N-Methyl-d-glucamine–Calix[4]resorcinarene Conjugates: Self-Assembly and Biological Properties
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization of N-methyl-d-glucamine-Based Calix[4]resorcinarenes
2.2. Conductometric Measurements
2.3. Investigation of Air-Water Interfacial Tension
2.4. UV-Spectroscopic Measurements
2.5. Self-Assembly Morphology
2.6. Macroccyle Aggregation Comparison
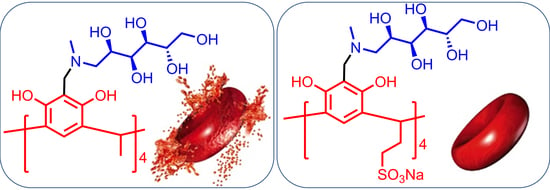
2.7. Evaluation of Toxicity and Biological Activity
3. Materials and Methods
3.1. General Information
3.2. Synthesis of N-methyl-d-glucaminemethyl Sulfonatoethylcalix[4]resorcinarene (GCR-1)
3.3. Synthesis of N-methyl-d-glucaminemethyl methylcalix[4]resorcinarene (GCR-2)
3.4. Tensiometry
3.5. Conductometry
3.6. Hydrophobic Dye Solubilization
3.7. Dynamic Light Scattering
3.8. Transmission Electron Microscopy
3.9. NMR Diffusion Spectroscopy
3.10. Antibacterial and Antifungal Activity
3.11. Cell Viability Evaluation
3.12. Hemolytic Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| GCR-1 | N-methyl-d-glucaminemethyl sulfonatoethylcalix[4]resorcinarene |
| GCR-2 | N-methyl-d-glucaminemethyl methylcalix[4]resorcinarene |
| CR-1 | Sulfonatoethylcalix[4]resorcinarene |
| CAC | Critical aggregation concentration |
| MG | N-methyl-d-glucamine |
| DLS | Dynamic light scattering |
| TEM | Transmission electron microscopy |
| MIC | Minimal inhibiting concentration |
| MBC | Minimal bactericidal concentration |
| MFC | Minimal fungicidal concentration |
References
- Grumezescu, A.M. Design and Development of New Nanocarriers; William Andrew Publishing: Norwich, UK, 2018. [Google Scholar]
- Yu, G.; Jie, K.; Huang, F. Supramolecular amphiphiles based on host − guest molecular recognition motifs. Chem. Rev. 2015, 115, 7240–7303. [Google Scholar] [CrossRef] [PubMed]
- Perret, F.; Coleman, A.W. Biochemistry of anionic calix[n]arenes. Chem. Commun. 2011, 47, 7303–7319. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.F.; Huang, L.Y.; Bu, J.H.; Li, S.Y.; Qin, S.; Xu, Y.W.; Liu, J.M.; Su, C.Y. A fluorescent calixarene-based dimeric capsule constructed: Via a MII-terpyridine interaction: Cage structure, inclusion properties and drug release. RSC Adv. 2018, 8, 22530–22535. [Google Scholar] [CrossRef]
- Nimse, S.B.; Kim, T. Biological applications of functionalized calixarenes. Chem. Soc. Rev. 2013, 42, 366–386. [Google Scholar] [CrossRef] [PubMed]
- Gangemi, C.M.A.; Pappalardo, A.; Trusso Sfrazzetto, G. Applications of supramolecular capsules derived from resorcin[4]arenes, calix[n]arenes and metallo-ligands: from biology to catalysis. RSC Adv. 2015, 5, 51919–51933. [Google Scholar] [CrossRef]
- Neri, P.; Wang, J.L.S.M. Calixarenes and Beyond; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Rahimi, M.; Karimian, R.; Mostafidi, E.; Bahojb Noruzi, E.; Taghizadeh, S.; Shokouhi, B.; Kafil, H.S. Highly branched amine-functionalized p-sulfonatocalix[4]arene decorated with human plasma proteins as a smart, targeted, and stealthy nano-vehicle for the combination chemotherapy of MCF7 cells. New J. Chem. 2018, 42, 13010–13024. [Google Scholar] [CrossRef]
- Chen, H.; Liu, F.; Qi, F.; Koh, K.; Wang, K. Fabrication of calix[4]arene derivative monolayers to control orientation of antibody immobilization. Int. J. Mol. Sci. 2014, 15, 5496–5507. [Google Scholar] [CrossRef]
- Durso, A.; Brancatelli, G.; Hickey, N.; Farnetti, E.; De Zorzi, R.; Bonaccorso, C.; Purrello, R.; Geremia, S. Interactions of a water-soluble calix[4]arene with spermine: Solution and solid-state characterisation. Supramol. Chem. 2016, 28, 499–505. [Google Scholar] [CrossRef]
- Athar, M.; Ranjan, P.; Jha, P.C. A DFT study of inclusion complexes of substituted calix[n]arenes with dasatinib and lapatinib. J. Mol. Graph. Model. 2018, 84, 160–165. [Google Scholar] [CrossRef]
- Yousaf, A.; Hamid, S.A.; Bunnori, N.M. Applications of calixarenes in cancer chemotherapy. Drug Des. Devel. Ther. 2015, 9, 2831–2838. [Google Scholar] [CrossRef]
- Cassimiro, D.L.; Ferreira, L.M.B.; Capela, J.M.V.; Crespi, M.S.; Ribeiro, C.A. Kinetic parameters for thermal decomposition of supramolecular polymers derived from diclofenac-meglumine supramolecular adducts. J. Pharm. Biomed. Anal. 2013, 73, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Aloisio, C.; Gomes De Oliveira, A.; Longhi, M. Characterization, inclusion mode, phase-solubility and in vitro release studies of inclusion binary complexes with cyclodextrins and meglumine using sulfamerazine as model drug. Drug Dev. Ind. Pharm. 2014, 40, 919–928. [Google Scholar] [CrossRef] [PubMed]
- Afsah, E.M.; Fadda, A.A.; Hanash, A.H. Synthesis of a new series of n-Mannich bases and polyhydroxy Mannich bases of pharmaceutical interest related to isatin and its schiff bases. J. Heterocycl. Chem. 2018, 55, 736–742. [Google Scholar] [CrossRef]
- Khavani, M.; Izadyar, M.; Housaindokht, M.R. Glucose derivatives substitution and cyclic peptide diameter effects on the stability of the self-assembled cyclic peptide nanotubes; a joint QM/MD study. J. Mol. Graph. Model. 2017, 71, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Nifantiev, N.E.; Yashunsky, D.V. Water-Soluble Porphyrin Derivatives for Photodynamic Therapy, Their Use and Manufacture. European Patent EP1404678B1, 7 April 2004. [Google Scholar]
- Nakajima, S.; Hayashi, H.; Omote, Y.; Yamazaki, Y.; Hirata, S.; Maeda, T.; Kubo, Y.; Takemura, T.; Kakiuchi, Y.; Shindo, Y.; et al. The tumour-localizing properties of porphyrin derivatives. J. Photochem. Photobiol. B Biol. 1990, 7, 189–198. [Google Scholar] [CrossRef]
- Pegaz, B.; Debefve, E.; Borle, F.; Ballini, J.-P.; Wagnières, G.; Spaniol, S.; Albrecht, V.; Scheglmann, D.; Nifantiev, N.E.; van den Bergh, H.; et al. Preclinical evaluation of a novel water-soluble chlorin e6 derivative (BLC 1010) as photosensitizer for the closure of the neovessels. Photochem. Photobiol. 2005, 81, 1505. [Google Scholar] [CrossRef]
- Kass, J.P.; Slasor, L.A.; Zambrano, C.H.; Dueno, E.E. Improved synthesis and purification of cavitands. Org. Prep. Proced. Int. New J. Org. Synth. 2006, 38, 480–483. [Google Scholar] [CrossRef]
- Kobayashi, K.; Asakawa, Y.; Kato, Y.; Aoyama, Y. Complexation of hydrophobic sugars and nucleosides in water with tetrasulfonate derivatives of resorcinol cyclic tetramer having a polyhydroxy aromatic cavity: importance of guest–host CH–π interaction. J. Am. Chem. Soc. 1992, 114, 10307–10313. [Google Scholar] [CrossRef]
- Kharlamov, S.V.; Kashapov, R.R.; Pashirova, T.N.; Zhiltsova, E.P.; Ziganshina, A.Y.; Zakharova, L.Y.; Latypov, S.K.; Konovalov, A.I. A supramolecular amphiphile based on calix[4]resorcinarene and cationic surfactant for controlled self-assembly. J. Phys. Chem. C. 2013, 117, 20280–20288. [Google Scholar] [CrossRef]
- Kashapov, R.R.; Pashirova, T.N.; Kharlamov, S.V.; Ziganshina, A.Y.; Ziltsova, E.P.; Lukashenko, S.S.; Zakharova, L.Y.; Habicher, W.D.; Latypov, S.K.; Konovalov, A.I. Novel self-assembling system based on resorcinarene and cationic surfactant. Phys. Chem. Chem. Phys. 2011, 13, 15891–15898. [Google Scholar] [CrossRef]
- Korshin, D.E.; Kashapov, R.R.; Murtazina, L.I.; Mukhitova, R.K.; Kharlamov, S.V.; Latypov, S.K.; Ryzhkina, I.S.; Ziganshina, A.Y.; Konovalov, A.I. Self-assembly of an aminoalkylated resorcinarene in aqueous media: Host-guest properties. New J. Chem. 2009, 33, 2397–2401. [Google Scholar] [CrossRef]
- Houmadi, S.; Coquière, D.; Legrand, L.; Fauré, M.C.; Goldmann, M.; Reinaud, O.; Rémita, S. Architecture-controlled “SMART” calix[6]arene self-assemblies in aqueous solution. Langmuir 2007, 23, 4849–4855. [Google Scholar] [CrossRef]
- Martin, A.D.; Houlihan, E.; Morellini, N.; Eggers, P.K.; James, E.; Stubbs, K.A.; Harvey, A.R.; Fitzgerald, M.; Raston, C.L.; Dunlop, S.A. Synthesis and toxicology of p-phosphonic acid calixarenes and O-alkylated analogues as potential calixarene-based phospholipids. Chempluschem 2012, 77, 308–313. [Google Scholar] [CrossRef]
- Semenov, V.E.; Voloshina, A.D.; Kulik, N.V.; Strobykina, A.S.; Giniyatullin, R.K.; Saifina, L.F.; Nikolaev, A.E.; Krylova, E.S.; Zobov, V.V.; Reznik, V.S. Macrocyclic and acyclic 1,3-bis[5-(trialkylammonio)pentyl]-5(6)-substituted uracil dibromides: synthesis, antimicrobial properties, and the structure—activity relationship. Russ. Chem. Bull. Int. Ed. 2015, 64, 2885–2896. [Google Scholar] [CrossRef]
- Voloshina, A.D.; Semenov, V.E.; Strobykina, A.S.; Kulik, N.V.; Krylova, E.S.; Zobov, V.V.; Reznik, V.S. Synthesis and antimicrobial and toxic properties of novel 1,3-bis(alkyl)-6-methyluracil derivatives containing 1,2,3- and 1,2,4-triazolium fragments. Russ. J. Bioorganic Chem. 2017, 43, 170–176. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |










| Substances | Tensiometry | Conductometry | UV Spectroscopy |
|---|---|---|---|
| GCR-1 | 15 | 14 | 10 |
| GCR-2 | 3.3 | 7.9 | 7.2 |
| Compounds | Minimal Inhibiting Concentration (MIC), mM | ||
|---|---|---|---|
| S. aureus 209P | B. cereus 8035 | C.albicans 855–653 | |
| Bacteriostatic and fungistatic activity | |||
| GCR-1 | 1.00 ± 0.07 | >1 | >1 |
| GCR-2 | 0.13 ± 0.01 | 0.25 | >0.5 |
| Minimal Bactericidal and Fungicidal Concentration (MBC, MFC), mM | |||
| Bactericidal and fungicidal activity | |||
| GCR-1 | 1.00 ± 0.06 | >1 | >1 |
| GCR-2 | 1.00 ± 0.08 | >1 | >0.5 |
| Compounds | Concentration, mM | % of Hemolysis | IC50 on Chang Liver Cells Line (mM) |
|---|---|---|---|
| GCR-1 | 5.00 ± 0.43 | 85.6 ± 7.3 | >0.1 |
| 2.00 ± 0.17 | 34.4 ± 3.2 | ||
| 1.00 ± 0.07 | 16.9 ± 1.4 | ||
| 0.50 ± 0.03 | 8.8 ± 0.7 | ||
| 0.25 ± 0.02 | 4.8 ± 0.4 | ||
| GCR-2 | 0.50 ± 0.04 | 38.1 ± 3.4 | >0.1 |
| 0.25 ± 0.02 | 31.6 ± 2.7 | ||
| 0.125 ± 0.01 | 21.3 ± 1.7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kashapov, R.R.; Razuvayeva, Y.S.; Ziganshina, A.Y.; Mukhitova, R.K.; Sapunova, A.S.; Voloshina, A.D.; Syakaev, V.V.; Latypov, S.K.; Nizameev, I.R.; Kadirov, M.K.; et al. N-Methyl-d-glucamine–Calix[4]resorcinarene Conjugates: Self-Assembly and Biological Properties. Molecules 2019, 24, 1939. https://doi.org/10.3390/molecules24101939
Kashapov RR, Razuvayeva YS, Ziganshina AY, Mukhitova RK, Sapunova AS, Voloshina AD, Syakaev VV, Latypov SK, Nizameev IR, Kadirov MK, et al. N-Methyl-d-glucamine–Calix[4]resorcinarene Conjugates: Self-Assembly and Biological Properties. Molecules. 2019; 24(10):1939. https://doi.org/10.3390/molecules24101939
Chicago/Turabian StyleKashapov, Ruslan R., Yuliya S. Razuvayeva, Albina Y. Ziganshina, Rezeda K. Mukhitova, Anastasiia S. Sapunova, Alexandra D. Voloshina, Victor V. Syakaev, Shamil K. Latypov, Irek R. Nizameev, Marsil K. Kadirov, and et al. 2019. "N-Methyl-d-glucamine–Calix[4]resorcinarene Conjugates: Self-Assembly and Biological Properties" Molecules 24, no. 10: 1939. https://doi.org/10.3390/molecules24101939