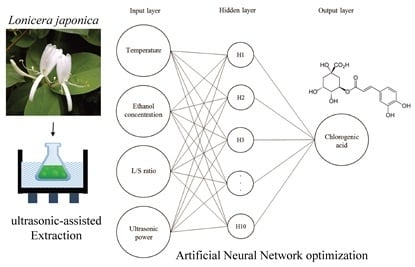
Comparison of Artificial Neural Networks and Response Surface Methodology towards an Efficient Ultrasound-Assisted Extraction of Chlorogenic Acid from Lonicera japonica
Abstract
:1. Introduction
2. Results and Discussion
2.1. Single-Factor Experiments
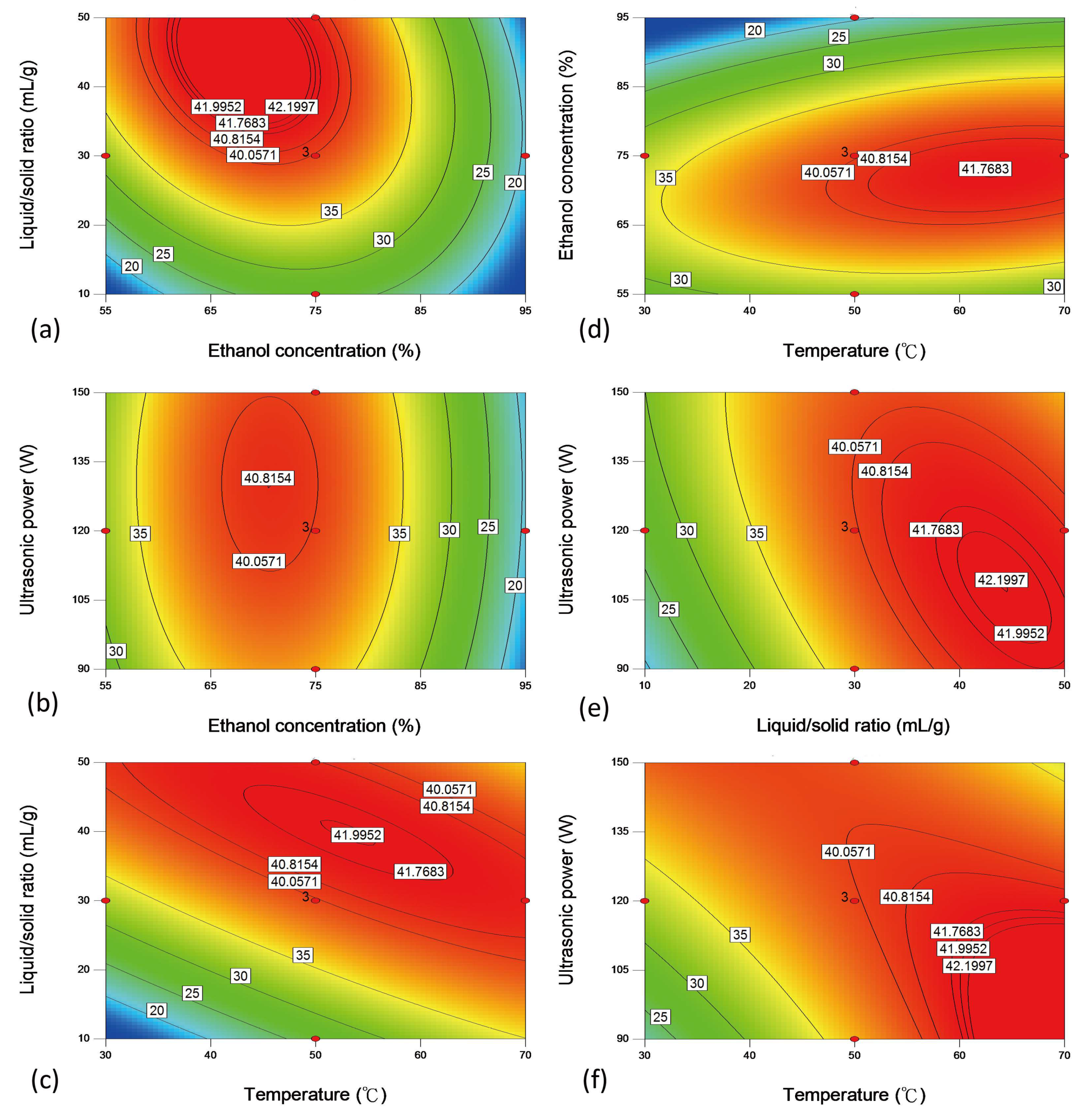
2.2. RSM Model
2.3. ANN Model
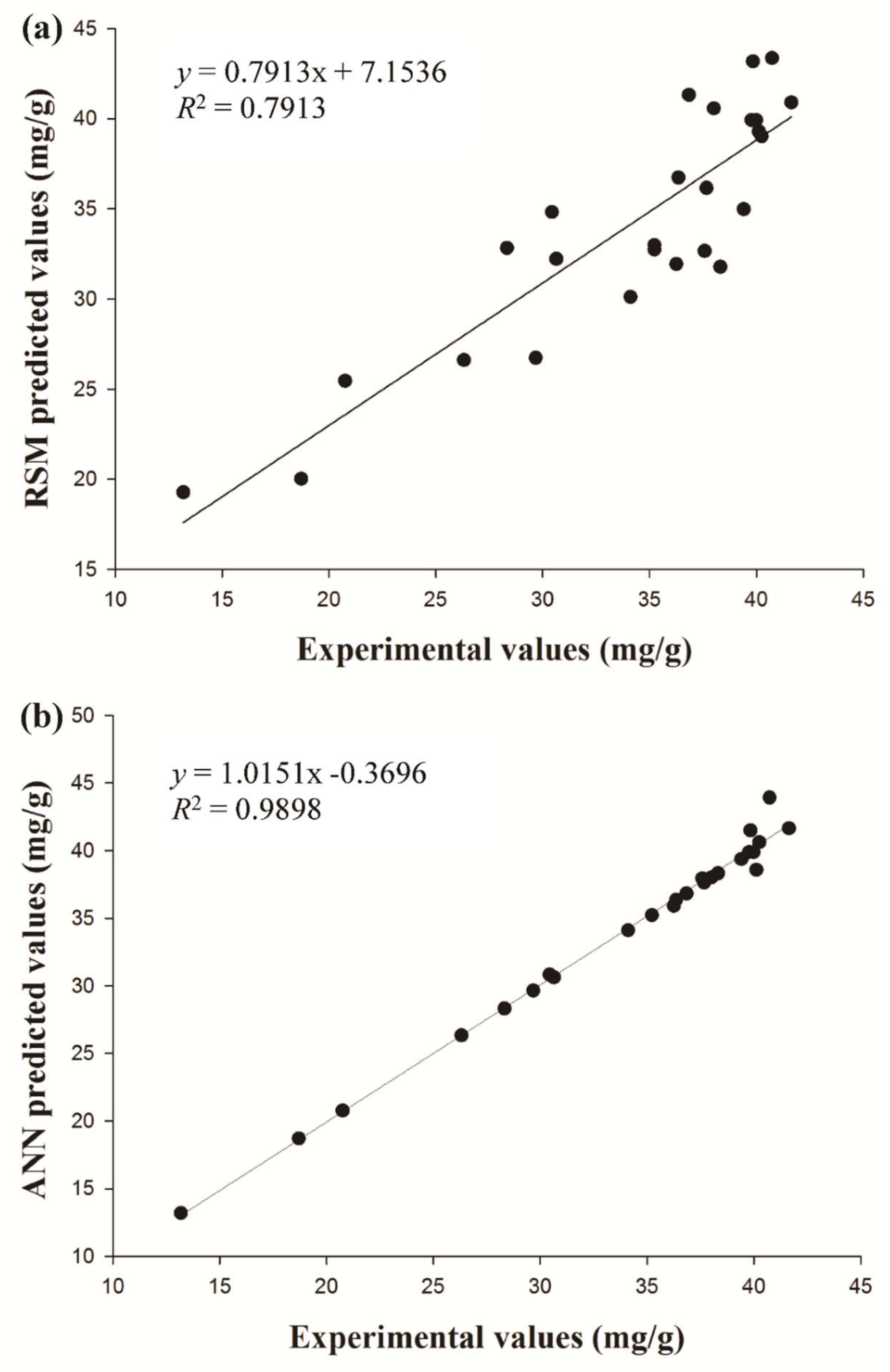
2.4. Verification, Comparison and Optimization
3. Materials and Methods
3.1. Materials
3.2. Conventional Shaking Extraction of CGA from Lonicera japonica
3.3. Ultrasonic-Assisted Extraction of CGA from Lonicera japonica
3.4. HPLC Analysis of Products Extracted from Lonicera japonica
3.5. Response Surface Methodology (RSM)
3.6. Artificial Neural Network (ANN)
3.7. Comparison of Prediction Capability between ANN and RSM for CGA Extraction
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liang, N.; Kitts, D.D. Role of Chlorogenic Acids in Controlling Oxidative and Inflammatory Stress Conditions. Nutrients 2016, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; Xia, F.; Modarresi-Ghazani, F.; et al. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Wianowska, D.; Gil, M. Recent advances in extraction and analysis procedures of natural chlorogenic acids. Phytochem. Rev. 2019, 18, 273–302. [Google Scholar] [CrossRef]
- Budryn, G.; Nebesny, E.; Oracz, J. Correlation between the stability of chlorogenic acids, antioxidant activity and acrylamide content in coffee beans roasted in different conditions. Int. J. Food Prop. 2015, 18, 290–302. [Google Scholar] [CrossRef]
- Wang, L.; Weller, C.L. Recent advances in extraction of nutraceuticals from plants. Trends Food Sci. Technol. 2006, 17, 300–312. [Google Scholar] [CrossRef]
- Vinatoru, M. An overview of the ultrasonically assisted extraction of bioactive principles from herbs. Ultrason. Sonochem. 2001, 8, 303–313. [Google Scholar] [CrossRef]
- Azmir, J.; Zaidul, I.; Rahman, M.; Sharif, K.; Mohamed, A.; Sahena, F.; Jahurul, M.; Ghafoor, K.; Norulaini, N.; Omar, A. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Srinath, D.; Maheswari, K. Ultrasound technology in food processing: A review. Int. J. Curr. Adv. Res. 2016, 5, 778–783. [Google Scholar]
- Dent, M.; Dragovic-Uzelac, V.; Garofulic, I.E.; Bosiljkov, T.; Jezek, D.; Brncic, M. Comparison of Conventional and Ultrasound-assisted Extraction Techniques on Mass Fraction of Phenolic Compounds from Sage (Salvia officinalis L.). Chem. Biochem. Eng. Q. 2015, 29, 475–484. [Google Scholar] [CrossRef]
- Li, H.; Chen, B.; Yao, S. Application of ultrasonic technique for extracting chlorogenic acid from Eucommia ulmodies Oliv. (E. ulmodies). Ultrason. Sonochem. 2005, 12, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Da Porto, C.; Porretto, E.; Decorti, D. Comparison of ultrasound-assisted extraction with conventional extraction methods of oil and polyphenols from grape (Vitis vinifera L.) seeds. Ultrason. Sonochem. 2013, 20, 1076–1080. [Google Scholar] [CrossRef] [PubMed]
- Goltz, C.; Ávila, S.; Barbieri, J.B.; Igarashi-Mafra, L.; Mafra, M.R. Ultrasound-assisted extraction of phenolic compounds from Macela (Achyrolcine satureioides) extracts. Ind. Crops Prod. 2018, 115, 227–234. [Google Scholar] [CrossRef]
- Mazvimba, M.T.; Yu, Y.; Cui, Z.-Q.; Zhang, Y. Optimization and orthogonal design of an ultrasonic-assisted aqueous extraction process for extracting chlorogenic acid from dry tobacco leaves. Chin. J. Nat. Med. 2012, 10, 311–320. [Google Scholar] [CrossRef]
- Chen, B.-Y.; Kuo, C.-H.; Liu, Y.-C.; Ye, L.-Y.; Chen, J.-H.; Shieh, C.-J. Ultrasonic-assisted extraction of the botanical dietary supplement resveratrol and other constituents of Polygonum cuspidatum. J. Nat. Prod. 2012, 75, 1810–1813. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-A.; Kuo, C.-H.; Chen, B.-Y.; Li, Y.; Liu, Y.-C.; Chen, J.-H.; Shieh, C.-J. A novel enzyme-assisted ultrasonic approach for highly efficient extraction of resveratrol from Polygonum cuspidatum. Ultrason. Sonochem. 2016, 32, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.-H.; Liu, T.-A.; Chen, J.-H.; Chang, C.-M. J.; Shieh, C.-J. Response surface methodology and artificial neural network optimized synthesis of enzymatic 2-phenylethyl acetate in a solvent-free system. Biocatal. Agric. Biotechnol. 2014, 3, 1–6. [Google Scholar] [CrossRef]
- Desai, K.M.; Survase, S.A.; Saudagar, P.S.; Lele, S.; Singhal, R.S. Comparison of artificial neural network (ANN) and response surface methodology (RSM) in fermentation media optimization: Case study of fermentative production of scleroglucan. Biochem. Eng. J. 2008, 41, 266–273. [Google Scholar] [CrossRef]
- Huang, S.-M.; Kuo, C.-H.; Chen, C.-A.; Liu, Y.-C.; Shieh, C.-J. RSM and ANN modeling-based optimization approach for the development of ultrasound-assisted liposome encapsulation of piceid. Ultrason. Sonochem. 2017, 36, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Guo, T.; Jiang, W.-J.; Dong, G.-L.; Chen, D.-W.; Yang, S.-L.; Li, H.-R. Effects of ultrahigh pressure extraction on yield and antioxidant activity of chlorogenic acid and cynaroside extracted from flower buds of Lonicera japonica. Chin. J. Nat. Med. 2015, 13, 445–453. [Google Scholar] [CrossRef]
- Cacace, J.; Mazza, G. Optimization of extraction of anthocyanins from black currants with aqueous ethanol. J. Food Sci. 2003, 68, 240–248. [Google Scholar] [CrossRef]
- Yu, H.C.; Tan, F.J. Optimization of ultrasonic-assisted enzymatic hydrolysis conditions for the production of antioxidant hydrolysates from porcine liver by using response surface methodology. Asian-Australas. J. Anim. Sci. 2017, 30, 1612–1619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ranjan, D.; Mishra, D.; Hasan, S. Bioadsorption of arsenic: An artificial neural networks and response surface methodological approach. Ind. Eng. Chem. Res. 2011, 50, 9852–9863. [Google Scholar] [CrossRef]
- Badkar, D.S.; Pandey, K.S.; Buvanashekaran, G. Development of RSM-and ANN-based models to predict and analyze the effects of process parameters of laser-hardened commercially pure titanium on heat input and tensile strength. Int. J. Adv. Manuf. Technol. 2013, 65, 1319–1338. [Google Scholar] [CrossRef]
- Xu, W.-J.; Zhai, J.-W.; Cui, Q.; Liu, J.-Z.; Luo, M.; Fu, Y.-J.; Zu, Y.-G. Ultra-turrax based ultrasound-assisted extraction of five organic acids from honeysuckle (Lonicera japonica Thunb.) and optimization of extraction process. Sep. Purif. Technol. 2016, 166, 73–82. [Google Scholar] [CrossRef]
- Pingret, D.; Fabiano-Tixier, A.-S.; Chemat, F. Degradation during application of ultrasound in food processing: A review. Food Control 2013, 31, 593–606. [Google Scholar] [CrossRef]
- Hsu, H.F.; Hsiao, P.C.; Kuo, T.C.; Chiang, S.T.; Chen, S.L.; Chiou, S.J.; Ling, X.H.; Liang, M.T.; Cheng, W.Y.; Houng, J.Y. Antioxidant and anti-inflammatory activities of Lonicera japonica Thunb. var. sempervillosa Hayata flower bud extracts prepared by water, ethanol and supercritical fluid extraction techniques. Ind. Crops Prod. 2016, 89, 543–549. [Google Scholar] [CrossRef]
- Dennis, J.E., Jr.; Schnabel, R.B. Numerical Methods for Unconstrained Optimization and Nonlinear Equations; Prentice Hall Inc.: Englewood Cliffs, NJ, USA, 1996. [Google Scholar]
- Priyadarshini, R.; Dash, N.; Swarnkar, T.; Misra, R. Functional analysis of artificial neural network for dataset classification. IJCCT 2010, 1, 49–54. [Google Scholar]
- Vogl, T.P.; Mangis, J.; Rigler, A.; Zink, W.; Alkon, D. Accelerating the convergence of the back-propagation method. Biol. Cybern. 1988, 59, 257–263. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds did not provide from the authors. |

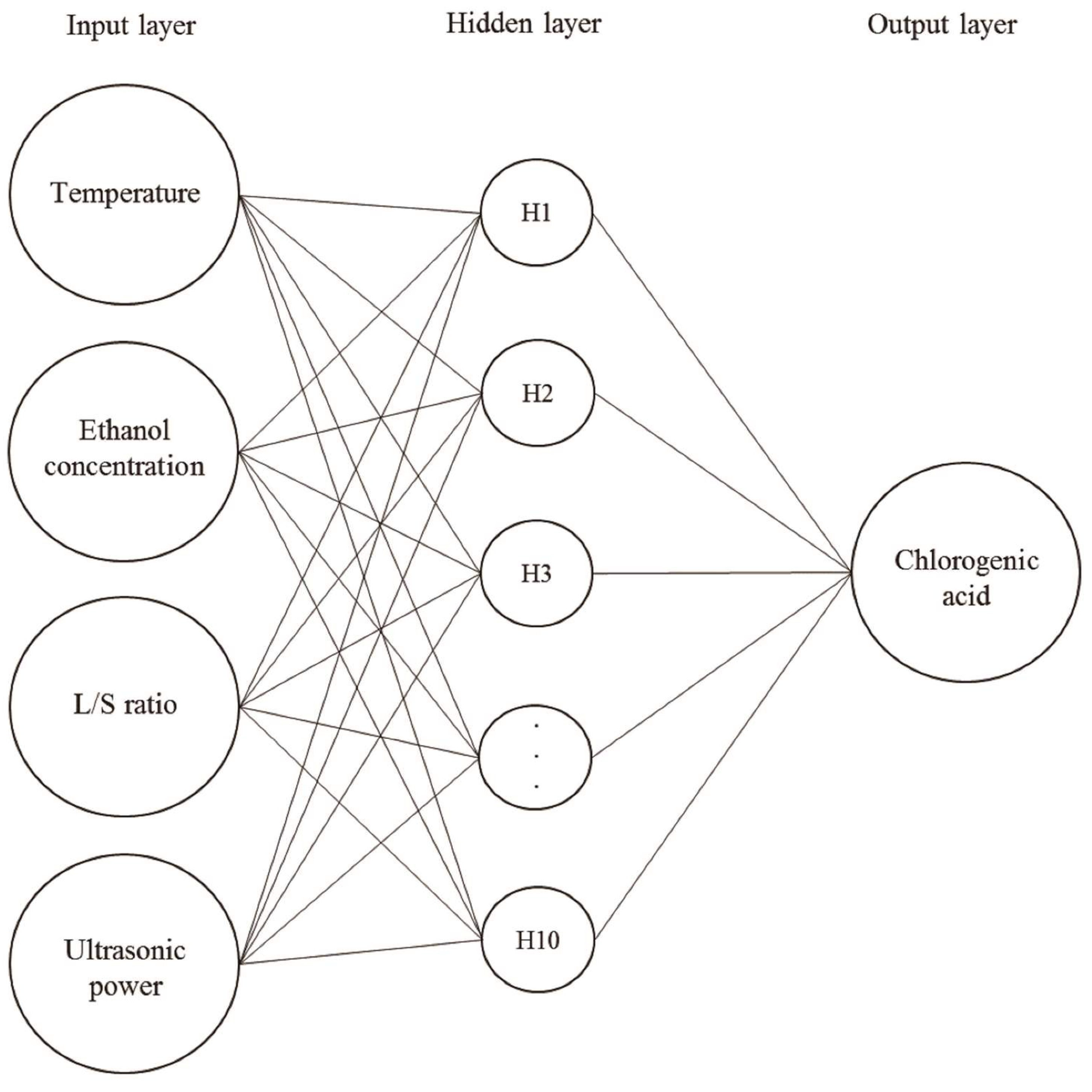

| Independent Variable | Unit | Symbols | Coded Values | ||||
|---|---|---|---|---|---|---|---|
| −2 | −1 | 0 | +1 | +2 | |||
| Temperature | °C | X1 | 30 | 40 | 50 | 60 | 70 |
| Ethanol concentration | % | X2 | 55 | 65 | 75 | 85 | 95 |
| L/S ratio | mL/g | X3 | 10 | 20 | 30 | 40 | 50 |
| Ultrasonic power | W | X4 | 90 | 105 | 120 | 135 | 150 |
| Run | Independent Variable a | Chlorogenic Acid Extraction Yield (mg/g) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| X1 | X2 | X3 | X4 | Experimental Data b | RSM-Predicted | RSM Deviation | ANN-Predicted | ANN Deviation | |
| 1 | 40 | 65 | 20 | 105 | 20.75 ± 3.60 | 25.45 | 4.70 | 20.77 | 0.02 |
| 2 | 60 | 65 | 20 | 105 | 39.41 ± 1.31 | 34.99 | 4.42 | 39.37 | 0.04 |
| 3 | 40 | 85 | 20 | 105 | 18.70 ± 1.92 | 20.01 | 1.30 | 18.70 | 0.01 |
| 4 | 60 | 85 | 20 | 105 | 35.23 ± 1.74 | 32.99 | 2.24 | 35.24 | 0.00 |
| 5 | 40 | 65 | 40 | 105 | 38.02 ± 3.10 | 40.56 | 2.55 | 38.01 | 0.00 |
| 6 | 60 | 65 | 40 | 105 | 39.84 ± 2.06 | 43.18 | 3.33 | 41.49 | 1.65 |
| 7 | 40 | 85 | 40 | 105 | 34.12 ± 2.26 | 30.11 | 4.01 | 34.12 | 0.00 |
| 8 | 60 | 85 | 40 | 105 | 37.67 ± 2.00 | 36.16 | 1.51 | 37.66 | 0.01 |
| 9 | 40 | 65 | 20 | 135 | 30.65 ± 1.29 | 32.23 | 1.58 | 30.64 | 0.00 |
| 10 | 60 | 65 | 20 | 135 | 30.44 ± 4.18 | 34.82 | 4.39 | 30.84 | 0.40 |
| 11 | 40 | 85 | 20 | 135 | 29.68 ± 3.48 | 26.72 | 2.96 | 29.66 | 0.01 |
| 12 | 60 | 85 | 20 | 135 | 35.23 ± 2.62 | 32.75 | 2.48 | 35.24 | 0.01 |
| 13 | 40 | 65 | 40 | 135 | 40.74 ± 1.79 | 43.36 | 2.62 | 43.92 | 3.19 |
| 14 | 60 | 65 | 40 | 135 | 40.25 ± 2.78 | 39.02 | 1.23 | 40.62 | 0.37 |
| 15 | 40 | 85 | 40 | 135 | 28.33 ± 3.10 | 32.83 | 4.50 | 28.32 | 0.01 |
| 16 | 60 | 85 | 40 | 135 | 36.25 ± 0.74 | 31.94 | 4.32 | 35.93 | 0.32 |
| 17 | 30 | 75 | 30 | 120 | 37.59 ± 1.16 | 32.67 | 4.92 | 37.94 | 0.36 |
| 18 | 70 | 75 | 30 | 120 | 36.85 ± 1.17 | 41.31 | 4.47 | 36.84 | 0.01 |
| 19 | 50 | 55 | 30 | 120 | 38.32 ± 0.77 | 31.79 | 6.53 | 38.32 | 0.00 |
| 20 | 50 | 95 | 30 | 120 | 13.18 ± 2.34 | 19.26 | 6.08 | 13.18 | 0.00 |
| 21 | 50 | 75 | 10 | 120 | 26.31 ± 0.95 | 26.60 | 0.29 | 26.33 | 0.01 |
| 22 | 50 | 75 | 50 | 120 | 41.64 ± 2.47 | 40.90 | 0.74 | 41.65 | 0.01 |
| 23 | 50 | 75 | 30 | 90 | 36.35 ± 1.39 | 36.73 | 0.38 | 36.36 | 0.00 |
| 24 | 50 | 75 | 30 | 150 | 40.12 ± 1.57 | 39.29 | 0.83 | 38.58 | 1.54 |
| 25 | 50 | 75 | 30 | 120 | 39.78 ± 2.38 | 39.92 | 0.14 | 39.89 | 0.12 |
| 26 | 50 | 75 | 30 | 120 | 39.99 ± 2.33 | 39.92 | 0.07 | 39.89 | 0.10 |
| 27 | 50 | 75 | 30 | 120 | 39.98 ± 0.35 | 39.92 | 0.07 | 39.89 | 0.09 |
| Source | Sum of Squares | DF | Mean Square | F Value | p-Value Prob > F |
|---|---|---|---|---|---|
| Model | 1113.03 | 14 | 79.5 | 3.25 | 0.0238 * |
| X1 | 112.13 | 1 | 112.13 | 4.58 | 0.0535 |
| X2 | 235.33 | 1 | 235.33 | 9.62 | 0.0092 * |
| X3 | 306.68 | 1 | 306.68 | 12.54 | 0.0041 * |
| X4 | 9.82 | 1 | 9.82 | 0.4 | 0.5382 |
| X1X2 | 11.83 | 1 | 11.83 | 0.48 | 0.5 |
| X1X3 | 48.02 | 1 | 48.02 | 1.96 | 0.1865 |
| X1X4 | 48.3 | 1 | 48.3 | 1.97 | 0.1853 |
| X2X3 | 25.19 | 1 | 25.19 | 1.03 | 0.3302 |
| X2X4 | 4.889 × 10−3 | 1 | 4.889 × 10−3 | 1.998 × 10−4 | 0.989 |
| X3X4 | 15.91 | 1 | 15.91 | 0.65 | 0.4356 |
| X12 | 11.41 | 1 | 11.41 | 0.47 | 0.5076 |
| X22 | 276.17 | 1 | 276.17 | 11.29 | 0.0057 * |
| X32 | 50.68 | 1 | 50.68 | 2.07 | 0.1756 |
| X42 | 4.84 | 1 | 4.84 | 0.2 | 0.6645 |
| Residual | 293.55 | 12 | 24.46 | ||
| Lack of Fit | 293.52 | 10 | 29.35 | 1983.46 | 0.0005 * |
| Pure Error | 0.03 | 2 | 0.015 | ||
| Cor Total | 1406.58 | 26 | |||
| Std. Dev. | 4.95 | R-Squared | 0.7913 | ||
| Mean | 34.27 | Adj R-Squared | 0.5478 | ||
| CV% | 14.43 | ||||
| PRESS | 1690.75 | ||||
| Run | Independent Variable a | Chlorogenic Acid Extraction Yield (mg/g) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| X1 | X2 | X3 | X4 | Experimental Data b | RSM-Predicted | RSM Deviation | ANN-Predicted | ANN Deviation | |
| 1 | 60 | 65 | 30 | 120 | 39.65 ± 0.97 | 40.02 | 0.37 | 39.98 | 0.33 |
| 2 | 50 | 75 | 20 | 135 | 34.45 ± 2.17 | 35.96 | 1.51 | 33.29 | 1.16 |
| 3 | 60 | 75 | 30 | 135 | 37.70 ± 3.13 | 39.77 | 2.07 | 36.69 | 1.01 |
| 4 | 50 | 65 | 20 | 120 | 33.70 ± 2.55 | 33.08 | 0.62 | 33.82 | 0.12 |
| Parameters a | RSM | ANN |
|---|---|---|
| R2 | 0.7913 | 0.9898 |
| RMSE | 1.9050 | 0.7006 |
| AAD | 1.6541 | 0.4204 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, H.-C.; Huang, S.-M.; Lin, W.-M.; Kuo, C.-H.; Shieh, C.-J. Comparison of Artificial Neural Networks and Response Surface Methodology towards an Efficient Ultrasound-Assisted Extraction of Chlorogenic Acid from Lonicera japonica. Molecules 2019, 24, 2304. https://doi.org/10.3390/molecules24122304
Yu H-C, Huang S-M, Lin W-M, Kuo C-H, Shieh C-J. Comparison of Artificial Neural Networks and Response Surface Methodology towards an Efficient Ultrasound-Assisted Extraction of Chlorogenic Acid from Lonicera japonica. Molecules. 2019; 24(12):2304. https://doi.org/10.3390/molecules24122304
Chicago/Turabian StyleYu, Hui-Chuan, Shang-Ming Huang, Wei-Min Lin, Chia-Hung Kuo, and Chwen-Jen Shieh. 2019. "Comparison of Artificial Neural Networks and Response Surface Methodology towards an Efficient Ultrasound-Assisted Extraction of Chlorogenic Acid from Lonicera japonica" Molecules 24, no. 12: 2304. https://doi.org/10.3390/molecules24122304