Influences of Extraction Methods on Physicochemical and Functional Characteristics of Three New Bulbil Starches from Dioscorea opposita Thunb. cv. Tiegun
Abstract
:1. Introduction
2. Results and Discussion
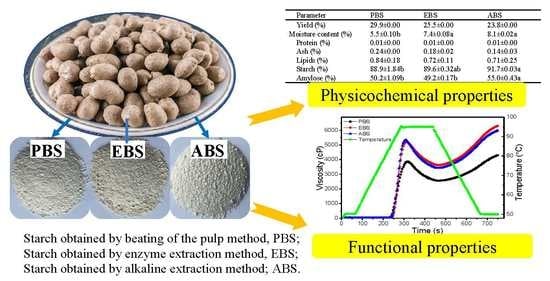
2.1. Starch Yield and Chemical Composition
2.2. X-ray Diffraction
2.3. FT-IR of Starch
2.4. Scanning Electron Microscopy
2.5. Thermo-Gravimetric Analysis
2.6. Swelling Power and Solubility
2.7. Percent Transmittancy
2.8. Freeze-Thaw Stability
2.9. Pasting Properties
3. Experimental
3.1. Materials and Reagents
3.2. Fractionation and Isolation of Starches
3.2.1. Aqueous Steeping Method
3.2.2. Enzyme Extraction Method
3.2.3. Alkaline Extraction Method
3.3. Characterization
3.3.1. Chemical Composition
3.3.2. Fourier Transform Infrared Spectroscopy (FT-IR)
3.3.3. Scanning Electron Microscopy
3.3.4. Differential Scanning Calorimetry (DSC)
3.3.5. X-ray Diffraction
3.3.6. Swelling Power and Solubility
3.3.7. Starch Paste Clarity
3.3.8. Freeze-Thaw Stability
3.3.9. Pasting Properties
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Commission, C.P. Pharmacopoeia of People’s Republic of China; Chem. Ind. Press: Beijing, China, 2015; Volume 1, pp. 28–29. [Google Scholar]
- Yu, X.R.; Zhang, J.; Shao, S.S.; Yu, H.; Xiong, F.; Wang, Z. Morphological and physicochemical properties of bulb and bulbil starches from Lilium lancifolium. Starch-Stärke 2015, 66, 1–11. [Google Scholar]
- Maniglia, B.C.; Tapia-Blácido, D.R. Isolation and characterization of starch from babassu mesocarp. Food Hydrocoll. 2016, 55, 47–55. [Google Scholar] [CrossRef]
- Correia, P.R.; Beirão-da-Costa, M.L. Starch isolation from chestnut and acorn flours through alkaline and enzymatic methods. Food Bioprod. Process. 2012, 90, 309–316. [Google Scholar] [CrossRef]
- Daiuto, É.; Cereda, M.; Sarmento, S.; Vilpoux, O. Effects of extraction methods on Yam (Dioscorea alata) starch characteristics. Starch-Stärke 2005, 57, 153–160. [Google Scholar] [CrossRef]
- Puri, M.; Sharma, D.; Barrow, C.J. Enzyme-assisted extraction of bioactives from plants. Trends Biotechnol 2012, 30, 37–44. [Google Scholar] [CrossRef]
- Cheetham, N.W.H.; Tao, L. Variation in crystalline type with amylose content in maize starch granules: An X-ray powder diffraction study. Carbohydr. Polym. 1998, 36, 277–284. [Google Scholar] [CrossRef]
- Hung, V.P.; Huong, N.T.M.; Phi, N.T.L.; Tien, N.N.T. Physicochemical characteristics and in vitro digestibility of potato and cassava starches under organic acid and heat-moisture treatments. Int. J. Biol. Macromol. 2017, 95, 299–305. [Google Scholar] [CrossRef]
- Kumar, L.; Brennan, M.; Zheng, H.T.; Brennan, C. The effects of dairy ingredients on the pasting, textural, rheological, freeze-thaw properties and swelling behaviour of oat starch. Food Chem. 2018, 245, 518–524. [Google Scholar] [CrossRef]
- Torruco-Uco, J.; Betancur-Ancona, D. Physicochemical and functional properties of makal (Xanthosoma yucatanensis) starch. Food Chem. 2007, 101, 1319–1326. [Google Scholar] [CrossRef]
- Zhou, H.Y.; Wang, J.H.; Fang, X.S.; Sun, Y.S.; Dou, X.H. Physicochemical properties of new starches isolated from Dioscorea opposita Thunb. bulbils. Starch-Stärke 2012, 64, 290–296. [Google Scholar] [CrossRef]
- Pascoal, A.M.; Di-Medeiros, M.C.B.; Batista, K.A.; Leles, M.I.G.; Lião, L.M.; Fernandes, K.F. Extraction and chemical characterization of starch from S. lycocarpum fruits. Carbohydr. Polym. 2013, 98, 1304–1310. [Google Scholar] [CrossRef] [PubMed]
- De Castro, D.S.; dos Santos Moreira, I.; de Melo Silva, L.M.; Lima, J.P.; da Silva, W.P.; Palmeira Gomes, J.; de Figueirêdo, R.M.F. Isolation and characterization of starch from pitomba endocarp. Food Res. Int. 2018, 98, 1304–1310. [Google Scholar] [CrossRef]
- Zhu, F. Isolation, composition, structure, properties, modifications, and uses of yam starch. Compr. Rev. Food Sci. Food Saf. 2015, 14, 357–386. [Google Scholar] [CrossRef]
- Zaidul, I.S.M.; Norulaini, N.; Omar, A.K.M.; Yamauchia, H.; Noda, T. Correlations of the composition, minerals, and RVA pasting properties of various potato starches. Starch-Stärke 2007, 59, 269–276. [Google Scholar] [CrossRef]
- Contreras-Jiménez, B.; Vázquez-Contreras, G.; de los Ángeles Cornejo-Villegas, M.; del Real-López, A.; Rodríguez-García, M.E. Structural, morphological, chemical, vibrational, pasting, rheological, and thermal characterization of isolated jicama (Pachyrhizus spp.) starch and jicama starch added with Ca(OH)2. Food Chem. 2019, 283, 83–91. [Google Scholar] [CrossRef]
- Contreras-Jiménez, B.; Del Real, A.; Millan-Malo, B.M.; Gaytán-Martínez, M.; Morales-Sánchez, E.; Rodríguez-García, M.E. Physicochemical changes in barley starch during malting. J. Inst. Brew. 2019, 125, 10–17. [Google Scholar] [CrossRef]
- Imberty, A.; Chanzy, H.; Pérez, S.; Bulèon, A.; Tran, V. The double-helical nature of the crystalline part of A-starch. J. Mol. Biol. 1988, 201, 365–378. [Google Scholar] [CrossRef]
- Takahashi, Y.; Kumano, T.; Nishikawa, S. Crystal structure of B-amylose. Macromolecules 2004, 37, 6827–6832. [Google Scholar] [CrossRef]
- Zhang, B.; Guo, K.; Lin, L.S.; Wei, C.X. Comparison of structural and functional properties of starches from the rhizome and bulbil of Chinese yam (Dioscorea opposita Thunb.). Molecules 2018, 23, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Londoño-Restrepo, S.M.; Rincón-Londoño, N.; Contreras-Padilla, M.; Millan-Malo, B.M.; Rodriguez-Garcia, M.E. Morphological, structural, thermal, compositional, vibrational, and pasting characterization of white, yellow, and purple Arracacha lego-like starches and flours (Arracacia xanthorrhiza). Int. J. Biol. Macromol. 2018, 113, 1188–1197. [Google Scholar] [CrossRef] [PubMed]
- Ali, T.M.; Hasnain, A. Morphological, physicochemical, and pasting properties of modified white Sorghum (Sorghum bicolor) starch. Int. J. Food Prop. 2014, 17, 523–535. [Google Scholar] [CrossRef]
- Craig, S.A.S.; Maningat, C.C.; Seib, P.A.; Hoseney, R.C. Starch paste clarity. Cereal Chem. 1989, 66, 173–182. [Google Scholar]
- Jarvis, C.E.; Walker, J.R.L. Simultaneous, rapid, spectrophotometric determination of total starch, amylose and amylopectin. J. Sci. Food Agric. 1993, 63, 53–57. [Google Scholar] [CrossRef]
- Carmona-García, R.; Bello-Pérez, L.A.; Aguirre-Cruz, A.; Aparicio-Saguilán, A.; Hernandez-Torres, J.; Alvarez-Ramirez, J. Effect of ultrasonic treatment on the morphological, physicochemical, functional and rheological properties of starches with different granule size. Starch-Stärke 2016, 68, 972–979. [Google Scholar] [CrossRef]
- Li, S.L.; Ward, R.; Gao, Q.Y. Effect of heat-moisture treatment on the formation and physicochemical properties of resistant starch from mung bean (Phaseolus radiatus) starch. Food Hydrocoll. 2011, 25, 1702–1709. [Google Scholar] [CrossRef]
- Jamir, K.; Seshagirirao, K. Isolation, characterization and comparative study of starches from selected Zingiberaceae species, a non-conventional source. Food Hydrocoll. 2017, 72, 247–253. [Google Scholar] [CrossRef]
- Findinier, J.; Laurent, S.; Duchêne, T.; Roussel, X.; Lancelon-Pin, C.; Cuiné, S.; Putaux, J.L.; Li-Beisson, Y.H.; D’Hulst, C.; Wattebled, F.; et al. Deletion of BSG1 in Chlamydomonas reinhardtii leads to abnormal starch granule size and morphology. Sci. Rep. 2019, 9, 1990. [Google Scholar] [CrossRef] [PubMed]
- Betancur, D.A.; Ancona, L.A.C.; Guerrero, R.I.; Matos, G.C.; Ortiz, D. Physicochemical and functional characterization of baby lima bean (Phaseolus lunatus) starch. Starch-Stärke 2001, 53, 219–226. [Google Scholar] [CrossRef]
- Yuan, R.C.; Thompson, D.B.; Boyer, C.D. Fine structure of amylopectin in relation to gelatinization and retrogradation behavior of maize starches from three wx-containing genotypes in two inbred lines. Cereal Chem. 1993, 70, 81–89. [Google Scholar]
- Moorthy, S.N. Physicochemical and functional properties of tropical tuber starches: A review. Starch-Stärke 2002, 54, 559–592. [Google Scholar] [CrossRef]
- Radosavljevic, M.; Jane, J.; Johnson, L.A. Isolation of amaranth starch by diluted alkaline-protease treatment. Cereal Chem. 1998, 75, 212–216. [Google Scholar] [CrossRef]
- Jane, J.; Chen, Y.Y.; Lee, L.F.; Mcpherson, A.E.; Wong, K.S.; Radosavljevic, M.; Kasemsuwan, T. Effects of amylopectin branch chain length and amylose content on the gelatinization and pasting properties of starch. Cereal Chem. 1999, 76, 629–637. [Google Scholar] [CrossRef]
- You, S.Y.; Lim, S.T.; Lee, J.H.; Chung, H.J. Impact of molecular and crystalline structures on in vitro digestibility of waxy rice starches. Carbohydr. Polym. 2014, 112, 729–735. [Google Scholar] [CrossRef] [PubMed]
- Tester, R.F.; Morrison, W.R. Swelling and gelatinization of cereal starches. I. Effects of amylopectin, amylose and lipids. Cereal Chem. 1990, 67, 551–557. [Google Scholar]
- Yuan, Y.; Zhang, L.; Dai, Y.J.; Yu, J.G. Physicochemical properties of starch obtained from Dioscorea nipponica Makino comparison with other tuber starches. J. Food Eng. 2007, 82, 436–442. [Google Scholar] [CrossRef]
- Ratnayake, W.S.; Hoover, R.; Warkentin, T. Pea starch: Composition, structure and properties-A review. Starch-Stärke 2002, 54, 217–234. [Google Scholar] [CrossRef]
- Leach, H.W.; McCowen, L.D.; Thomas, C.J. Structure of the starch granule I. Swelling and solubility patterns of various starches. Cereal Chem. 1959, 36, 534–544. [Google Scholar]
- Miao, M.; Zhang, T.; Jiang, B. Characterisations of kabuli and desi chickpea starches cultivated in China. Food Chem. 2009, 113, 1025–1032. [Google Scholar] [CrossRef]
- Zavareze, E.D.R.; Storck, C.R.; Castro, L.A.S.D.; Schirmer, M.A.; Dias, A.R.G. Effect of heat-moisture treatment on rice starch of varying amylose content. Food Chem. 2010, 121, 358–365. [Google Scholar] [CrossRef]
- Singh, N.; Singh, J.; Kaur, L.; Sodhi, N.S.; Gill, B.S. Morphological, thermal and rheological properties of starches from different botanical sources. Food Chem. 2003, 81, 219–231. [Google Scholar] [CrossRef]
- Hong, J.; Zeng, X.A.; Buckow, R.; Han, Z. Structural, thermodynamic and digestible properties of maize starches esterified by conventional and dual methods: Differentiation of amylose contents. Food Hydrocoll. 2018, 83, 419–429. [Google Scholar] [CrossRef]
- Rincón-Londoño, N.; Vega-Rojas, L.J.; Contreras-Padilla, M.; Acosta-Osoriod, A.A.; Rodríguez-García, M.E. Analysis of the pasting profile in corn starch: Structural, morphological, and thermal transformations, Part I. Int. J. Biol. Macromol. 2016, 91, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Wani, I.A.; Sogi, D.S.; Wani, A.A.; Gill, B.S.; Shivhare, U.S. Physico-chemical properties of starches from Indian kidney bean (Phaseolus vulgaris) cultivars. Int. J. Food Sci. Technol. 2010, 45, 2176–2185. [Google Scholar] [CrossRef]
- Charles, A.L.; Chang, Y.H.; Ko, W.C.; Sriroth, K.; Huang, T.C. Some physical and chemical properties of starch isolates of cassava genotypes. Starch-Stärke 2004, 56, 413–418. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 18th ed.; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Hoover, R.; Senanayake, S. Composition and physicochemical properties of oat starches. Food Res. Int. 1996, 29, 15–26. [Google Scholar] [CrossRef] [Green Version]








| Parameter | SBS | EBS | ABS |
|---|---|---|---|
| Yield (%) | 29.9 ± 0.54a | 25.5 ± 0.78b | 23.8 ± 0.21c |
| Moisture content (%) | 5.5 ± 0.10b | 7.4 ± 0.08a | 8.1 ± 0.02a |
| Protein (%) | 0.01 ± 0.00a | 0.01 ± 0.00a | 0.01 ± 0.00a |
| Ash (%) | 0.24 ± 0.00a | 0.18 ± 0.02a | 0.14 ± 0.03a |
| Lipids (%) | 0.84 ± 0.18a | 0.72 ± 0.11a | 0.71 ± 0.25a |
| Starch (%) | 88.9 ± 1.84b | 89.6 ± 0.32ab | 91.7 ± 0.03a |
| Amylose (%) | 50.2 ± 1.09b | 49.2 ± 0.17b | 55.0 ± 0.43a |
| Mineral elements (mg/kg) | |||
| P | 127 ± 4.61b | 160 ± 6.29a | 174 ± 16.32a |
| B | 1.13 ± 0.01a | 1.11 ± 0.04a | 1.08 ± 0.06a |
| Na | 30.39 ± 20.07b | 58.06 ± 13.07a | 4.08 ± 0.001c |
| Mg | 87.65 ± 23.82a | 96.08 ± 16.6a | 39.4 ± 17.88b |
| K | - | - | - |
| Ca | 404.22 ± 85.49ab | 464.01 ± 83.71a | 255.93 ± 88.42c |
| Fe | - | 17.25 ± 0.2 | - |
| Cu | 0.7 ± 0.01b | 1.32 ± 0.09a | 0.18 ± 0.004c |
| Zn | 12.88 ± 0.27b | 10.49 ± 0.15c | 55.59 ± 0.75a |
| Se | 0.06 ± 0.008b | 0.21 ± 0.08a | 0.04 ± 0.02b |
| Parameter | SBS | ABS | EBS |
|---|---|---|---|
| To (°C) | 65.8 ± 0.2c | 67.9 ± 0.3a | 66.8 ± 0.1b |
| Tp (°C) | 70.3 ± 0.3b | 69.3 ± 0.2c | 73.0 ± 0.4a |
| Tc (°C) | 76.3 ± 0.3b | 73.0 ± 0.1c | 82.0 ± 0.2a |
| ΔHgel (J/g) | 2.0 ± 0.1c | 13.8 ± 0.1a | 11.5 ± 0.3b |
| (Tc−To) (°C) | 10.5 ± 0.4b | 5.1 ± 0.3c | 15.2 ± 0.5a |
| Sample | Peak Viscosity (cP) | Trough (cP) | Breakdown (cP) | Final Viscosity (cP) | Setback (cP) | Pasting Temperature (°C) |
|---|---|---|---|---|---|---|
| SBS | 3919 ± 53.0c | 2556 ± 8.5c | 1364 ± 61.5c | 4344 ± 53.5c | 1788 ± 62c | 83.58 ± 0.03c |
| EBS | 5192 ± 2.5b | 3626 ± 9.5a | 1571 ± 12b | 6287 ± 13.5a | 2666 ± 4a | 85.55 ± 0.45a |
| ABS | 5293 ± 30.0a | 3388 ± 32.0b | 1905 ± 2a | 5914 ± 61.5b | 2526 ± 29.5b | 84.35 ± 0.05b |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, P.; Wang, L.; Qian, Y.; Wang, X.; Zhang, S.; Chang, J.; Ruan, Y.; Ma, B. Influences of Extraction Methods on Physicochemical and Functional Characteristics of Three New Bulbil Starches from Dioscorea opposita Thunb. cv. Tiegun. Molecules 2019, 24, 2232. https://doi.org/10.3390/molecules24122232
Zhang P, Wang L, Qian Y, Wang X, Zhang S, Chang J, Ruan Y, Ma B. Influences of Extraction Methods on Physicochemical and Functional Characteristics of Three New Bulbil Starches from Dioscorea opposita Thunb. cv. Tiegun. Molecules. 2019; 24(12):2232. https://doi.org/10.3390/molecules24122232
Chicago/Turabian StyleZhang, Pengzhan, Li Wang, Yanyan Qian, Xuguang Wang, Shaoning Zhang, Jiping Chang, Yuan Ruan, and Bingji Ma. 2019. "Influences of Extraction Methods on Physicochemical and Functional Characteristics of Three New Bulbil Starches from Dioscorea opposita Thunb. cv. Tiegun" Molecules 24, no. 12: 2232. https://doi.org/10.3390/molecules24122232