β-Caryophyllene as a Potential Protective Agent Against Myocardial Injury: The Role of Toll-Like Receptors
Abstract
:1. Introduction
2. Results
2.1. Effects of β-Caryophyllene on Myocardial Infarct Size
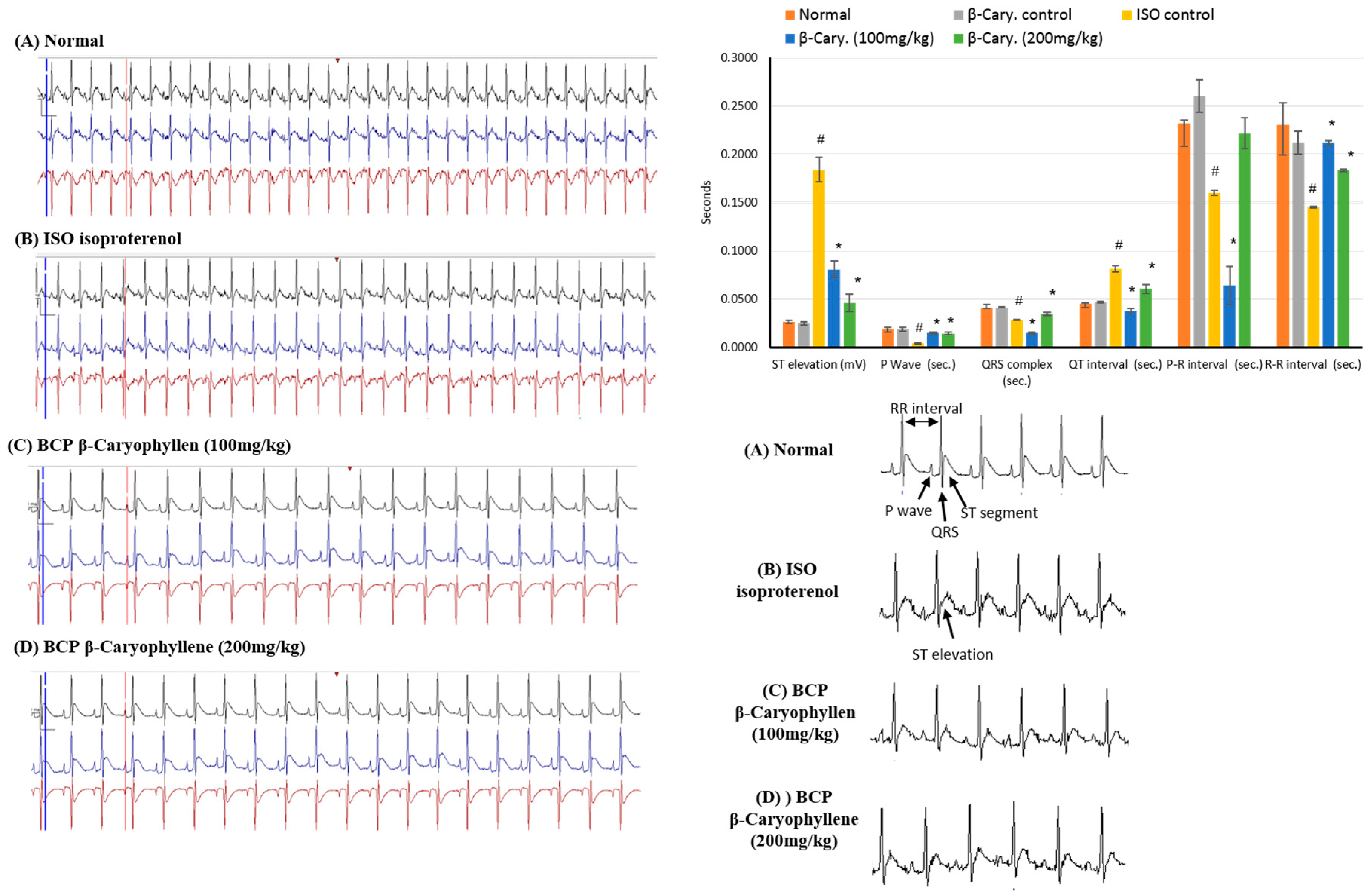
2.2. Effects of β-Caryophyllene on Electrocardiographic (ECG) Traces
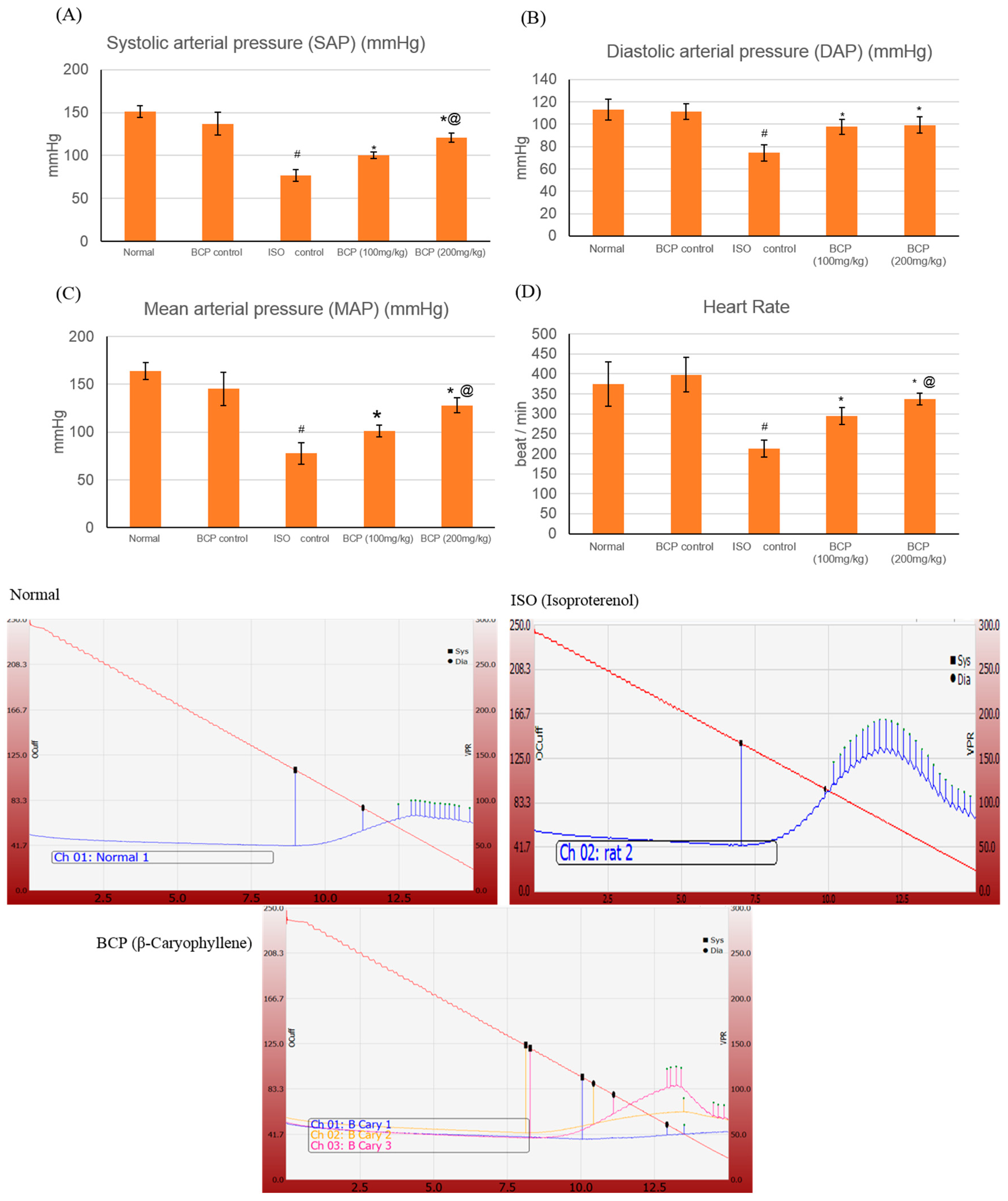
2.3. Effects of β-Caryophyllene on Blood Pressure (BP) Indices
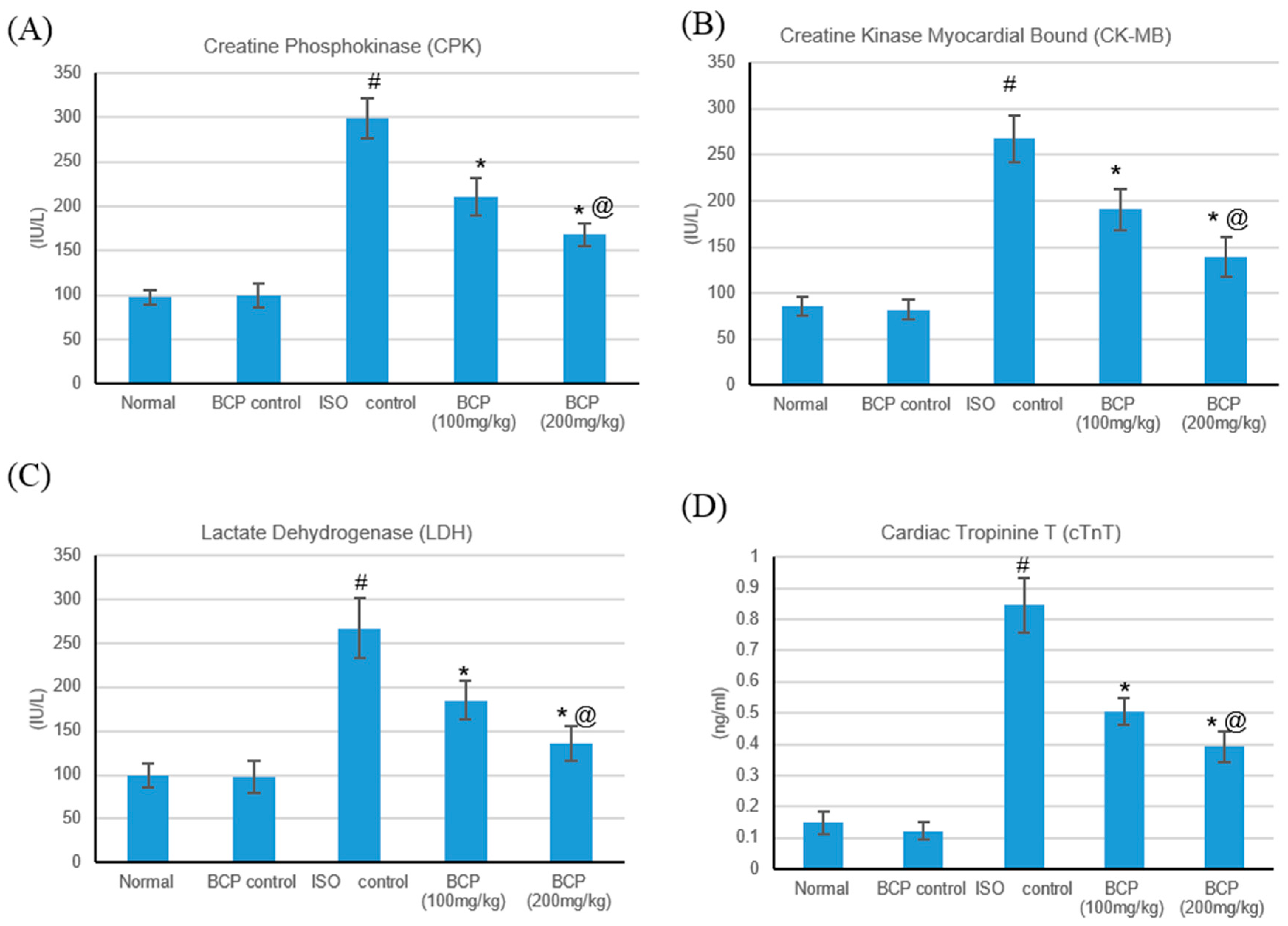
2.4. Effects of β-Caryophyllene on Cardiac Marker Enzymes
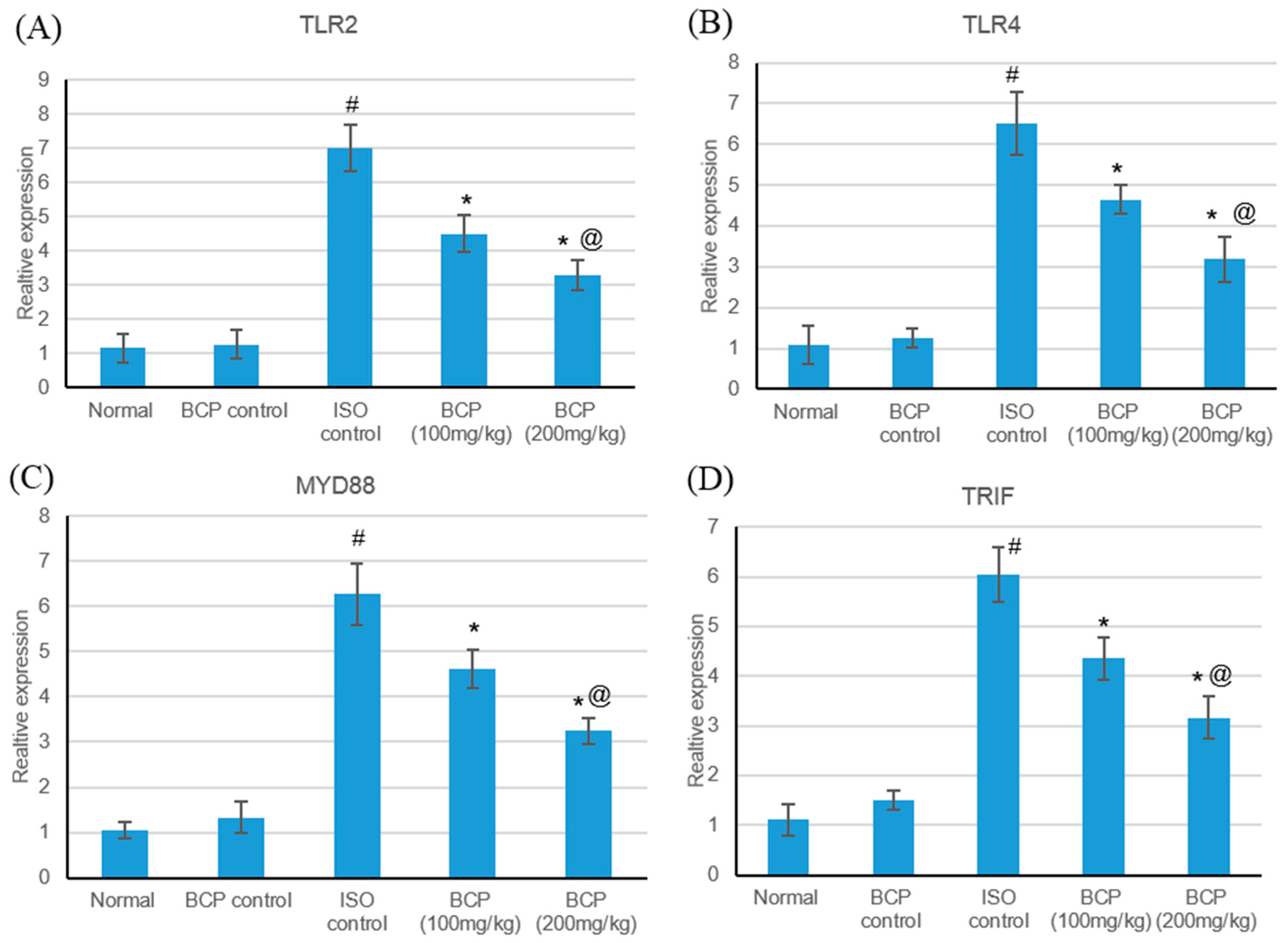
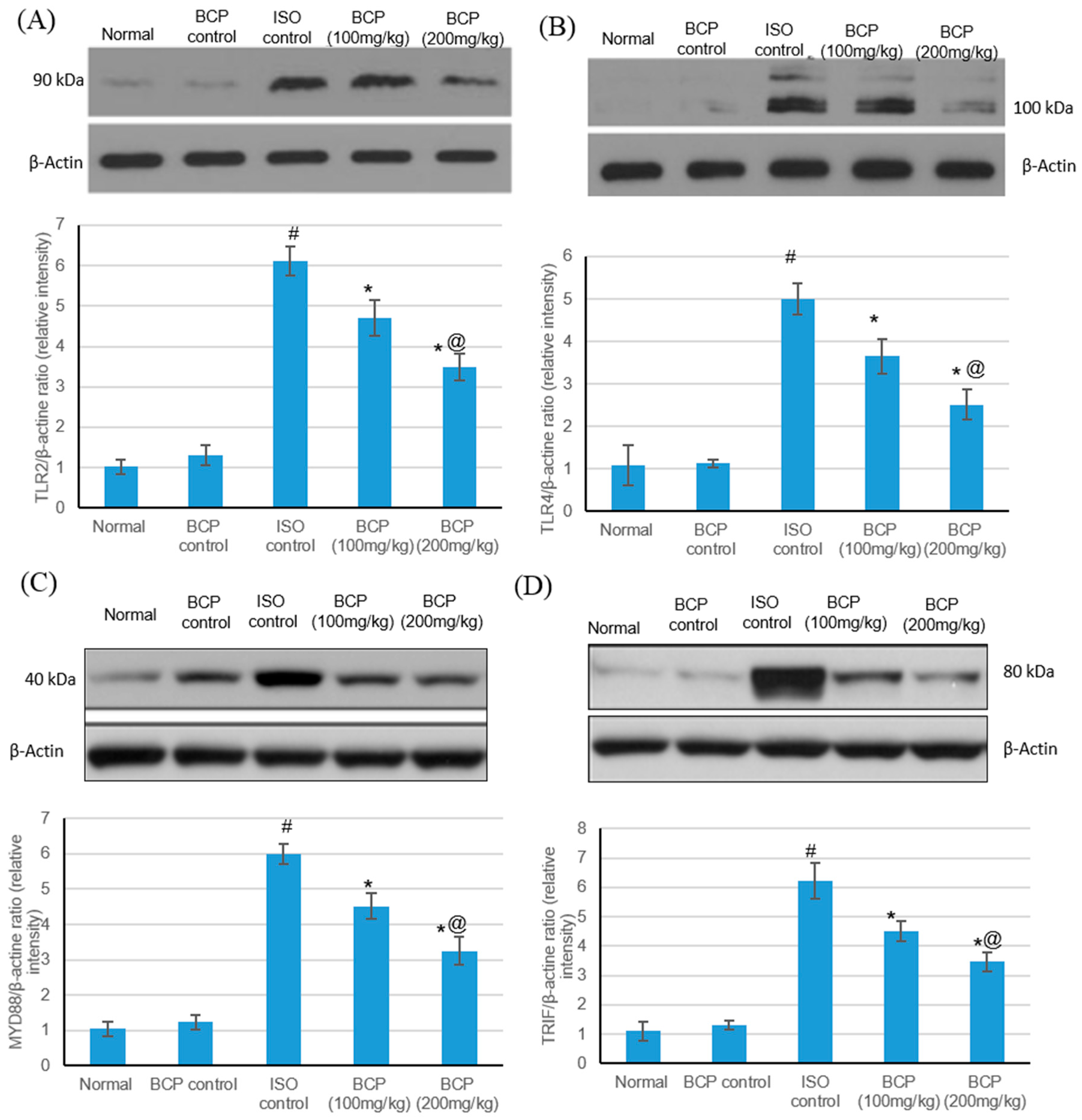
2.5. Effects of β-Caryophyllene Treatment on TLR4, TLR2, MyD88, and TRIF Expression Levels
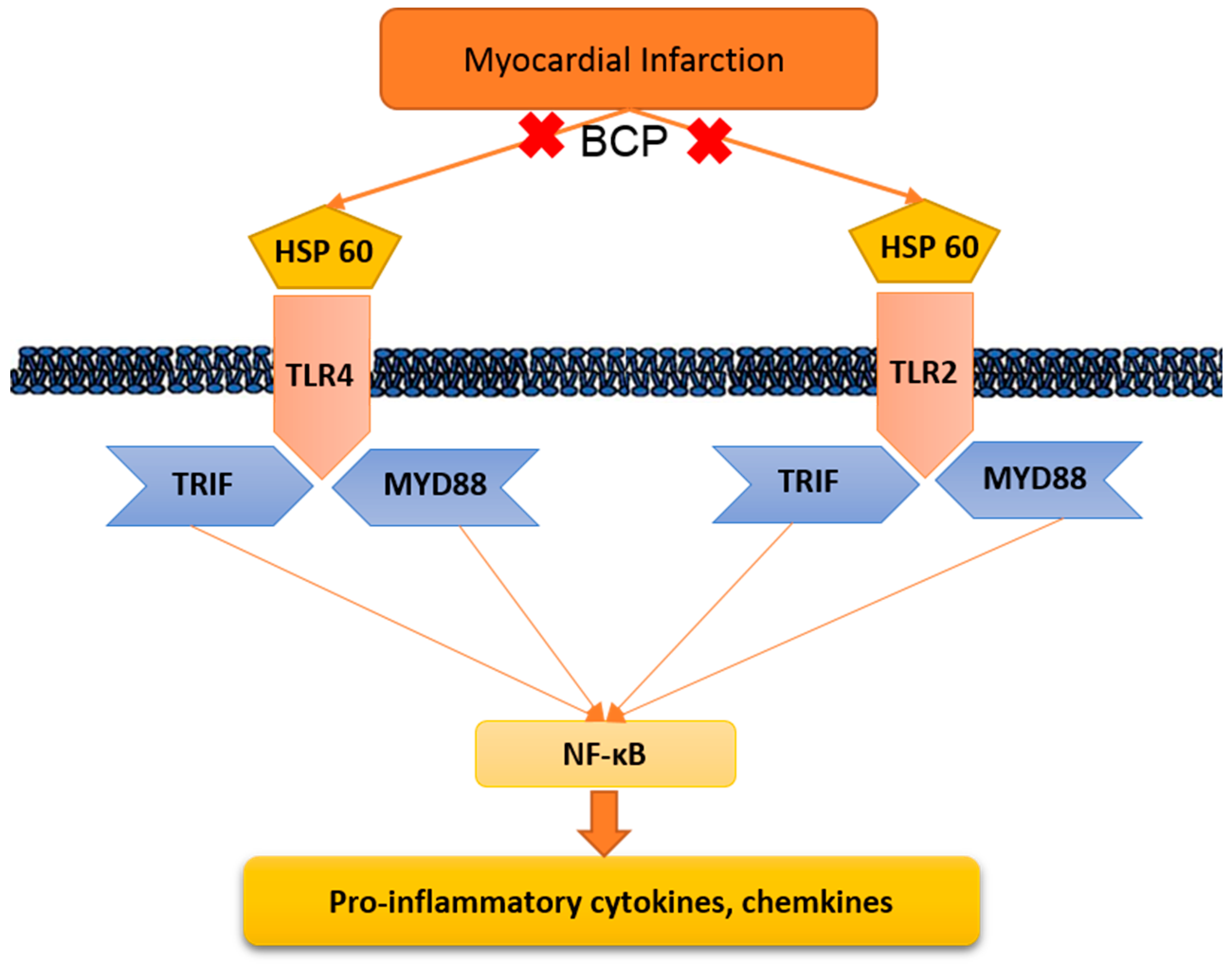
2.6. Effect of β-Caryophyllene Treatment on Toll-Like Receptor Pathway
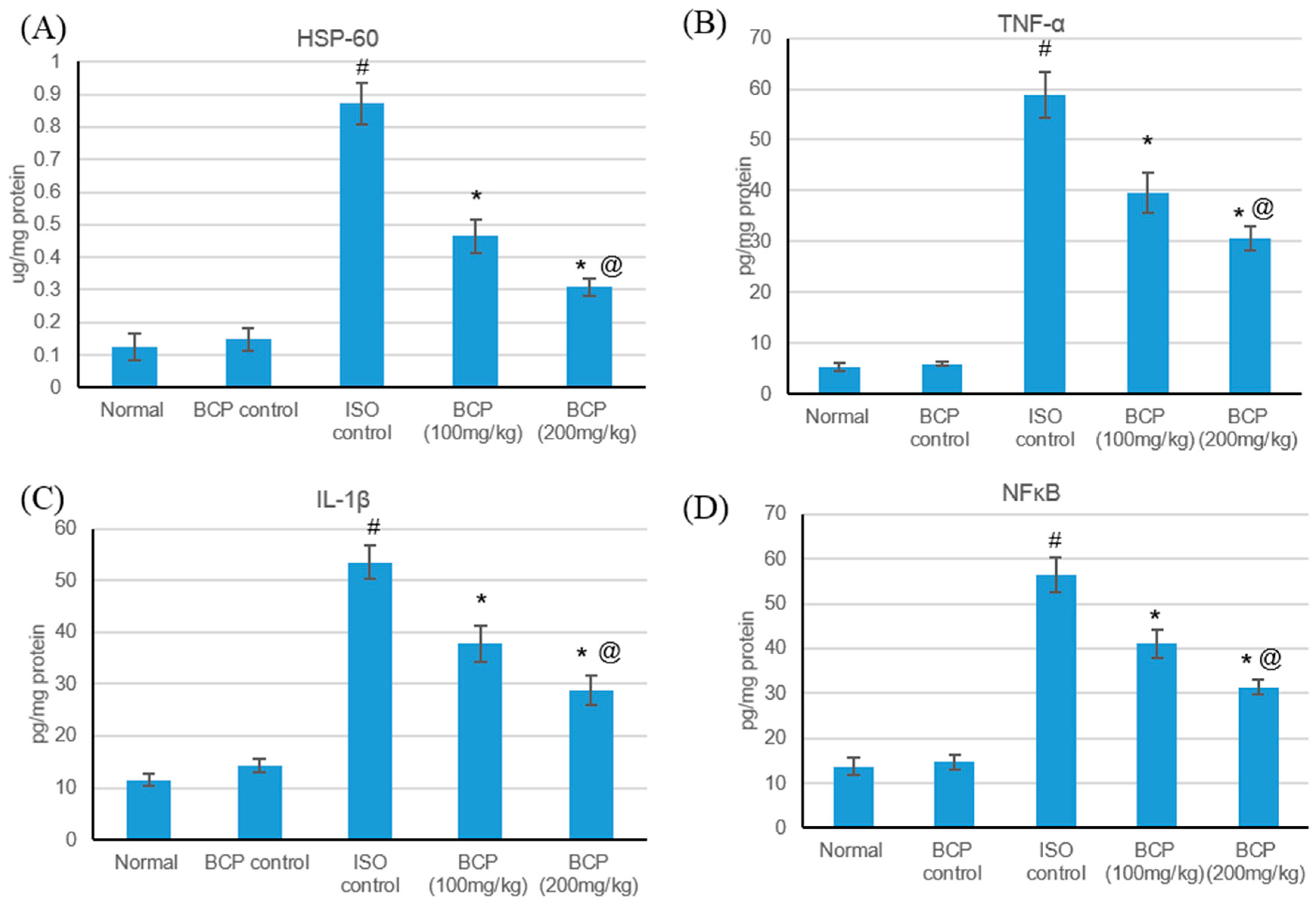
2.7. Effect of β-Caryophyllene Treatment on HSP-60 and Inflammatory Mediators
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Animals
4.3. Experimental Design
4.4. Electrocardiogram (ECG) and Blood Pressure (BP) Recording and Measurement
4.5. Tissue Handling and Biochemical Estimation
4.6. Measurement of Myocardial Infarct Size
4.7. Determination of Cardiac Marker Enzymes and Inflammatory Mediators
4.8. Quantitative Analysis of TLR Pathway
4.9. Detection of the Toll-Like Receptors Pathway Protein Expressions
4.10. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yang, Y.; Lv, J.; Jiang, S.; Ma, Z.; Wang, D.; Hu, W.; Deng, C.; Fan, C.; Di, S.; Sun, Y.; et al. The emerging role of Toll-like receptor 4 in myocardial inflammation. Cell Death Dis. 2016, 7, e2234. [Google Scholar] [CrossRef] [PubMed]
- Frangogiannis, N.G.; Smith, C.W.; Entman, M.L. The inflammatory response in myocardial infarction. Cardiovasc. Res. 2002, 531, 31–47. [Google Scholar] [CrossRef]
- Srivastava, R.A.; Pinkosky, S.L.; Filippov, S.; Hanselman, J.C.; Cramer, C.T.; Newton, R.S. AMP-activated protein kinase: An emerging drug target to regulate imbalances in lipid and carbohydrate metabolism to treat cardio-metabolic diseases. J. Lipid Res. 2012, 5312, 2490–2514. [Google Scholar] [CrossRef]
- Ren, G.; Dewald, O.; Frangogiannis, N.G. Inflammatory mechanisms in myocardial infarction. Curr. Drug Targets Inflamm. Allergy 2003, 23, 242–256. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. The immune system and cardiac repair. Pharm. Res. 2008, 582, 88–111. [Google Scholar] [CrossRef] [PubMed]
- Christia, P.; Frangogiannis, N.G. Targeting inflammatory pathways in myocardial infarction. Eur. J. Clin. Investig. 2013, 439, 986–995. [Google Scholar] [CrossRef]
- Land, W.G. The Role of Damage-Associated Molecular Patterns (DAMPs) in Human Diseases: Part II: DAMPs as diagnostics, prognostics and therapeutics in clinical medicine. Sultan Qaboos Univ. Med. J. 2015, 152, e157–e170. [Google Scholar]
- Nollen, E.A.; Morimoto, R.I. Chaperoning signaling pathways: Molecular chaperones as stress-sensing ‘heat shock’ proteins. J. Cell Sci. 2002, 115, 2809–2816. [Google Scholar]
- Satoh, M.; Shimoda, Y.; Akatsu, T.; Ishikawa, Y.; Minami, Y.; Nakamura, M. Elevated circulating levels of heat shock protein 70 are related to systemic inflammatory reaction through monocyte Toll signal in patients with heart failure after acute myocardial infarction. Eur. J. Heart Fail. 2006, 88, 810–815. [Google Scholar] [CrossRef]
- Ding, H.S.; Yang, J.; Chen, P.; Yang, J.; Bo, S.Q.; Ding, J.W.; Yu, Q.Q. The HMGB1-TLR4 axis contributes to myocardial ischemia/reperfusion injury via regulation of cardiomyocyte apoptosis. Gene 2013, 5271, 389–393. [Google Scholar] [CrossRef]
- Vallejo Jesus, G. Role of Toll-like receptors in cardiovascular diseases. Clin. Sci. 2011, 1211, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Barakat, W.; Safwet, N.; El-Maraghy, N.N.; Zakaria, M.N. Candesartan and glycyrrhizin ameliorate ischemic brain damage through downregulation of the TLR signaling cascade. Eur. J. Pharmacol. 2014, 724, 43–50. [Google Scholar] [CrossRef]
- Hori, M.; Nishida, K. Toll-like receptor signaling: Defensive or offensive for the heart? Circ. Res. 2008, 1022, 137–139. [Google Scholar] [CrossRef] [PubMed]
- Kolodzinska, A.; Czarzasta, K.; Szczepankiewicz, B.; Glowczynska, R.; Fojt, A.; Ilczuk, T.; Budnik, M.; Krasuski, K.; Folta, M.; Cudnoch-Jędrzejewska, A.; et al. Toll-like receptor expression and apoptosis morphological patterns in female rat hearts with takotsubo syndrome induced by isoprenaline. Life Sci. 2018, 199, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Gertsch, J.; Leonti, M.; Raduner, S.; Racz, I.; Chen, J.Z.; Xie, X.Q.; Altmann, K.H.; Karsak, M.; Zimmer, A. β-caryophyllene is a dietary cannabinoid. Proc. Natl. Acad. Sci. USA 2008, 10526, 9099–9104. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, D.; Levy, R.; Carroll, B. Toxicological Evaluation of β-Caryophyllene Oil: Subchronic Toxicity in Rats. Int. J. Toxicol. 2016, 355, 558–567. [Google Scholar] [CrossRef] [PubMed]
- Sharma, C.; Al Kaabi, J.M.; Nurulain, S.M.; Goyal, S.N.; Kamal, M.A.; Ojha, S. Polypharmacological Properties and Therapeutic Potential of β-Caryophyllene: A Dietary Phytocannabinoid of Pharmaceutical Promise. Curr. Pharm. Des. 2016, 2221, 3237–3264. [Google Scholar] [CrossRef]
- Basha, R.H.; Sankaranarayanan, C. β-Caryophyllene, a natural sesquiterpene lactone attenuates hyperglycemia mediated oxidative and inflammatory stress in experimental diabetic rats. Chem. Biol. Interact. 2016, 245, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Kubo, I.; Chaudhuri, S.K.; Kubo, Y.; Sanchez, Y.; Ogura, T.; Saito, T.; Ishikawa, H.; Haraguchi, H. Cytotoxic and antioxidative sesquiterpenoids from Heterotheca inuloides. Planta Med. 1996, 625, 427–430. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Marimuthu, P.; de Heluani, C.S.; Catalan, C.A. Antioxidant and biocidal activities of Carum nigrum (seed) essential oil, oleoresin, and their selected components. J. Agric. Food Chem. 2006, 541, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Dahham, S.S.; Tabana, Y.M.; Iqbal, M.A.; Ahamed, M.B.; Ezzat, M.O.; Majid, A.S. The Anticancer, Antioxidant and Antimicrobial Properties of the Sesquiterpene β-Caryophyllene from the Essential Oil of Aquilaria crassna. Molecules 2015, 207, 11808–11829. [Google Scholar] [CrossRef]
- Leonhardt, V.; Leal-Cardoso, J.H.; Lahlou, S.; Albuquerque, A.A.; Porto, R.S.; Celedônio, N.R.; Oliveira, A.C.; Pereira, R.F.; Silva, L.P.; Garcia-Teófilo, T.M.; et al. Antispasmodic effects of essential oil of Pterodon polygalaeflorus and its main constituent β-caryophyllene on rat isolated ileum. Fundam. Clin. Pharmacol. 2010, 246, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Baldissera, M.D.; Souza, C.F.; Grando, T.H.; Doleski, P.H.; Boligon, A.A.; Stefani, L.M.; Monteiro, S.G. Hypolipidemic effect of β-caryophyllene to treat hyperlipidemic rats. Naunyn Schmiedeberg’s Arch. Pharmacol. 2017, 390, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Lou, J.; Cao, G.; Li, R.; Liu, J.; Dong, Z.; Xu, L. β-Caryophyllene Attenuates Focal Cerebral Ischemia-Reperfusion Injury by Nrf2/HO-1 Pathway in Rats. Neurochem. Res. 2016, 416, 1291–1304. [Google Scholar] [CrossRef]
- Li, Y.; Si, R.; Feng, Y.; Chen, H.H.; Zou, L.; Wang, E.; Zhang, M.; Warren, H.S.; Sosnovik, D.E.; Chao, W. Myocardial ischemia activates an injurious innate immune signaling via cardiac heat shock protein 60 and Toll-like receptor 4. J. Biol. Chem. 2011, 28636, 31308–31319. [Google Scholar] [CrossRef]
- Soraya, H.; Clanachan, A.S.; Rameshrad, M.; Maleki-Dizaji, N.; Ghazi-Khansari, M.; Garjani, A. Chronic treatment with metformin suppresses toll-like receptor 4 signaling and attenuates left ventricular dysfunction following myocardial infarction. Eur. J. Pharmacol. 2014, 737, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Soraya, H.; Farajnia, S.; Khani, S.; Rameshrad, M.; Khorrami, A.; Banani, A.; Maleki-Dizaji, N.; Garjani, A. Short-term treatment with metformin suppresses toll like receptors (TLRs) activity in isoproterenol-induced myocardial infarction in rat: Are AMPK and TLRs connected? Int. Immunopharmacol. 2012, 144, 785–791. [Google Scholar] [CrossRef]
- Shukla, S.K.; Sharma, S.B.; Singh, U.R. β-Adrenoreceptor Agonist Isoproterenol Alters Oxidative Status, Inflammatory Signaling, Injury Markers and Apoptotic Cell Death in Myocardium of Rats. Indian J. Clin. Biochem. 2015, 301, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Haleagrahara, N.; Varkkey, J.; Chakravarthi, S. Cardioprotective effects of glycyrrhizic acid against isoproterenol-induced myocardial ischemia in rats. Int. J. Mol. Sci. 2011, 1210, 7100–7113. [Google Scholar] [CrossRef] [PubMed]
- Arslan, F.; Smeets, M.; O’Neill, L.A.; Keogh, B.; McGuirk, P.; Timmers, L.; Hoefer, I.E.; Doevendans, P.A.; Pasterkamp, G.; De Kleijn, D. Myocardial ischemia/reperfusion injury is mediated by leukocytic toll-like receptor-2 and reduced by systemic administration of a novel anti-toll-like receptor-2 antibody. Circulation 2010, 1211, 80–90. [Google Scholar] [CrossRef]
- Chong, A.J.; Shimamoto, A.; Hampton, C.R.; Takayama, H.; Spring, D.J.; Rothnie, C.L.; Yada, M.; Pohlman, T.H.; Verrier, E.D. Toll-like receptor 4 mediates ischemia/reperfusion injury of the heart. J. Thorac. Cardiovasc. Surg. 2004, 1282, 170–179. [Google Scholar] [CrossRef]
- Van Hout, G.P.; Arslan, F.; Pasterkamp, G.; Hoefer, I.E. Targeting danger-associated molecular patterns after myocardial infarction. Expert Opin. Ther. Targets 2016, 202, 223–239. [Google Scholar] [CrossRef] [PubMed]
- Ren, M.; Li, R.; Luo, M.; Chen, N.; Deng, X.; Yan, K.; Zeng, M.; Wu, J. Endothelial cells but not platelets are the major source of Toll-like receptor 4 in the arterial thrombosis and tissue factor expression in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 3077, R901–R907. [Google Scholar] [CrossRef]
- Chao, W. Toll-like receptor signaling: A critical modulator of cell survival and ischemic injury in the heart. Am. J. Physiol. Heart Circ. Physiol. 2009, 2961, H1–H12. [Google Scholar] [CrossRef]
- Cho, H.I.; Hong, J.M.; Choi, J.W.; Choi, H.S.; Kwak, J.H.; Lee, D.U.; Lee, S.K.; Lee, S.M. β-Caryophyllene alleviates d-galactosamine and lipopolysaccharide-induced hepatic injury through suppression of the TLR4 and RAGE signaling pathways. Eur. J. Pharmacol. 2015, 764, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, Y.; Cao, Z.Y.; Wang, M.M.; Liu, X.M.; Gao, T.; Hu, Q.K.; Yuan, W.J.; Lin, L. Up-regulated TLR4 in cardiomyocytes exacerbates heart failure after long-term myocardial infarction. J. Cell. Mol. Med. 2015, 1912, 2728–2740. [Google Scholar] [CrossRef]
- Tian, J.; Guo, X.; Liu, X.M.; Liu, L.; Weng, Q.F.; Dong, S.J.; Knowlton, A.A.; Yuan, W.J.; Lin, L. Extracellular HSP60 induces inflammation through activating and up-regulating TLRs in cardiomyocytes. Cardiovasc. Res. 2013, 983, 391–401. [Google Scholar] [CrossRef]
- Kim, S.C.; Stice, J.P.; Chen, L.; Jung, J.S.; Gupta, S.; Wang, Y.; Baumgarten, G.; Trial, J.; Knowlton, A.A. Extracellular heat shock protein 60, cardiac myocytes, and apoptosis. Circ. Res. 2009, 10512, 1186–1195. [Google Scholar] [CrossRef]
- Guo, K.; Mou, X.; Huang, J.; Xiong, N.; Li, H. Trans-caryophyllene suppresses hypoxia-induced neuroinflammatory responses by inhibiting NF-kappaB activation in microglia. J. Mol. Neurosci. 2014, 541, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Basha, R.H.; Sankaranarayanan, C. β-Caryophyllene, a natural sesquiterpene, modulates carbohydrate metabolism in streptozotocin-induced diabetic rats. Acta Histochem. 2014, 1168, 1469–1479. [Google Scholar] [CrossRef] [PubMed]
- Holmbom, B.; Naslund, U.; Eriksson, A.; Virtanen, I.; Thornell, L.E. Comparison of triphenyltetrazolium chloride (TTC) staining versus detection of fibronectin in experimental myocardial infarction. Histochemistry 1993, 994, 265–275. [Google Scholar] [CrossRef]
- Li, X.; Yang, J.; Yang, J.; Dong, W.; Li, S.; Wu, H.; Li, L. RP105 protects against myocardial ischemia-reperfusion injury via suppressing TLR4 signaling pathways in rat model. Exp. Mol. Pathol. 2016, 1002, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yang, J.; Ding, J.W.; Chen, L.H.; Wang, Y.L.; Li, S.; Wu, H. Sequential expression of TLR4 and its effects on the myocardium of rats with myocardial ischemia-reperfusion injury. Inflammation 2008, 315, 304–312. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of β-Caryophyllene is available from the authors. |


| Primer Sequence (5′ to 3′) | |
|---|---|
| TLR4 | F AGTGTATCGGTGGTCAGTGTGCT R AAACTCCAGCCACACATTCC |
| TLR 2 | F AAACTGTGTTCGTGCTTTCTGA R CTTTCTTCTCAATGGGTTCCAG |
| MyD88 | F GAGATCCGCGAGTTTGAGAC R CTGTTTCTGCTGGTTGCGTA |
| TRIF | F TCAGCCATTCTCCGTCCTCTTC R GGTCAGCAGAAGGATAAGGAA |
| β-Actin | F CACGATGGAGGGGCCGGACTCATC R TAAAGACCTCTATGCCAACACAGT |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Younis, N.S.; Mohamed, M.E. β-Caryophyllene as a Potential Protective Agent Against Myocardial Injury: The Role of Toll-Like Receptors. Molecules 2019, 24, 1929. https://doi.org/10.3390/molecules24101929
Younis NS, Mohamed ME. β-Caryophyllene as a Potential Protective Agent Against Myocardial Injury: The Role of Toll-Like Receptors. Molecules. 2019; 24(10):1929. https://doi.org/10.3390/molecules24101929
Chicago/Turabian StyleYounis, Nancy S., and Maged E. Mohamed. 2019. "β-Caryophyllene as a Potential Protective Agent Against Myocardial Injury: The Role of Toll-Like Receptors" Molecules 24, no. 10: 1929. https://doi.org/10.3390/molecules24101929





