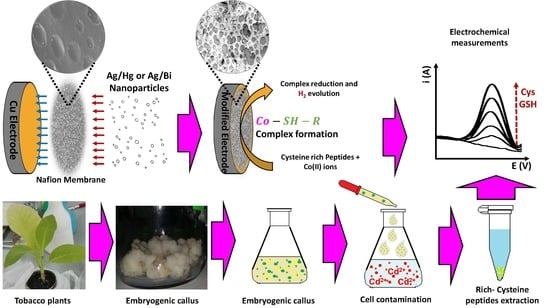
Development and Evaluation of Copper Electrodes, Modified with Bimetallic Nanoparticles, to be Used as Sensors of Cysteine-Rich Peptides Synthesized by Tobacco Cells Exposed to Cytotoxic Levels of Cadmium
Abstract
:1. Introduction
2. Experimental
2.1. Reagents
2.2. Instrumentation
2.3. Electrode Development
2.3.1. Electrode Construction
2.3.2. Bimetallic Deposits Formation
2.4. Nicotiana tabacum Plants and Cells Culturing
2.5. Analytical Procedure
3. Results and Discussion
3.1. Electrode Characterization
3.1.1. Scanning Electron Microscopy (SEM)
3.1.2. Electrode’s Electrochemical Characterization
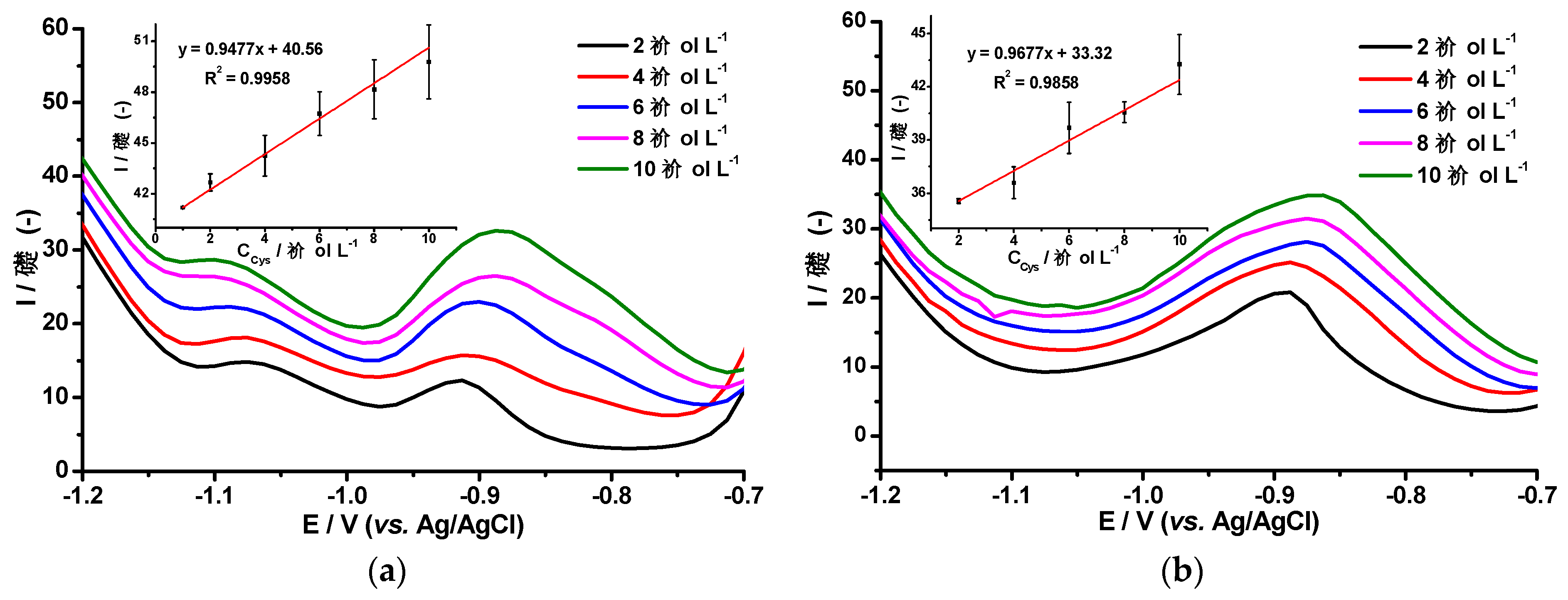
3.2. Differential Pulse Voltammetry of L-cysteine and Co(II) Ions using the AgHgNf/Cu and AgBiNf/Cu Modified Electrodes
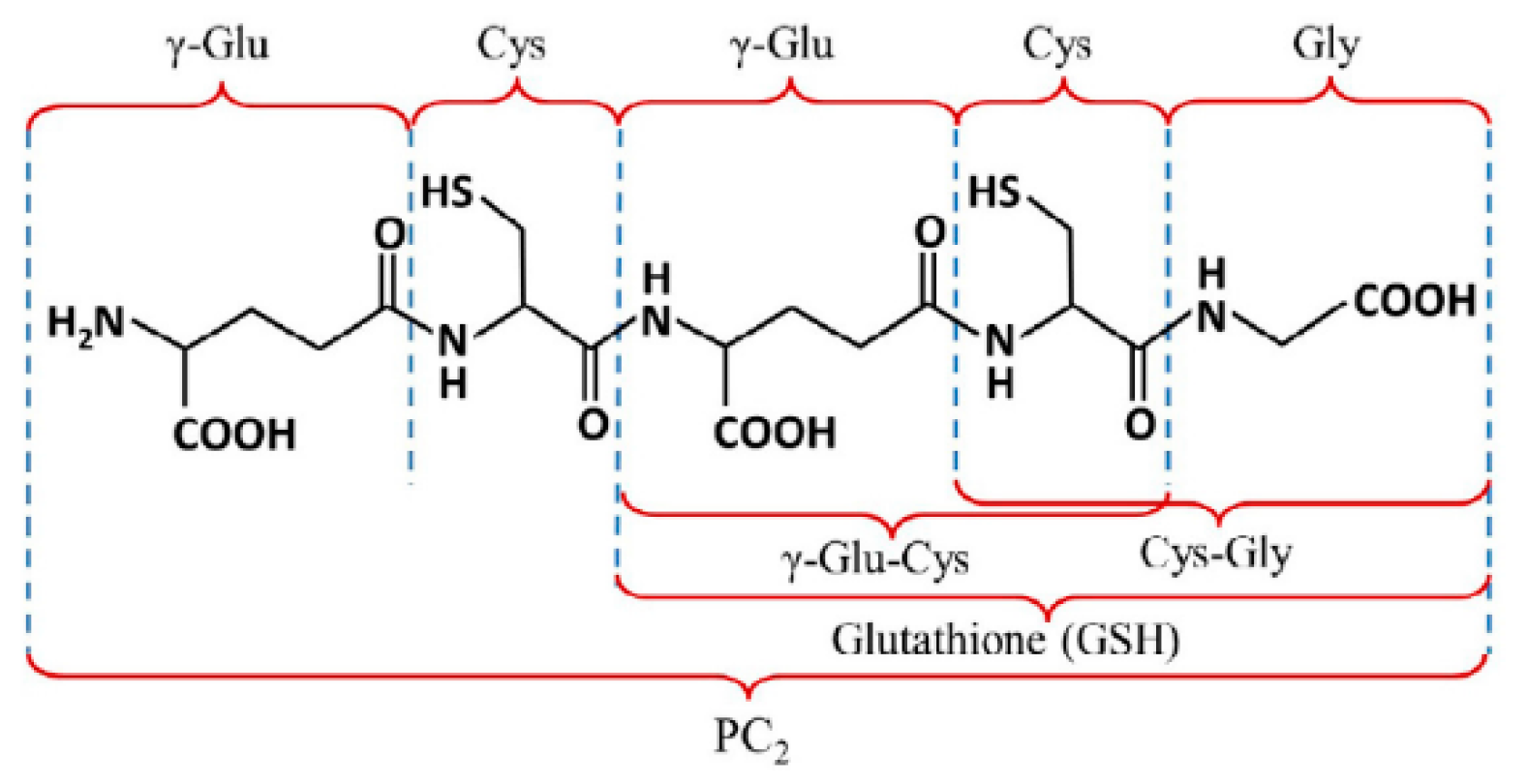
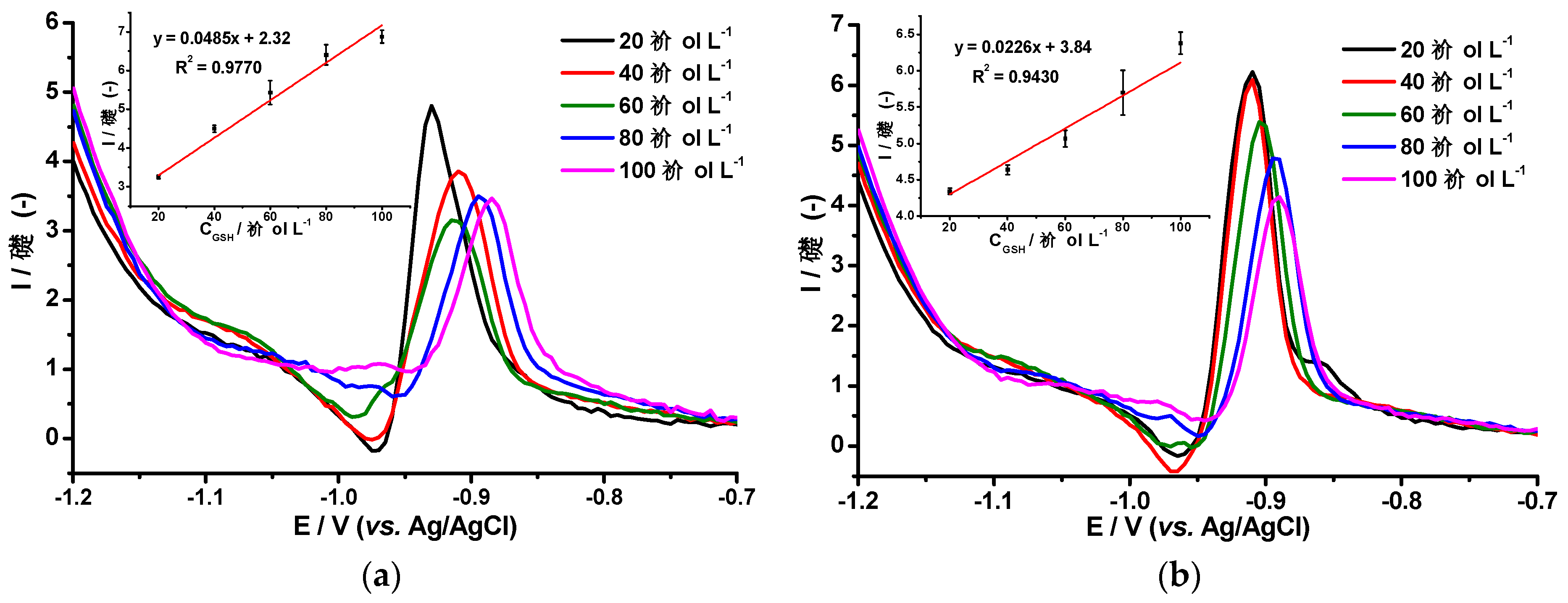
3.3. Differential Pulse Voltammetry of Glutathione and Co(II) Ions Using the AgHgNf/Cu and the AgBiNf/Cu Modified Electrodes
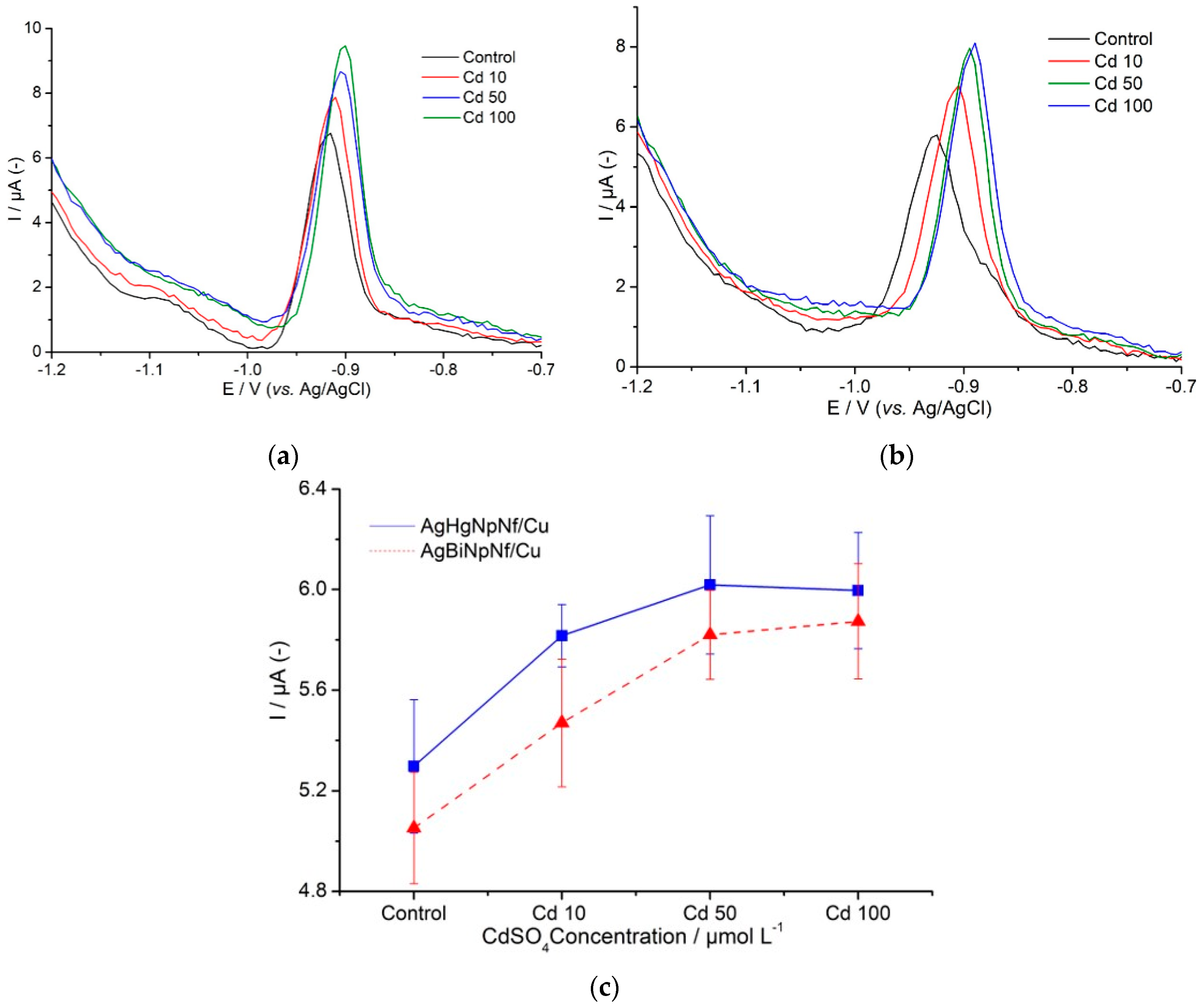
3.4. Determination of CRp Generated by Nicotiana tabacum Cells Exposed to Cytotoxic Levels of Cd
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yadav, S. Heavy metals toxicity in plants: An overview of the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. S. Afr. J. Bot. 2010, 76, 167–179. [Google Scholar] [CrossRef]
- Adigun, F.; Raab, A.; Voigt, M.; Malta, L.; Krupp, E.; Feldmann, J. The importance of glutathione and phytochelatins on the selenite and arsenate detoxification in Arabidopsis thaliana. J. Environ. Sci. 2016, 49, 150–161. [Google Scholar] [CrossRef]
- Krystofova, O.; Zitka, O.; Krizkova, S.; Hynek, D.; Shestivska, V.; Adam, V.; Hubalek, J.; Mackova, M.; Macek, T.; Zehnalek, J.; et al. Accumulation of cadmium by transgenic tobacco plants (Nicotiana tabacum L.) carrying yeast metallothionein gene revealed by electrochemistry. Int. J. Electrochem. Sci. 2012, 7, 886–907. Available online: https://www.scopus.com/record/display.uri?eid=2s2.084857855467&origin=inward&txGid=2e19b186c09c5755641580e9f6a2d0df# (accessed on 10 April 2018).
- Serrano, N.; Díaz-Cruz, M.; Ariño, C.; Esteban, M. Recent contributions to the study of phytochelatins with an analytical approach. Trends Anal. Chem. 2015, 73, 129–145. [Google Scholar] [CrossRef]
- Fojta, M.; Fojtová, M.; Havran, L.; Pivoňková, H.; Dorčáka, V.; Šestákovác, I. Electrochemical monitoring of phytochelatin accumulation in nicotiana tabacum cells exposed to sub-cytotoxic and cytotoxic levels of cadmium. Anal. Chim. Acta 2006, 558, 171–178. [Google Scholar] [CrossRef]
- Wójcik, M.; Vangronsveld, J.; Tukiendorf, A. Cadmium tolerance in Thlaspi caerulescens: I. Growth parameters, metal accumulation, and phytochelatin synthesis in response to cadmium. Environ. Exp. Bot. 2005, 53, 151–161. [Google Scholar] [CrossRef]
- Brdićka, R. Polarographic studies with the dropping mercury cathode. Part XXXI. A new test for proteins in the presence of cobalt salts in ammoniacal solutions of ammonium chloride. Collect. Czech. Chem. Commun. 1933, 1, 112–128. [Google Scholar] [CrossRef]
- Dorćák, V.; Šestáková, I. Electrochemical behavior of phytochelatins and related peptides at the hanging mercury drop electrode in the presence of cobalt (II) ions. Bioelectrochemistry 2006, 68, 14–21. [Google Scholar] [CrossRef]
- Vacek, J.; Petřek, J.; Kizek, R.; Havel, L.; Klejdus, B. Electrochemical determination of lead and glutathione in a plant cell culture. Bioelectrochemistry 2004, 63, 347–351. [Google Scholar] [CrossRef]
- Yosypchuk, B.; Šestáková, I.; Novotný, L. Voltammetric determination of phytochelatin using copper solid amalgam electrode. Talanta 2003, 59, 1253–1258. [Google Scholar] [CrossRef]
- Nesakumar, N.; Berchmans, S.; Subbiah, A. Chemically modified carbon-based electrodes for the detection of reduced glutathione. Sens. Actuators B Chem. 2018, 264, 448–466. [Google Scholar] [CrossRef]
- Xing, S.; Xu, H.; Chen, J.; Shi, G.; Jin, L. Nafion stabilized silver nanoparticles modified electrode and its application to Cr (VI) detection. J. Electroanal. Chem. 2011, 652, 60–65. [Google Scholar] [CrossRef]
- Fernandez-Carretero, F. Síntesis y Caracterización de Membranas Híbridas Organo-Inorgánicas Para Su Uso en Pilas de Combustible. Ph.D. Thesis, Universidad Politécnica de Valencia, Valencia, España, 2008. Recuperado de la base de datos Researchgate. (50838442). [Google Scholar]
- Pifferi, V.; Marona, V.; Longhi, M.; Falciola, L. Characterization of polymer stabilized silver nanoparticles modified glassy carbon electrodes for electroanalytical applications. Electrochim. Acta 2013, 109, 447–453. [Google Scholar] [CrossRef]
- Valera, D.; Espinoza-Montero, P.; Alvarado, J.; Carrera, P.; Bonilla, P.; Cumbal, L.; Fernández, L. Development and evaluation of a glassy carbon electrode modified with silver and mercury nanoparticles for quantification of cysteine rich peptides. Sens. Actuators B Chem. 2017, 253, 1170–1179. [Google Scholar] [CrossRef]
- Legeai, S.; Vittori, O. A Cu/Nf/Bi electrode for on-site monitoring of trace heavy metals in natural waters using anodic stripping voltammetry: An alternative to mercury-based electrodes. Anal. Chim. Acta 2006, 560, 184–190. [Google Scholar] [CrossRef]
- Alberich, A.; Serrano, N.; Ariño, C.; Díaz-Cruz, M.; Esteban, M. Bismuth film electrodes for the study of metal thiolate complexation: An alternative to mercury electrodes. Talanta 2009, 78, 1017–1022. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Li, J.; Lu, X.; Xi, G.; Yan, Y. Reliable synthesis of bismuth nanoparticles for heavy metal detection. Mater. Res. Bull. 2013, 48, 4718–4722. [Google Scholar] [CrossRef]
- Liu, B.; Ma, C.; Li, Y.; Kou, Y.; Lu, J.; Jiang, X.; Tan, L. Voltammetric determination of reduced glutathione using poly (thionine) as a mediator in the presence of Fenton-type reaction. Talanta 2017, 170, 399–405. [Google Scholar] [CrossRef]
- Šelešovská, R.; Fojta, M.; Navrátil, T.; Chýlková, J. Brdićka-type processes of cysteine and cysteine-containing peptides on silver amalgam electrodes. Anal. Chim. Acta 2007, 582, 344–352. [Google Scholar] [CrossRef]
- Brett, C.; Alves, V.; Fungaro, D. Nafion-coated mercury thin film and glassy carbon electrodes for electroanalysis: Characterization by electrochemical impedance. Electroanalysis 2001, 3, 212–218. Available online: https://www.ipen.br/biblioteca/2001/08421.pdf (accessed on 22 May 2018). [CrossRef]
- Roca, W.; Mroginski, L. Cultivo de Tejidos en la Agricultura: Fundamentos y Aplicaciones; Centro Internacional de Agricultura Tropical (CIAT): Cali, Colombia, 1991. [Google Scholar]
- Alanazi, A.; Mostafa, G.; Al-Badr, A. Chapter two—Glutathione. In Profiles of Drug Substances, Excipients and Related Methodology; Elsevier: Amsterdam, The Netherlands, 2015; Volume 40, pp. 43–158. [Google Scholar] [CrossRef]
- Diniz, M.; Santos, H.; Costa, P.; Peres, I.; Costa, M.; Alves, S.; Capelo-Martinez, J. Efectos de la exposición al arsénico en Corbicula fluminea: Evaluación de las respuestas histológicas, histoquímicas y bioquímicas. Cienc. Mar. 2008, 34, 307–316. Available online: http://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S0185-38802008000300004 (accessed on 13 May 2018). [CrossRef]
- Gouveia-Caridade, C.; Brett, C. Electrochemical impedance characterization of nafion-coated carbon film resistor electrode for electroanalysis. Electroanalysis 2005, 7, 549–555. [Google Scholar] [CrossRef]
- Yuan, S.; Hu, S. Characterization and electrochemical studies of Nafion/nano-TiO2 film modified electrodes. Electrochim. Acta 2004, 49, 4287–4293. [Google Scholar] [CrossRef]
- Raspor, B.; Paić, M.; Erk, M. Analysis of metallothioneins by the modified Brdićka procedure. Talanta 2001, 55, 109–115. [Google Scholar] [CrossRef]
- Armbruster, A.; Terry, P. Limit of blank, limit of detection and limit of quantitation. Clin. Biochem. Rev. 2008, 29, 49–52. [Google Scholar]
- Lee, P.; Goncalves, L.; Compton, R. Electrochemical determination of free and total glutathione in human saliva samples. Sens. Actuators B Chem. 2015, 221, 962–968. [Google Scholar] [CrossRef]
- Ensafi, A.; Tai, M.; Khayamian, T.; Karimi-Maleh, H.; Hasanpour, F. Voltammetric measurement of trace amounts of glutathione using multiwall carbon nanotubes as a sensor and chlorpromazine as a mediator. J. Solid State Electrochem. 2010, 14, 1415–1423. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velasco-Medina, C.; Espinoza-Montero, P.J.; Montero-Jimenez, M.; Alvarado, J.; Jadán, M.; Carrera, P.; Fernandez, L. Development and Evaluation of Copper Electrodes, Modified with Bimetallic Nanoparticles, to be Used as Sensors of Cysteine-Rich Peptides Synthesized by Tobacco Cells Exposed to Cytotoxic Levels of Cadmium. Molecules 2019, 24, 2200. https://doi.org/10.3390/molecules24122200
Velasco-Medina C, Espinoza-Montero PJ, Montero-Jimenez M, Alvarado J, Jadán M, Carrera P, Fernandez L. Development and Evaluation of Copper Electrodes, Modified with Bimetallic Nanoparticles, to be Used as Sensors of Cysteine-Rich Peptides Synthesized by Tobacco Cells Exposed to Cytotoxic Levels of Cadmium. Molecules. 2019; 24(12):2200. https://doi.org/10.3390/molecules24122200
Chicago/Turabian StyleVelasco-Medina, Carlos, Patricio J. Espinoza-Montero, Marjorie Montero-Jimenez, José Alvarado, Mónica Jadán, Patricio Carrera, and Lenys Fernandez. 2019. "Development and Evaluation of Copper Electrodes, Modified with Bimetallic Nanoparticles, to be Used as Sensors of Cysteine-Rich Peptides Synthesized by Tobacco Cells Exposed to Cytotoxic Levels of Cadmium" Molecules 24, no. 12: 2200. https://doi.org/10.3390/molecules24122200