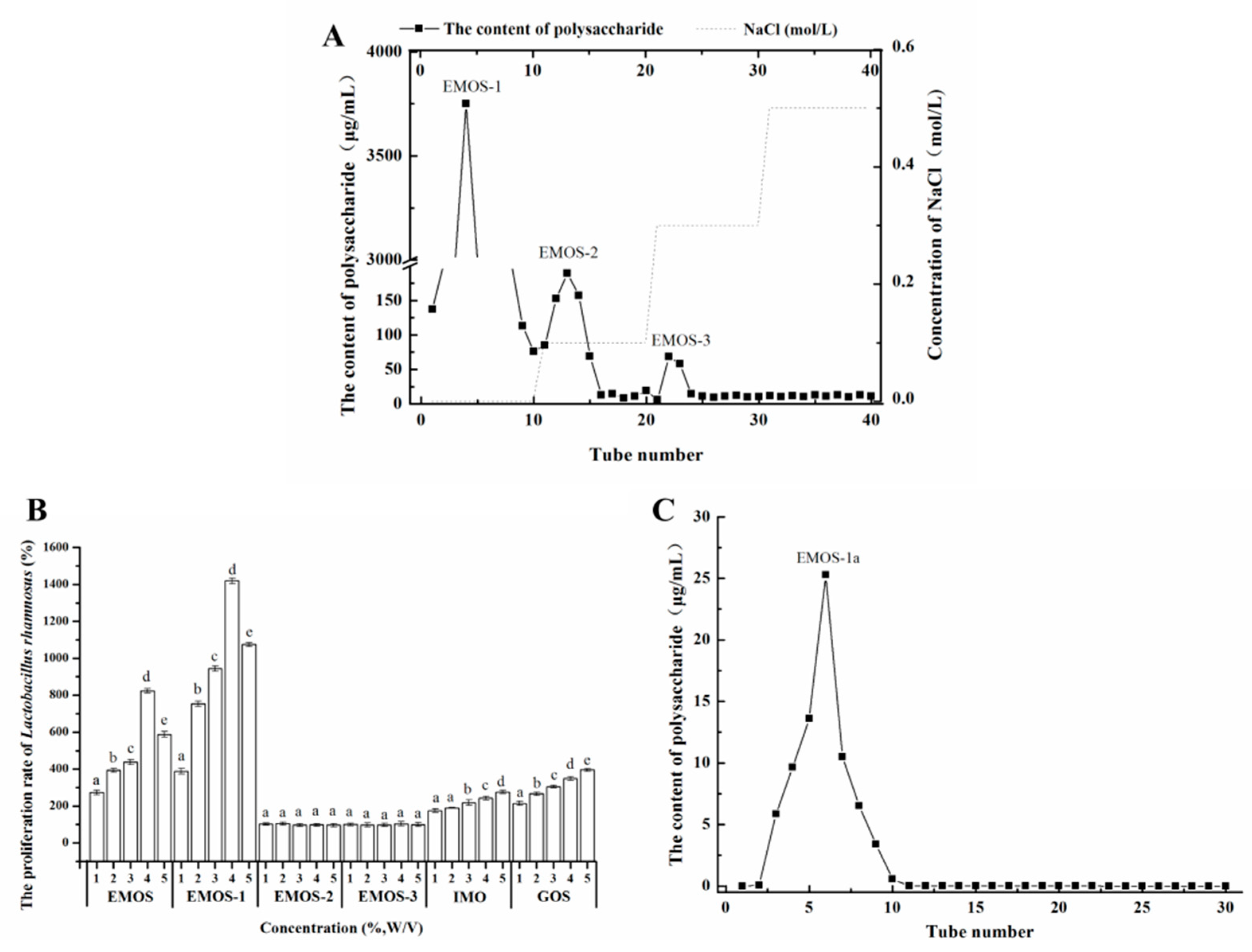
2.1. Isolation and Purification of Oligosaccharides
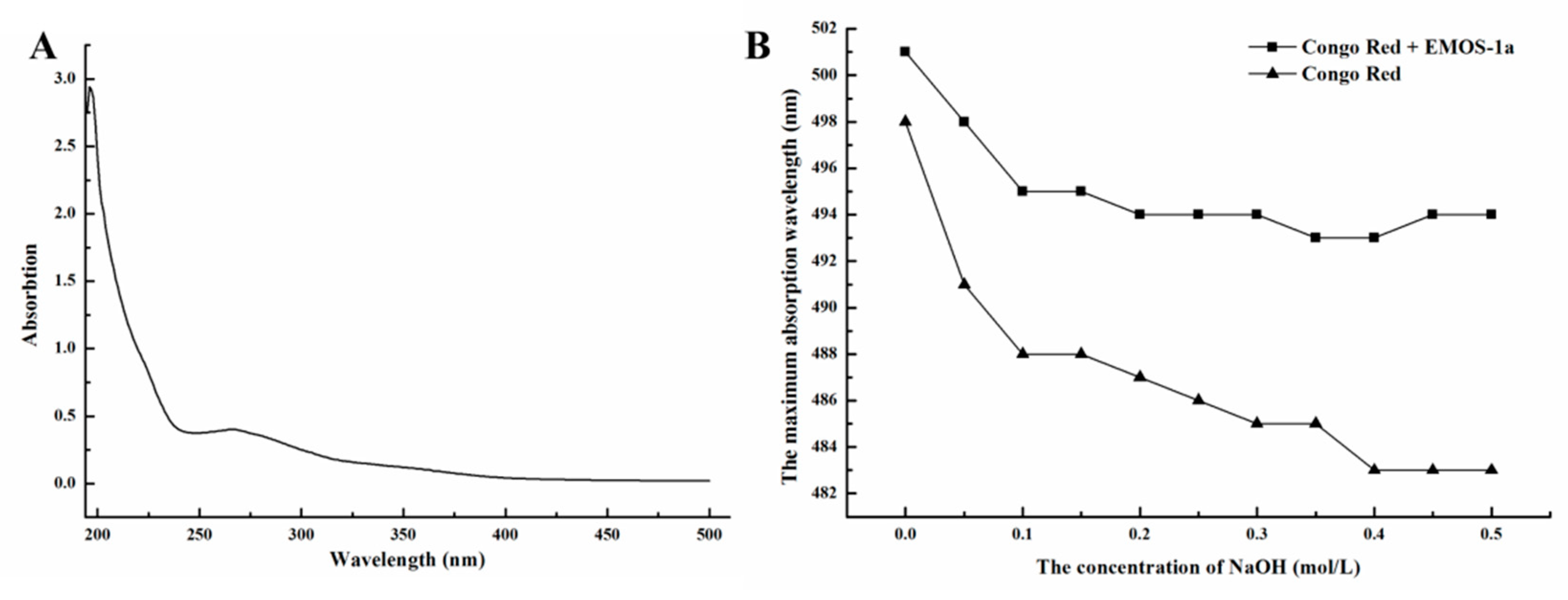
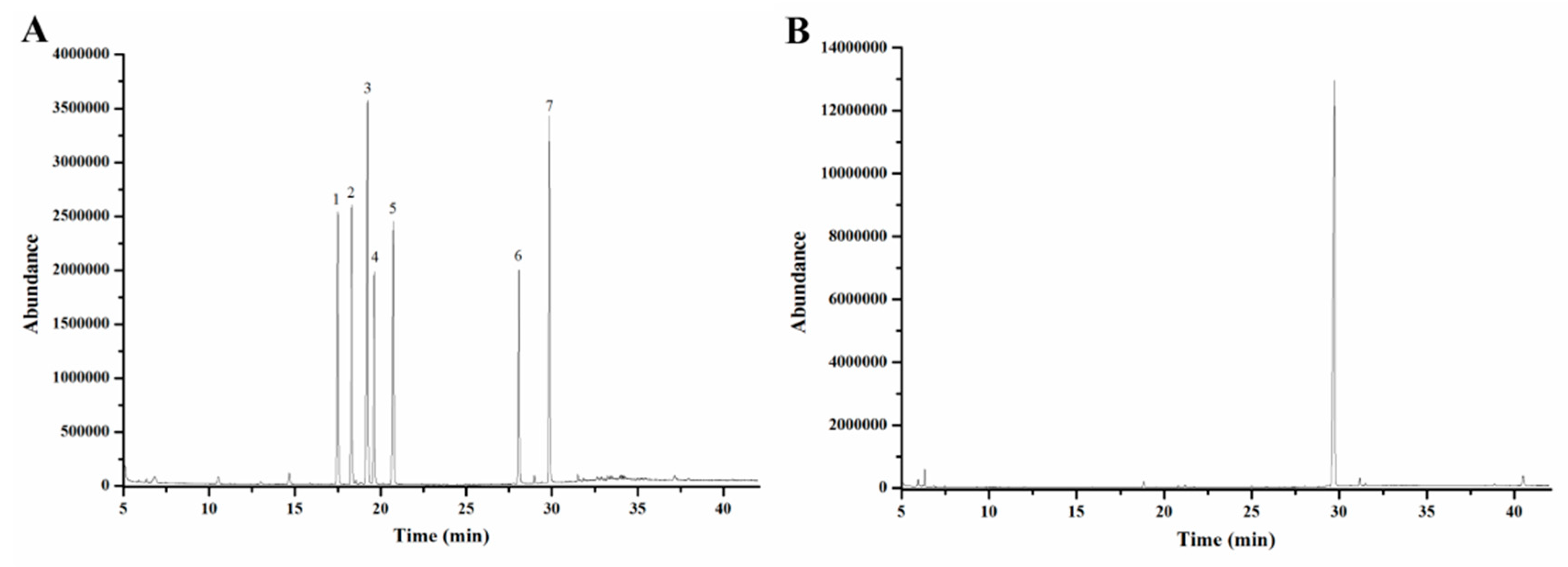
2.2. Characterization of EMOS-1a
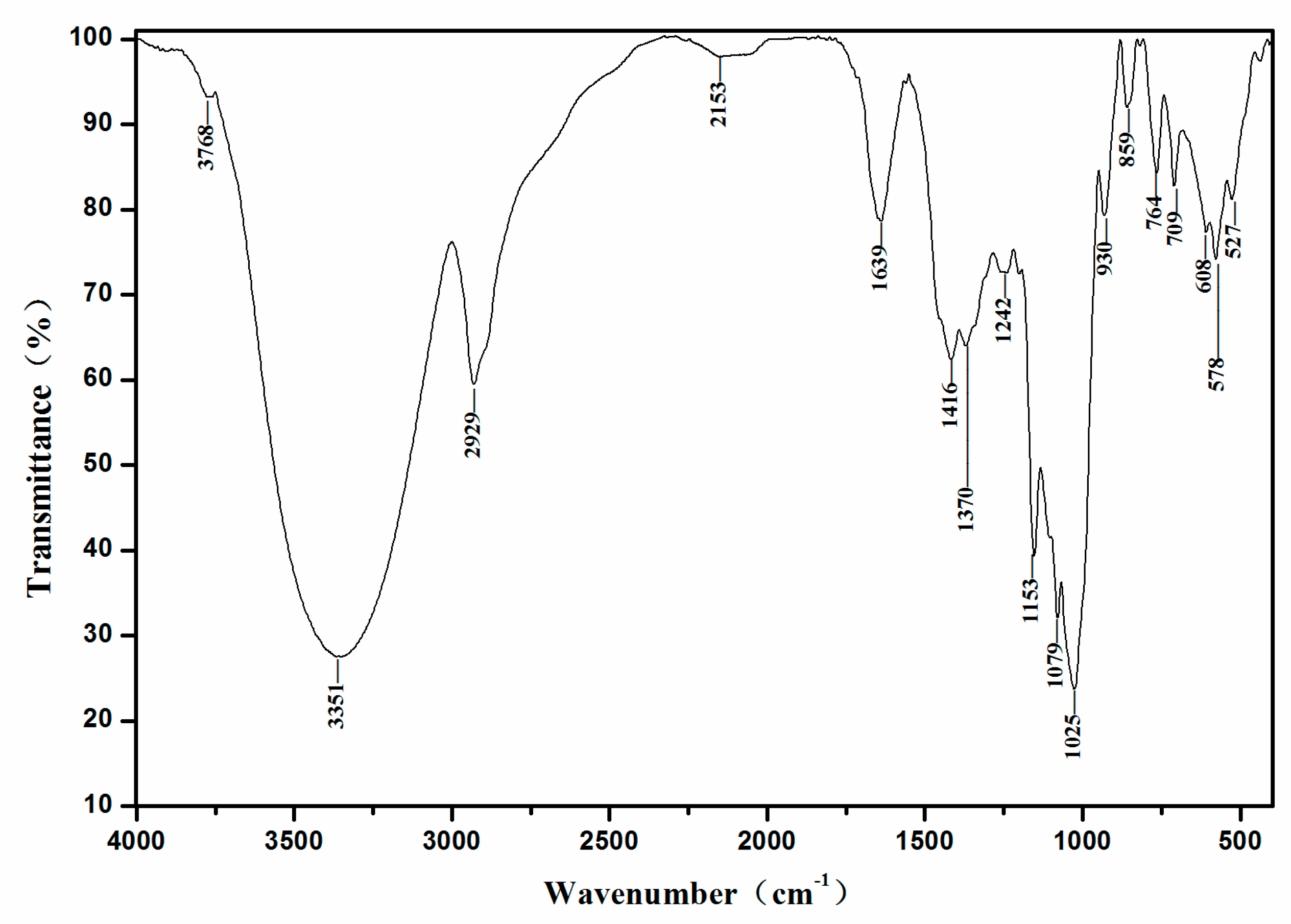
2.3. FT-IR Analysis
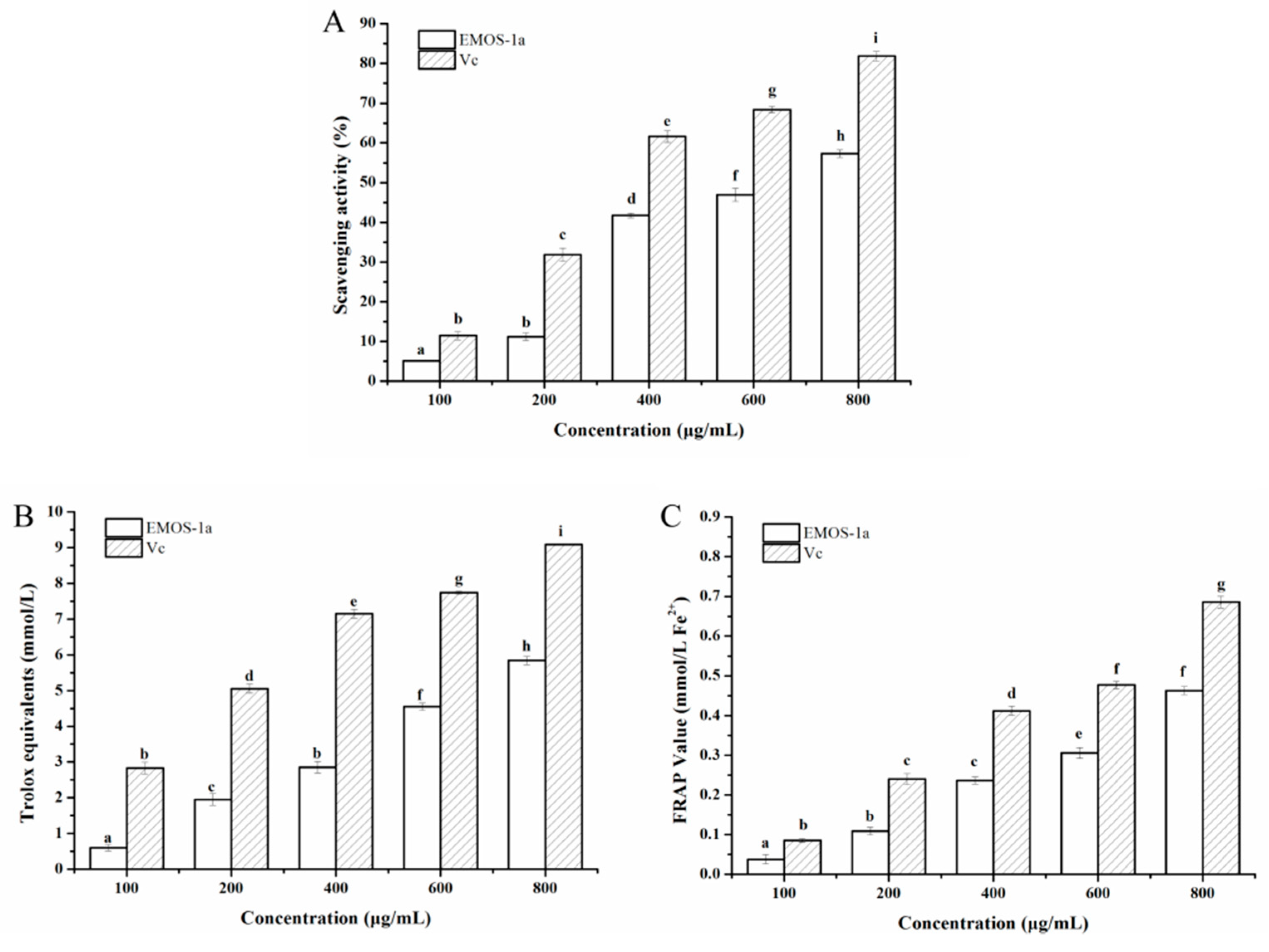
2.4. Antioxidant Activity
3. Material and Methods
3.1. Material and Chemicals
3.2. Bacterial Strain
3.3. Preparation, Isolation, and Purification of Mulberry Oligosaccharides
3.4. Effect of Oligosaccharides on the Growth of L. rhamnosus
3.5. Chemical Characterization of EMOS-1a
3.5.1. Determination of Chemical Composition
3.5.2. UV Spectroscopy
3.5.3. Determination of Structure
3.5.4. Fourier-Transform Infrared (FT-IR) Spectroscopy
3.5.5. Molecular Weight Determination
3.5.6. Analysis of Monosaccharide Composition
3.6. Antioxidant Activity of EMOS-1a In Vitro
3.6.1. DPPH Radical Scavenging Assay
3.6.2. ABTS [2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic) Acid] Assay
3.6.3. FRAP (Ferric Reducing Antioxidant Power) Assay
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nomura, T.; Fukai, T.; Matsumoto, J.; Imashimizu, A.; Terada, S.; Hama, M. Constituents of the cultivated mulberry tree. Planta Med. 1982, 46, 28–32. [Google Scholar] [CrossRef]
- Butt, M.S.; Nazir, A.; Sultan, M.T.; Schroën, K. Morus alba L. nature’s functional tonic. Trends Food Sci. Technol. 2008, 19, 505–512. [Google Scholar] [CrossRef]
- Yildiz, O. Physicochemical and sensory properties of mulberry products: Gümüşhane pestil and köme. Turkish J. Agr. Forest. 2013, 37, 762–771. [Google Scholar] [CrossRef]
- Bonini, S.A.; Premoli, M.; Tambaro, S.; Kumar, A.; Maccarinelli, G.; Memo, M.; Mastinu, A. Cannabis sativa: A comprehensive ethnopharmacological review of a medicinal plant with a long history. J. Ethnopharmacol. 2018, 227, 300–315. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Premoli, M.; Aria, F.; Bonini, S.A.; Maccarinelli, G.; Gianoncelli, A.; Memo, M.; Mastinu, A. Cannabimimetic plants: Are they new cannabinoidergic modulators? Planta 2019, 249, 1681–1694. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.X.; Hua, J.L.; Wang, B. Hypoglycemic Function of Mulberry Leaf Polysaccharide and 1-Deoxynojirimycin (DNJ). Adv. Mater. Res. 2012, 361–363, 808–812. [Google Scholar]
- Ercisli, S.; Orhan, E. Chemical composition of white (Morus Alba), red (Morus rubra), and black (Morus nigra) mulberry fruits. Food Chem. 2007, 103, 1380–1384. [Google Scholar] [CrossRef]
- Naoko, Y.; Takako, A.; Chihiro, I.; Masao, F.; Masami, S.; Ritsuo, N.; Lait, C.G.; Tumlinson, J.H.; Naoki, M. Fatty acid amides, previously identified in caterpillars, found in the cricket Teleogryllus taiwanemma and fruit fly Drosophila melanogaster larvae. J. Chem. Ecol. 2007, 33, 1376–1381. [Google Scholar]
- Chieh-Jung, L.; Jin-Yuarn, L. Anti-inflammatory and anti-apoptotic effects of strawberry and mulberry fruit polysaccharides on lipopolysaccharide-stimulated macrophages through modulating pro-/anti-inflammatory cytokines secretion and Bcl-2/Bak protein ratio. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2012, 50, 3032–3039. [Google Scholar]
- Aramwit, P.; Bang, N.; Srichana, T. The properties and stability of anthocyanins in mulberry fruits. Food Res. Int. 2010, 43, 1093–1097. [Google Scholar] [CrossRef]
- Chen, C.; You, L.J.; Abbasi, A.M.; Xiong, F.; Rui, H.L. Optimization for ultrasound extraction of polysaccharides from mulberry fruits with antioxidant and hyperglycemic activity in vitro. Carbohydr. Polym. 2015, 130, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.J.; Lin, J.Y. Protective effects of strawberry and mulberry fruit polysaccharides on inflammation and apoptosis in murine primary splenocytes. J. Food Drug Anal. 2014, 22, 210–219. [Google Scholar] [CrossRef]
- Chen, C.; Huang, Q.; Fu, X.; Liu, R.H. In vitro fermentation of mulberry fruit polysaccharides by human fecal inocula and impact on microbiota. Food Func. 2016, 7, 4637–4643. [Google Scholar] [CrossRef] [PubMed]
- Kuusk, S.; Bissaro, B.; Kuusk, P.; Forsberg, Z.; Eijsink, V.G.; Sørlie, M.; Väljamäe, P. Kinetics of H2O2-driven degradation of chitin by a bacterial lytic polysaccharide monooxygenase. J. Biol. Chem. 2018, 293, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Li, N.; Qiao, X.; Qiu, Z.; Liu, P. Effects of thermal treatment on polysaccharide degradation during black garlic processing. LWT 2018, 95, 223–229. [Google Scholar] [CrossRef]
- Schultz-Johansen, M.; Bech, P.K.; Hennessy, R.C.; Glaring, M.A.; Barbeyron, T.; Czjzek, M.; Stougaard, P. A novel enzyme portfolio for red algal polysaccharide degradation in the marine bacterium Paraglaciecola hydrolytica S66T encoded in a sizeable polysaccharide utilization locus. Front. Microbiol. 2018, 9, 839. [Google Scholar] [CrossRef]
- Sun, Y.; Liang, H.; Cai, G.; Guan, S.; Tong, H.; Yang, X.; Liu, J. Sulfated modification of the water-soluble polysaccharides from Polyporus albicans mycelia and its potential biological activities. Int. J. Biol. Macromol. 2009, 44, 14–17. [Google Scholar] [CrossRef]
- Li, E.; Yang, H.; Zou, Y.; Wang, H.; Hu, T.; Li, Q.; Liao, S. In-vitro digestion by simulated gastrointestinal juices of Lactobacillus rhamnosus cultured with mulberry oligosaccharides and subsequent fermentation with human fecal inocula. LWT 2019, 101, 61–68. [Google Scholar] [CrossRef]
- Wang, X.; Huang, M.; Yang, F.; Sun, H.; Zhou, X.; Guo, Y.; Wang, X.; Zhang, M. Rapeseed polysaccharides as prebiotics on growth and acidifying activity of probiotics in vitro. Carbohydr. Polym. 2015, 125, 232–240. [Google Scholar] [CrossRef]
- Li, D.; Jin, M.K.; Jin, Z.; Jie, Z. Prebiotic effectiveness of inulin extracted from edible burdock. Anaerobe 2008, 14, 29–34. [Google Scholar] [CrossRef]
- Biedrzycka, E.; Bielecka, M. Prebiotic effectiveness of fructans of different degrees of polymerization. Trends Food Sci. Technol. 2004, 15, 170–175. [Google Scholar] [CrossRef]
- Manning, T.S.; Gibson, G.R. Prebiotics. Best Practice Res. Clin. Gastroenterol. 2004, 18, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Stewart, M.L.; Timm, D.A.; Slavin, J.L. Fructooligosaccharides exhibit more rapid fermentation than long-chain inulin in an in vitro fermentation system. Nutr. Res. 2008, 28, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, M.J.; Isaacs, M.; Matsuhiro, B.; Mendoza, L.; Zúñiga, E.A. Characterization of a neutral polysaccharide with antioxidant capacity from red wine. Carbohydr. Res. 2009, 344, 1095–1101. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, G.; Lai, F.; Wu, H. Structural Characterization and Immunomodulatory Activity of a Novel Polysaccharide from Lepidium meyenii. J. Agric. Food Chem. 2016, 64, 1921–1931. [Google Scholar] [CrossRef] [PubMed]
- Nep, E.I.; Sims, I.M.; Morris, G.A.; Kontogiorgos, V.; Smith, A.M. Evaluation of some important physicochemical properties of starch free grewia gum. Food Hydrocoll. 2016, 53, 134–140. [Google Scholar] [CrossRef]
- Yamassaki, F.T.; Lenzi, R.M.; Campestrini, L.H.; Bovo, F.; Seyfried, M.; Soldera-Silva, A.; Stevan-Hancke, F.R.; Zawadzki-Baggio, S.F.; Pettolino, F.A.; Bacic, A. Effect of the native polysaccharide of cashew-nut tree gum exudate on murine peritoneal macrophage modulatory activities. Carbohydr. Polym. 2015, 125, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Villena, J.F.; Domínguez, E.; Heredia, A. Monitoring Biopolymers Present in Plant Cuticles by FT-IR Spectroscopy. J. Plant Physiol. 2000, 156, 419–422. [Google Scholar] [CrossRef]
- Chen, Y.; Xie, M.Y.; Nie, S.P.; Li, C.; Wang, Y.X. Purification, composition analysis and antioxidant activity of a polysaccharide from the fruiting bodies of Ganoderma atrum. Food Chem. 2008, 107, 231–241. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Sun, H.; Jiang, S.; Zi, M.; Qi, D. Purification, chemical composition, and in vitro antioxidant activity of two protein-bound polysaccharides from rapeseed meal. Food Sci. Biotechnol. 2009, 18, 1386–1391. [Google Scholar]
- Wood, P.J.; Erfle, J.D.; Teather, R.M. Use of complex formation between Congo Red and polysaccharides in detection and assay of polysaccharide hydrolases. Methods Enzymol. 1988, 160, 59–74. [Google Scholar]
- Chen, Y.; Huang, Y.; Cui, Z.; Liu, J. Purification, characterization and biological activity of a novel polysaccharide from Inonotus obliquus. Int. J. Biol. Macromol. 2015, 79, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. Lwt Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, E.; Yang, S.; Zou, Y.; Cheng, W.; Li, B.; Hu, T.; Li, Q.; Wang, W.; Liao, S.; Pang, D. Purification, Characterization, Prebiotic Preparations and Antioxidant Activity of Oligosaccharides from Mulberries. Molecules 2019, 24, 2329. https://doi.org/10.3390/molecules24122329
Li E, Yang S, Zou Y, Cheng W, Li B, Hu T, Li Q, Wang W, Liao S, Pang D. Purification, Characterization, Prebiotic Preparations and Antioxidant Activity of Oligosaccharides from Mulberries. Molecules. 2019; 24(12):2329. https://doi.org/10.3390/molecules24122329
Chicago/Turabian StyleLi, Erna, Shiyuan Yang, Yuxiao Zou, Weiwei Cheng, Bing Li, Tenggen Hu, Qian Li, Weifei Wang, Sentai Liao, and Daorui Pang. 2019. "Purification, Characterization, Prebiotic Preparations and Antioxidant Activity of Oligosaccharides from Mulberries" Molecules 24, no. 12: 2329. https://doi.org/10.3390/molecules24122329




