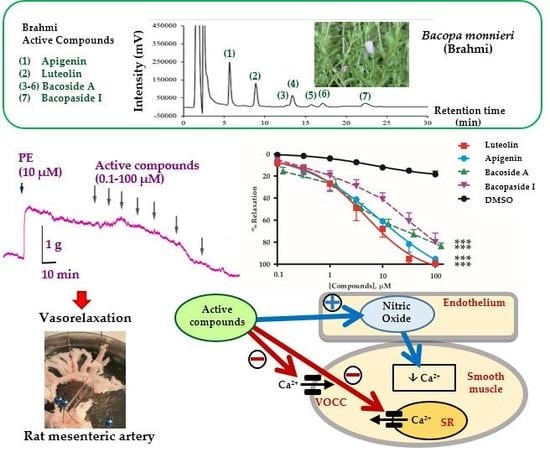
Vasodilatory Effects and Mechanisms of Action of Bacopa monnieri Active Compounds on Rat Mesenteric Arteries
Abstract
:1. Introduction
2. Results
2.1. Vasorelaxant Effects of the B. monnieri Active Compounds
2.2. Mechanisms of Vasorelaxation by B. monnieri Compounds
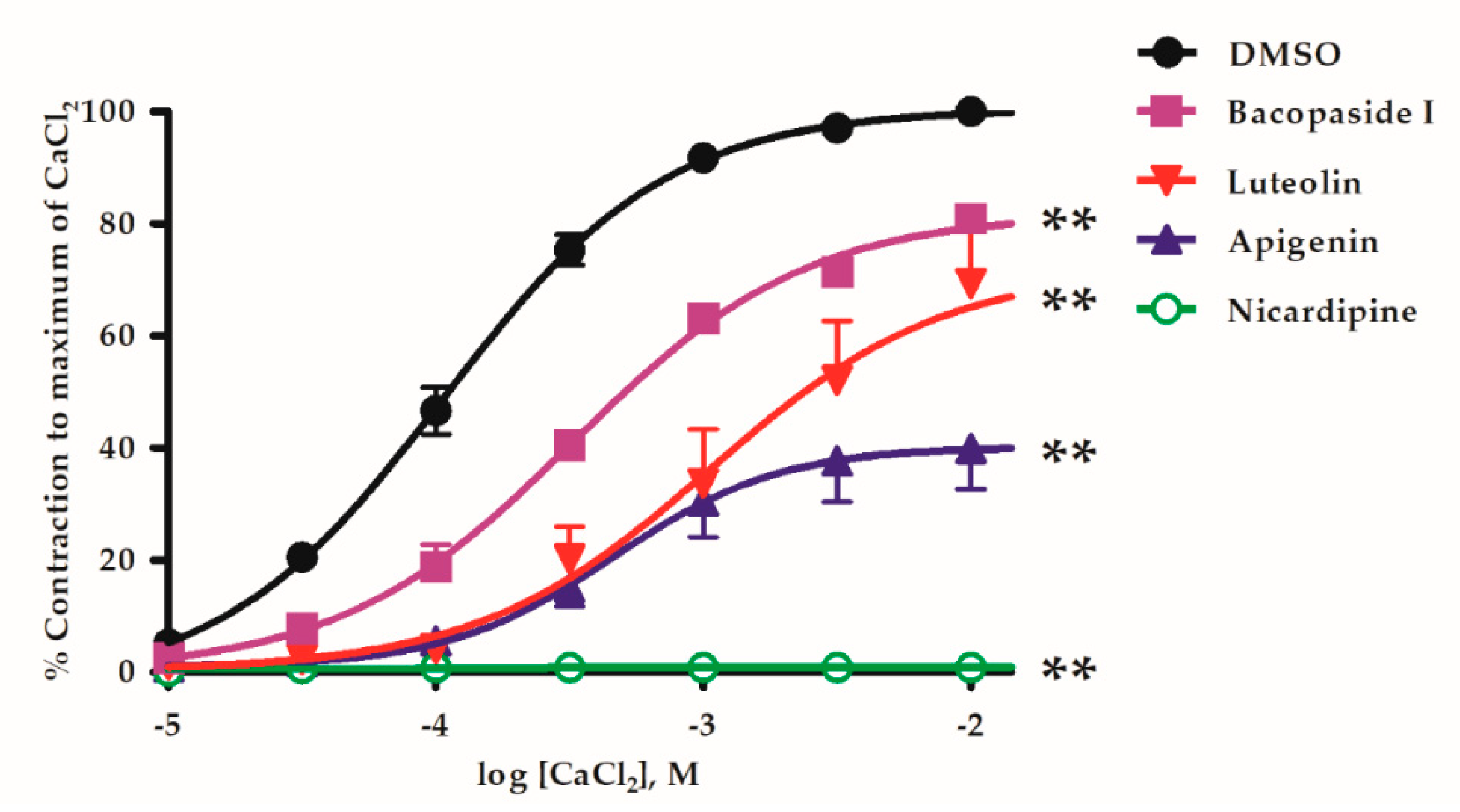
2.3. B. monnieri Compounds and Ca2+ Influx
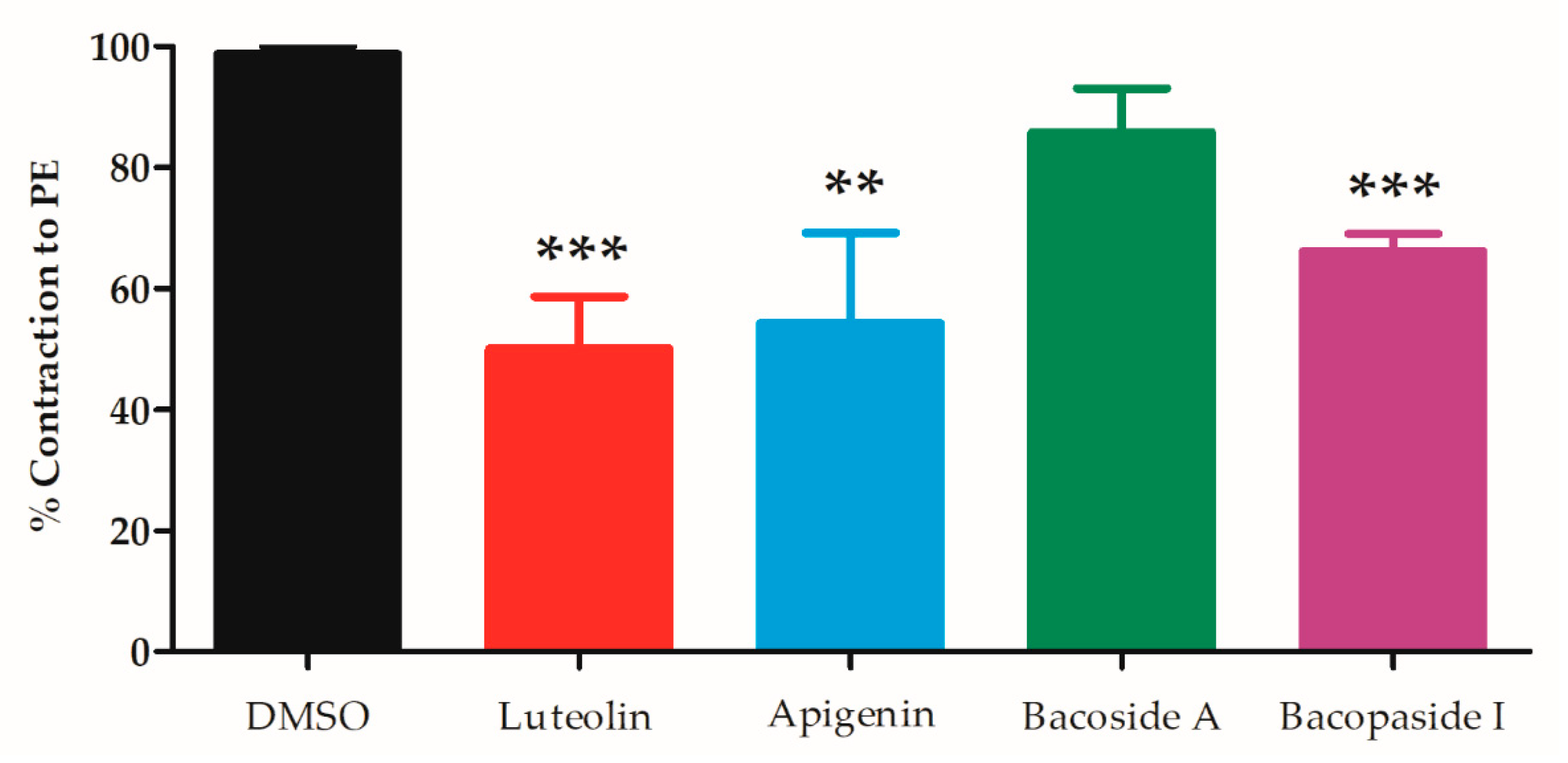
2.4. B. monnieri Compounds and Intracellular Ca2+ Release
3. Discussion
4. Materials and Methods
4.1. General Information
4.2. Vasorelaxant Effects of B. monnieri Active Compounds on Endothelial Intact Arteries
4.3. Vasorelaxant Effects of B. monnieri Active Compounds on Endothelial Denuded Arteries
4.4. Study of Vasorelaxant Mechanisms of B. monnieri Active Compounds via eNOS Pathway
4.5. Study of Vasorelaxant Mechanisms of B. monnieri Active Compounds on Extracellular Ca2+ Influx
4.6. Study of Vasorelaxant Mechanisms of B. monnieri Active Compounds on Intracellular Ca2+ Release
4.7. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bacopa monniera. Monograph. Altern. Med. Rev. J. Clin. Ther. 2004, 9, 79–85.
- Russo, A.; Borrelli, F. Bacopa monniera, a reputed nootropic plant: An overview. Phytomedicine 2005, 12, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V. Potential medicinal plants for CNS disorders: An overview. Phytother. Res. PTR 2006, 20, 1023–1035. [Google Scholar] [CrossRef]
- Rajan, K.E.; Preethi, J.; Singh, H.K. Molecular and functional characterization of Bacopa monniera: A retrospective review. Evid.-Based Complement. Altern. Med. eCAM 2015, 2015, 945217. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, S.; Borowski, T. Neuropharmacological review of the nootropic herb Bacopa monnieri. Rejuvenation Res. 2013, 16, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.K. Brain enhancing ingredients from Ayurvedic medicine: Quintessential example of Bacopa monniera, a narrative review. Nutrients 2013, 5, 478–497. [Google Scholar] [CrossRef] [PubMed]
- Mathur, D.; Goyal, K.; Koul, V.; Anand, A. The molecular links of re-emerging therapy: A review of evidence of Brahmi (Bacopa monniera). Front. Pharmacol. 2016, 7, 44. [Google Scholar] [CrossRef]
- Das, A.; Shanker, G.; Nath, C.; Pal, R.; Singh, S.; Singh, H. A comparative study in rodents of standardized extracts of Bacopa monniera and Ginkgo biloba: Anticholinesterase and cognitive enhancing activities. Pharmacol. Biochem. Behav. 2002, 73, 893–900. [Google Scholar] [CrossRef]
- Dhanasekaran, M.; Tharakan, B.; Holcomb, L.A.; Hitt, A.R.; Young, K.A.; Manyam, B.V. Neuroprotective mechanisms of ayurvedic antidementia botanical Bacopa monniera. Phytother. Res. PTR 2007, 21, 965–969. [Google Scholar] [CrossRef]
- Limpeanchob, N.; Jaipan, S.; Rattanakaruna, S.; Phrompittayarat, W.; Ingkaninan, K. Neuroprotective effect of Bacopa monnieri on beta-amyloid-induced cell death in primary cortical culture. J. Ethnopharmacol. 2008, 120, 112–117. [Google Scholar] [CrossRef]
- Uabundit, N.; Wattanathorn, J.; Mucimapura, S.; Ingkaninan, K. Cognitive enhancement and neuroprotective effects of Bacopa monnieri in Alzheimer’s disease model. J. Ethnopharmacol. 2010, 127, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Vollala, V.R.; Upadhya, S.; Nayak, S. Enhancement of basolateral amygdaloid neuronal dendritic arborization following Bacopa monniera extract treatment in adult rats. Clinics (Sao Paulo Brazil) 2011, 66, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Vollala, V.R.; Upadhya, S.; Nayak, S. Learning and memory-enhancing effect of Bacopa monniera in neonatal rats. Bratisl. Lek. Listy 2011, 112, 663–669. [Google Scholar] [PubMed]
- Vollala, V.R.; Upadhya, S.; Nayak, S. Enhanced dendritic arborization of hippocampal CA3 neurons by Bacopa monniera extract treatment in adult rats. Rom. J. Morphol. Embryol. 2011, 52, 879–886. [Google Scholar] [PubMed]
- Vollala, V.R.; Upadhya, S.; Nayak, S. Enhanced dendritic arborization of amygdala neurons during growth spurt periods in rats orally intubated with Bacopa monniera extract. Anat. Sci. Int. 2011, 86, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Kongkeaw, C.; Dilokthornsakul, P.; Thanarangsarit, P.; Limpeanchob, N.; Norman Scholfield, C. Meta-analysis of randomized controlled trials on cognitive effects of Bacopa monnieri extract. J. Ethnopharmacol. 2014, 151, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Anbarasi, K.; Vani, G.; Balakrishna, K.; Devi, C.S. Effect of bacoside A on brain antioxidant status in cigarette smoke exposed rats. Life Sci. 2006, 78, 1378–1384. [Google Scholar] [CrossRef] [PubMed]
- Jyoti, A.; Sharma, D. Neuroprotective role of Bacopa monniera extract against aluminium-induced oxidative stress in the hippocampus of rat brain. Neurotoxicology 2006, 27, 451–457. [Google Scholar] [CrossRef]
- Mannan, A.; Abir, A.B.; Rahman, R. Antidepressant-like effects of methanolic extract of Bacopa monniera in mice. BMC Complement. Altern. Med. 2015, 15, 337. [Google Scholar] [CrossRef]
- Sairam, K.; Dorababu, M.; Goel, R.K.; Bhattacharya, S.K. Antidepressant activity of standardized extract of Bacopa monniera in experimental models of depression in rats. Phytomedicine 2002, 9, 207–211. [Google Scholar] [CrossRef]
- Kadali, S.R.M.; Das, M.C.; Rao, A.S.; Sri, G.K. Antidepressant activity of brahmi in albino mice. J. Clin. Diagn. Res. JCDR 2014, 8, 35–37. [Google Scholar] [CrossRef] [PubMed]
- Udhaya Lavinya, B.; Sabina, E.P. Anti-hyperglycaemic effect of Brahmi (Bacopa monnieri L.) in streptozotocininduced diabetic rats: A study involving antioxidant, biochemical and haematological parameters. J. Chem. Pharm. Res. 2015, 7, 531–534. [Google Scholar]
- Kamesh, V.; Sumathi, T. Antihypercholesterolemic effect of Bacopa monniera Linn. on high cholesterol diet induced hypercholesterolemia in rats. Asian Pac. J. Trop. Med. 2012, 5, 949–955. [Google Scholar] [CrossRef]
- Sireeratawong, S.; Jaijoy, K.; Khonsung, P.; Lertprasertsuk, N.; Ingkaninan, K. Acute and chronic toxicities of Bacopa monnieri extract in Sprague-Dawley rats. BMC Complement. Altern. Med. 2016, 16, 249. [Google Scholar] [CrossRef] [PubMed]
- Joshua Allan, J.; Damodaran, A.; Deshmukh, N.S.; Goudar, K.S.; Amit, A. Safety evaluation of a standardized phytochemical composition extracted from Bacopa monnieri in Sprague–Dawley rats. Food Chem. Toxicol. 2007, 45, 1928–1937. [Google Scholar] [CrossRef] [PubMed]
- Pravina, K.; Ravindra, K.R.; Goudar, K.S.; Vinod, D.R.; Joshua, A.J.; Wasim, P.; Venkateshwarlu, K.; Saxena, V.S.; Amit, A. Safety evaluation of BacoMind in healthy volunteers: A phase I study. Phytomedicine 2007, 14, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Srimachai, S.; Devaux, S.; Demougeot, C.; Kumphune, S.; Ullrich, N.D.; Niggli, E.; Ingkaninan, K.; Kamkaew, N.; Scholfield, C.N.; Tapechum, S.; et al. Bacopa monnieri extract increases rat coronary flow and protects against myocardial ischemia/reperfusion injury. BMC Complement. Altern. Med. 2017, 17, 117. [Google Scholar] [CrossRef]
- Nandave, M.; Ojha, S.K.; Sujata, J.; Kumari, S.; Arya, D.S. Cardioprotective effect of Bacopa monneira against isoproterenol-induced myocardial necrosis in rats. Int. J. Pharmacol. 2007, 3, 385–392. [Google Scholar]
- Kamkaew, N.; Scholfield, C.N.; Ingkaninan, K.; Maneesai, P.; Parkington, H.C.; Tare, M.; Chootip, K. Bacopa monnieri and its constituents is hypotensive in anaesthetized rats and vasodilator in various artery types. J. Ethnopharmacol. 2011, 137, 790–795. [Google Scholar] [CrossRef]
- Kamkaew, N.; Norman Scholfield, C.; Ingkaninan, K.; Taepavarapruk, N.; Chootip, K. Bacopa monnieri increases cerebral blood flow in rat independent of blood pressure. Phytother. Res. PTR 2013, 27, 135–138. [Google Scholar] [CrossRef]
- Phrompittayarat, W.; Putalun, W.; Tanaka, H.; Jetiyanon, K.; Wittaya-Areekul, S.; Ingkaninan, K. Determination of pseudojujubogenin glycosides from Brahmi based on immunoassay using a monoclonal antibody against bacopaside I. Phytochem. Anal. PCA 2007, 18, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Phrompittayarat, W.; Putalun, W.; Tanaka, H.; Wittaya-Areekul, S.; Jetiyanon, K.; Ingkaninan, K. An enzyme-linked immunosorbant assay using polyclonal antibodies against bacopaside I. Anal. Chim. Acta 2007, 584, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Nuengchamnong, N.; Sookying, S.; Ingkaninan, K. LC-ESI-QTOF-MS based screening and identification of isomeric jujubogenin and pseudojujubogenin aglycones in Bacopa monnieri extract. J. Pharm. Biomed. Anal. 2016, 129, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Honnegowda, S.; Bagul, M.S.; Padh, H.; Rajani, M. A rapid densitometric method for the quantification of luteolin in medicinal plants using HPTLC. Chromatographia 2004, 60, 131–134. [Google Scholar]
- Deepak, M.; Sangli, G.K.; Arun, P.C.; Amit, A. Quantitative determination of the major saponin mixture bacoside A in Bacopa monnieri by HPLC. Phytochem. Anal. PCA 2005, 16, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Rajasekaran, A. Simultaneous estimation of luteolin and apigenin in methanol leaf extract of Bacopa monnieri Linn by HPLC. Br. J. Pharm. Res. 2014, 4, 1629–1637. [Google Scholar] [CrossRef]
- Chan, E.C.; Pannangpetch, P.; Woodman, O.L. Relaxation to flavones and flavonols in rat isolated thoracic aorta: Mechanism of action and structure-activity relationships. J. Cardiovasc. Pharmacol. 2000, 35, 326–333. [Google Scholar] [CrossRef]
- Calderone, V.; Chericoni, S.; Martinelli, C.; Testai, L.; Nardi, A.; Morelli, I.; Breschi, M.C.; Martinotti, E. Vasorelaxing effects of flavonoids: Investigation on the possible involvement of potassium channels. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2004, 370, 290–298. [Google Scholar] [CrossRef]
- Je, H.D.; Kim, H.-D.; La, H.-O. The inhibitory effect of apigenin on the agonist-induced regulation of vascular contractility via calcium desensitization-related pathways. Biomol. Ther. 2014, 22, 100–105. [Google Scholar] [CrossRef]
- Jiang, H.; Xia, Q.; Wang, X.; Song, J.; Bruce, I.C. Luteolin induces vasorelaxion in rat thoracic aorta via calcium and potassium channels. Die Pharm. 2005, 60, 444–447. [Google Scholar]
- Si, H.; Wyeth, R.P.; Liu, D. The flavonoid luteolin induces nitric oxide production and arterial relaxation. Eur. J. Nutr. 2014, 53, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Saesong, T.; Temkitthawon, P.; Nangngam, P.; Ingkaninan, K. Pharmacognostic and physico-chemical investigations of the aerial part of Bacopa monnieri (L.) Wettst. SJST 2019, 41, 397–404. [Google Scholar]
- Wu, H.; Jiang, H.; Wang, L.; Hu, Y. Relationship between vasorelaxation of flavonoids and their retention index in RP-HPLC. Die Pharm. 2006, 61, 667–669. [Google Scholar]
- Jin, B.H.; Qian, L.B.; Chen, S.; Li, J.; Wang, H.P.; Bruce, I.C.; Lin, J.; Xia, Q. Apigenin protects endothelium-dependent relaxation of rat aorta against oxidative stress. Eur. J. Pharmacol. 2009, 616, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Ko, E.A.; Han, J.; Jung, I.D.; Park, W.S. Physiological roles of K+ channels in vascular smooth muscle cells. J. Smooth Muscle Res. 2008, 44, 65–81. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Li, Y.F.; Gao, Q.; Ye, Z.G.; Lu, X.J.; Wang, H.P.; Jiang, H.D.; Bruce, I.C.; Xia, Q. Inhibition of superoxide anion-mediated impairment of endothelium by treatment with luteolin and apigenin in rat mesenteric artery. Life Sci. 2008, 83, 110–117. [Google Scholar] [CrossRef]
- Qian, L.B.; Wang, H.P.; Chen, Y.; Chen, F.X.; Ma, Y.Y.; Bruce, I.C.; Xia, Q. Luteolin reduces high glucose-mediated impairment of endothelium-dependent relaxation in rat aorta by reducing oxidative stress. Pharmacol. Res. 2010, 61, 281–287. [Google Scholar] [CrossRef]
- El-Bassossy, H.M.; Abo-Warda, S.M.; Fahmy, A. Chrysin and luteolin attenuate diabetes-induced impairment in endothelial-dependent relaxation: Effect on lipid profile, AGEs and NO generation. Phytother. Res. PTR 2013, 27, 1678–1684. [Google Scholar] [CrossRef]
- Wisutthathum, S.; Kamkaew, N.; Inchan, A.; Chatturong, U.; Paracha, T.U.; Ingkaninan, K.; Wongwad, E.; Chootip, K. Extract of Aquilaria crassna leaves and mangiferin are vasodilators while showing no cytotoxicity. J. Tradit. Complement. Med. 2018, in press. [Google Scholar] [CrossRef]
- Wisutthathum, S.; Demougeot, C.; Totoson, P.; Adthapanyawanich, K.; Ingkaninan, K.; Temkitthawon, P.; Chootip, K. Eulophia macrobulbon extract relaxes rat isolated pulmonary artery and protects against monocrotaline-induced pulmonary arterial hypertension. Phytomedicine 2018, 50, 157–165. [Google Scholar] [CrossRef]
- Wisutthathum, S.; Chootip, K.; Martin, H.; Ingkaninan, K.; Temkitthawon, P.; Totoson, P.; Demougeot, C. Vasorelaxant and hypotensive effects of an ethanolic extract of Eulophia macrobulbon and Its main compound 1-(4′-hydroxybenzyl)-4,8-dimethoxyphenanthrene-2,7-diol. Front. Pharmacol. 2018, 9, 484. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds, luteolin, apigenin, bacoside A and bacopaside I are not available from the authors. |
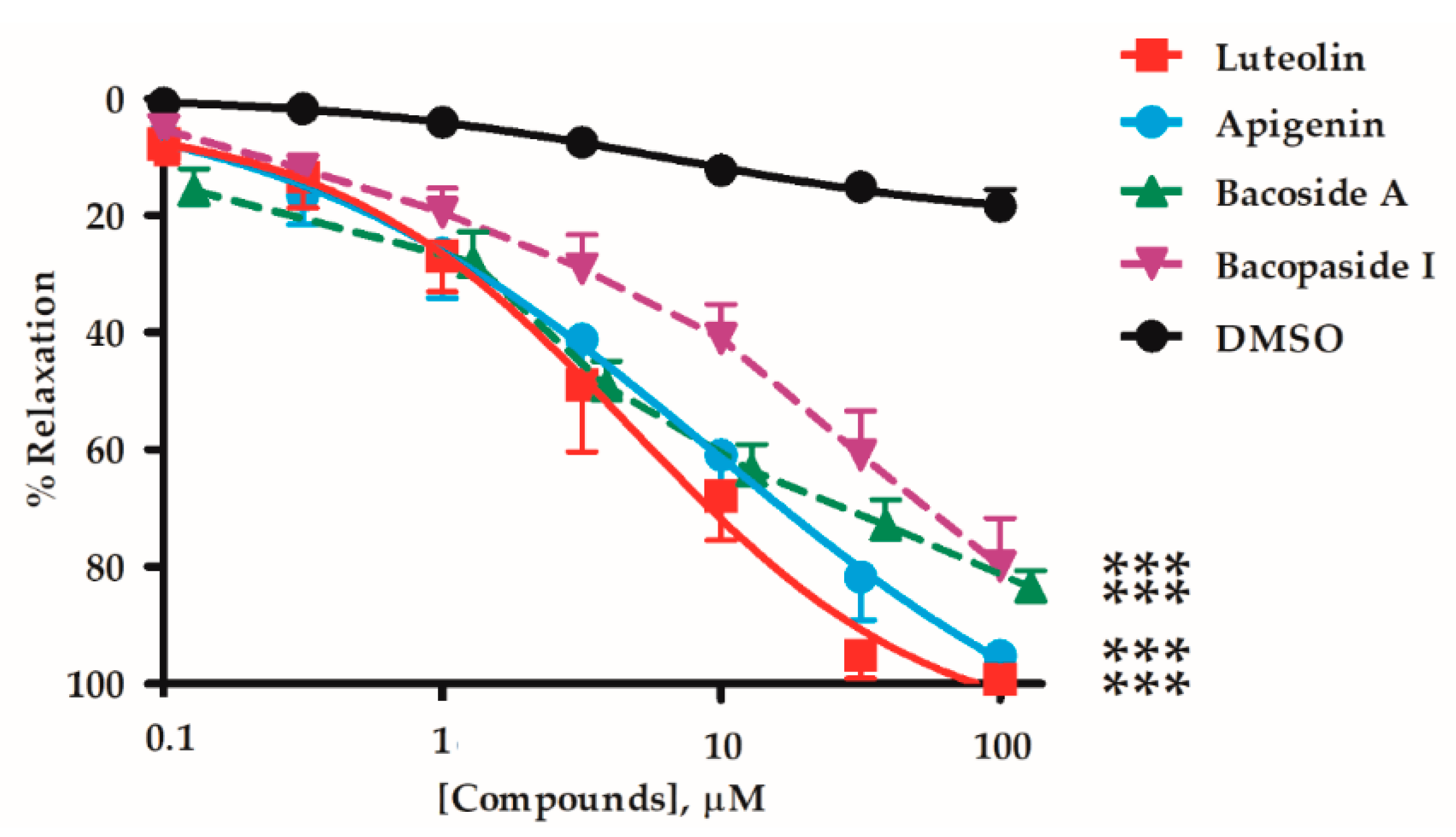

| Active Compounds | EC50 (µM) | Emax (%) | n | p-Value Whole Graph Curves | |
|---|---|---|---|---|---|
| Flavonoids | Luteolin | 4.35 ± 1.31 | 99.4 ± 0.7 | 6 | - |
| Apigenin | 8.93 ± 3.33 | 95.3 ± 2.6 | 9 | NS | |
| Saponins | Bacoside A | 10.8 ± 5.9 | 83.6 ± 2.9 †† | 7 | < 0.05 † |
| Bacopaside I | 14.6 ± 5.4 | 79.9 ± 8.2 † | 7 | < 0.01 †† | |
| Vehicle | DMSO | - | 17.4 ± 3.1 †† | 7 | < 0.01 †† |
| Active Compounds | EC50 (µM) | Emax (%) | n |
|---|---|---|---|
| Luteolin | |||
| +EC | 4.35 ± 1.31 | 99.35 ± 0.66 | 6 |
| -EC | 21.90 ± 5.86 † | 82.42 ± 4.65 †† | 6 |
| +EC plus L-NAME | 14.99 ± 3.56 † | 90.85 ± 5.85 | 6 |
| Apigenin | |||
| +EC | 8.93 ± 3.33 | 95.27 ± 2.61 | 9 |
| -EC | 12.80 ± 2.54 | 98.81 ± 1.19 | 8 |
| +EC plus L-NAME | 25.62 ± 3.38 †† | 94.40 ± 2.10 | 7 |
| Bacoside A | |||
| +EC | 10.81 ± 5.95 | 83.60 ± 2.86 | 7 |
| -EC | 14.50 ± 6.30 | 37.90 ± 4.72 †† | 6 |
| +EC plus L-NAME | 33.81 ± 6.25 † | 33.16 ± 8.41 †† | 5 |
| Bacopaside I | |||
| +EC | 14.63 ± 5.36 | 79.94 ± 8.17 | 7 |
| -EC | 17.29 ± 4.75 | 58.97 ± 7.05 † | 7 |
| +EC plus L-NAME | 25.38 ± 4.33 | 58.45 ± 4.21 † | 7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamkaew, N.; Paracha, T.U.; Ingkaninan, K.; Waranuch, N.; Chootip, K. Vasodilatory Effects and Mechanisms of Action of Bacopa monnieri Active Compounds on Rat Mesenteric Arteries. Molecules 2019, 24, 2243. https://doi.org/10.3390/molecules24122243
Kamkaew N, Paracha TU, Ingkaninan K, Waranuch N, Chootip K. Vasodilatory Effects and Mechanisms of Action of Bacopa monnieri Active Compounds on Rat Mesenteric Arteries. Molecules. 2019; 24(12):2243. https://doi.org/10.3390/molecules24122243
Chicago/Turabian StyleKamkaew, Natakorn, Tamkeen Urooj Paracha, Kornkanok Ingkaninan, Neti Waranuch, and Krongkarn Chootip. 2019. "Vasodilatory Effects and Mechanisms of Action of Bacopa monnieri Active Compounds on Rat Mesenteric Arteries" Molecules 24, no. 12: 2243. https://doi.org/10.3390/molecules24122243