Solid-Phase Synthesis of Phosphorothioate/Phosphonothioate and Phosphoramidate/Phosphonamidate Oligonucleotides
Abstract
:1. Introduction
2. Results and Discussion
2.1. Study on Model Dimers
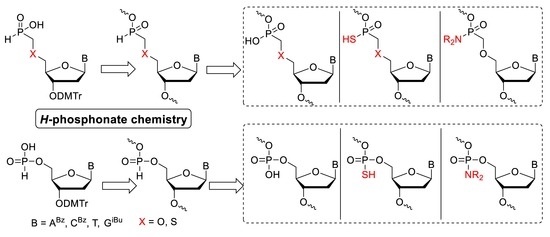
2.1.1. H-Phosphonate Coupling
2.1.2. Oxidation/Amidation/Sulfurization of H-Phosphinate Internucleotide Linkage
2.1.3. Stability of Modified Internucleotide Linkages
2.2. Synthetic Protocols for Oligonucleotide Synthesis
2.2.1. Synthetic Protocol A
2.2.2. Synthetic Protocol B
2.2.3. Synthetic Protocol C
2.2.4. Synthesis of Hetero-Oligonucleotides
3. Materials and Methods
3.1 General Information
3.2. H-Phosphonate Chemistry
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Šípová, H.; Špringer, T.; Rejman, D.; Šimák, O.; Petrová, M.; Novák, P.; Rosenbergová, Š.; Páv, O.; Liboska, R.; Barvík, I.; et al. 5′-O-Methylphosphonate nucleic acids—new modified DNAs that increase the Escherichia coli RNase H cleavage rate of hybrid duplexes. Nucleic Acids Res. 2014, 42, 5378–5389. [Google Scholar] [CrossRef]
- Kostov, O.; Páv, O.; Buděšínský, M.; Liboska, R.; Šimák, O.; Petrová, M.; Novák, P.; Rosenberg, I. 4-Toluenesulfonyloxymethyl-(H)-phosphinate: A reagent for the introduction of O- and S-Methyl-(H)-phosphinate Moieties. Org. Lett. 2016, 18, 2704–2707. [Google Scholar]
- Kostov, O.; Páv, O.; Rosenberg, I. Nucleoside-O-methyl-(H)-phosphinates: Novel monomers for the synthesis of methylphosphonate oligonucleotides using H-phosphonate chemistry. Curr. Protoc. Nucleic Acid Chem. 2017, 70, 4.76.1–4.76.22. [Google Scholar]
- Garegg, P.J.; Lindh, I.; Regberg, T.; Stawinski, J.; Strömberg, R.; Henrichson, C. Nucleoside H-phosphonates. III. Chemical synthesis of oligodeoxyribonucleotides by the hydrogenphosphonate approach. Tetrahedron Lett. 1986, 27, 4051–4054. [Google Scholar] [CrossRef]
- Froehler, B.C.; Ng, P.G.; Matteucci, M.D. Synthesis of DNA via deoxynudeoside H-phosphonate intermediates. Nucleic Acids Res. 1986, 14, 5399–5407. [Google Scholar] [CrossRef]
- Liu, C.; Cozens, C.; Jaziri, F.; Rozenski, J.; Maréchal, A.; Dumbre, S.; Pezo, V.; Marlière, P.; Pinheiro, V.B.; Groaz, E.; et al. Phosphonomethyl oligonucleotides as backbone-modified artificial genetic polymers. J. Am. Chem. Soc. 2018, 140, 6690–6699. [Google Scholar] [CrossRef]
- Garegg, P.J.; Regberg, T.; Stawinski, J.; Stromberg, R. Nucleoside phosphonates: Part 7. Studies on the oxidation of nucleoside phosphonate esters. J. Chem. Soc. Perkin Trans. 1 1987, 1269–1273. [Google Scholar] [CrossRef]
- Cullis, P.M.; Lee, M. The mechanism of iodine-water oxidation of H-phosphonate diesters. J. Chem. Soc. Chem. Commun. 1992, 1207–1208. [Google Scholar] [CrossRef]
- Wallin, R.; Kalek, M.; Bartoszewicz, A.; Thelin, M.; Stawinski, J. On the sulfurization of H-phosphonate diesters and phosphite triesters using elemental sulfur. PhosphorusSulfurSilicon Relat. Elem. 2009, 184, 908–916. [Google Scholar] [CrossRef]
- Mohe, N.U.; Padiya, K.J.; Salunkhe, M.M. An efficient oxidizing reagent for the synthesis of mixed backbone oligonucleotides via the H-phosphonate approach. Bioorg. Med. Chem. 2003, 11, 1419–1431. [Google Scholar] [CrossRef]
- Nilsson, J.; Stawinski, J. Oxidative coupling of H-phosphonate and H-phosphonothioate diesters. Iodine as a reagent and a catalyst. Collect. Symp. Ser. 2002, 5, 87–92. [Google Scholar]
- Nilsson, J.; Stawinski, J. Controlling stereochemistry during oxidative coupling. Preparation of Rp or Sp phosphoramidates from one P-chiral precursor. Chem. Commun. 2004, 2566–2567. [Google Scholar] [CrossRef]
- Stawinski, J.; Stromberg, R. Di- and oligonucleotide synthesis using H-phosphonate chemistry. Methods Mol. Biol. (CliftonN.J.) 2005, 288, 81–100. [Google Scholar] [CrossRef]
- Maier, M.A.; Guzaev, A.P.; Manoharan, M. Synthesis of chimeric oligonucleotides containing phosphodiester, phosphorothioate, and phosphoramidate linkages. Org. Lett. 2000, 2, 1819–1822. [Google Scholar] [CrossRef]
- Bartoszewicz, A.; Kalek, M.; Stawinski, J. The Case for the intermediacy of monomeric metaphosphate analogues during oxidation of H-phosphonothioate, H-phosphonodithioate, and H-phosphonoselenoate monoesters: Mechanistic and synthetic studies. J. Org. Chem. 2008, 73, 5029–5038. [Google Scholar] [CrossRef]
- Le Corre, S.S.; Berchel, M.; Couthon-Gourvès, H.; Haelters, J.-P.; Jaffrès, P.-A. Atherton–Todd reaction: Mechanism, scope and applications. Beilstein J. Org. Chem. 2014, 10, 1166–1196. [Google Scholar] [CrossRef]
- Letsinger, R.L.; Singman, C.N.; Histand, G.; Salunkhe, M. Cationic oligonucleotides. J. Am. Chem. Soc. 1988, 110, 4470–4471. [Google Scholar] [CrossRef]
- Jung, P.M.; Histand, G.; Letsinger, R.L. Hybridization of alternating cationic/anionic oligonucleotides to RNA segments. Nucleosides Nucleotides 1994, 13, 1597–1605. [Google Scholar] [CrossRef]
- Paul, S.; Roy, S.; Monfregola, L.; Shang, S.; Shoemaker, R.; Caruthers, M.H. Oxidative substitution of boranephosphonate diesters as a route to post-synthetically modified DNA. J. Am. Chem. Soc. 2015, 137, 3253–3264. [Google Scholar] [CrossRef]
- Brill, W.K.D. Thioalkylation of nucleoside-H-phosphonates and its application to solid phase synthesis of oligonucleotides. Tetrahedron Lett. 1995, 36, 703–706. [Google Scholar] [CrossRef]
- Reese, C.B.; Yan, H. Solution phase synthesis of ISIS 2922 (Vitravene) by the modified H-phosphonate approach. J. Chem. Soc. Perkin Trans. 1 2002, 2619–2633. [Google Scholar] [CrossRef]
- Alefelder, S.; Patel, B.K.; Eckstein, F. Incorporation of terminal phosphorothioates into oligonucleotides. Nucleic Acids Res. 1998, 26, 4983–4988. [Google Scholar] [CrossRef] [Green Version]
- Andrus, A.; Efcavitch, J.W.; McBride, L.J.; Giusti, B. Novel activating and capping reagents for improved hydrogen-phosphonate DNA synthesis. Tetrahedron Lett. 1988, 29, 861–864. [Google Scholar] [CrossRef]
- Ugi, I.; Jacob, P.; Landgraf, B.; Ruppf, C.; Lemmen, P.; Verfürth, U. Phosphite oxidation and the preparation of five-membered cyclic phosphorylating reagents via the phosphites. Nucleosides Nucleotides 1988, 7, 605–608. [Google Scholar] [CrossRef]
- Reynolds, M.A.; Hogrefe, R.I.; Jaeger, J.A.; Schwartz, D.A.; Riley, T.A.; Marvin, W.B.; Daily, W.J.; Vaghefi, M.M.; Beck, T.A.; Knowles, S.K.; et al. Synthesis and thermodynamics of oligonucleotides containing chirally pure R P methylphosphonate linkages. Nucleic Acids Res. 1996, 24, 4584–4591. [Google Scholar] [CrossRef]
- Dellinger, D.J.; Sheehan, D.M.; Christensen, N.K.; Lindberg, J.G.; Caruthers, M.H. Solid-phase chemical synthesis of phosphonoacetate and thiophosphonoacetate oligodeoxynucleotides. J. Am. Chem. Soc. 2003, 125, 940–950. [Google Scholar] [CrossRef]
- Glen Research Unicap Phosphoramidite, An Alternative to Acetic Anhydride Capping. Available online: http://www.glenresearch.com/GlenReports/GR17-13.html (accessed on 9 October 2018).
- Vlaho, D.; Damha, M.J. Synthesis of chimeric oligonucleotides having modified internucleotide linkages via an automated H-phosphonate/phosphoramidite approach. Curr. Protoc. Nucleic Acid Chem. 2018, 73, e53. [Google Scholar] [CrossRef]
Sample Availability: Not available. |






| Code | Mixture | Oxidation Time (min) | |
|---|---|---|---|
| H-Phosphonate | H-Phosphinate | ||
| Ox-A | CCl4/MeOH/MeIm (7:2:1) | 15 | 30 |
| Ox-B | CCl4/MeOH/MeIm/Et3N (7:2:0.5:0.5) | 10 | 20 |
| Ox-C | 0.1 M HOCH2CH2CN in CCl4/DCM/MeIm (6:2:2) | 15 | 30 |
| Ox-D | 0.5 M sulfur in pyridine | 20 | 20 |
| Ox-E | 0.2 M CSS in ACN/BSTFA (29:1) | 30 | 30 |
| Ox-F | CCl4/amine */DCM (3:2:5) | 180 | 180 |
| Internucleotide Linkages | Code | Sequence 5′→3′ | Synthetic Protocol (HPLC Purity) | MALDI TOF Calcd Found | |
|---|---|---|---|---|---|
 | dT101 | d(T T T T T T T T T T) | A (70%), B (74%), C (64%) | 3020.5516 | 3020.5733 |
| dT102 | d(T T T T T T T T T T) | A (59%), B (77%) | 3164.3460 | 3164.3219 | |
 | dT103 | d(T T T T T T T T T T) | B (36%), C (88%) | 3187.3049 | 3187.1703 |
| dT104 | d(T T T T T T T T T T) | B (42%), C (70%) | 3229.3859 | 3229.1063 | |
 | dT105 | d(T T T T T T T T T T) | C (30%) | 3070.2479 | 3070.7329 |
| dT106 | d(T T T T T T T T T T) | C (75%) | 2979.9839 | 2980.3970 | |
| dT107 | d(T T T T T T T T T T) | C (77%) | 3028.1696 | 3028.4048 | |
| Internucleotide Linkages | Code | Sequence 5′→3′ | Synthetic Protocol (HPLC Purity) | MALDI TOF | |
|---|---|---|---|---|---|
| Calcd | Found | ||||
 | ON-1 | d(G C A T A T C A C) | B (86%) | 2729.4322 | 2729.5331 |
| ON-2 | d(G C A T A T C A C) | B (84%) | 2761.3865 | 2761.1589 | |
| ON-3 | d(G C A T A T C A C) | B (81%) | 2809.3180 | 2809.3292 | |
 | ON-4 | d(G C A T A T C A C) | B (21%) | 2771.4792 | 2771.3983 |
| ON-5 | d(G C A T A T C A C) | B (56%) | 2899.2964 | 2899.2376 | |
| ON-6 | d(G C A T A T C A C) | B (51%) | 2819.4106 | 2819.2887 | |
| ON-7 | d(G C A T A T C A C) | B (43%) | 2851.3650 | 2851.3559 | |
 | ON-8 | d(G C A T A T C A C) | A (76%) | 2723.5477 | 2723.7076 |
| ON-9 | d(G C A T A T C A C) | A (84%) | 2851.3650 | 2851.2513 | |
| ON-10 | d(G C A T A T C A C) | B (86%) | 2771.4791 | 2771.3636 | |
| ON-11 | d(G C A T A T C A C) | B (86%) | 2803.4335 | 2803.5659 | |
 | ON-12 | d(G C A T A T C A C) | C (59%) | 2930.7213 | 2930.6797 |
| ON-13 | d(G C A T A T C A C) | C (66%) | 2975.8631 | 2975.7741 | |
| ON-14 | d(G C A T A T C A C) | C (78%) | 2888.6743 | 2888.8365 | |
| ON-15 | d(G C A T A T C A C) | C (79%) | 2933.8162 | 2933.7591 | |
| Procedure | Reagents | Volume/Time |
|---|---|---|
| H-Phosphonate/H-Phosphinate Condensation Step | ||
| Deblocking | 3% DCA in DCM | Flow through the column 6 mL/2 min |
| Condensation | 0.1M monomer in ACN/Py (1:1) | 120 µL/10min |
| 0.3M DMOCP in ACN/Py (95:5) | ||
| Oxidation | Ox-A, Ox-B, Ox-D | 220 µL/30 min |
| Methyl-ester cleavage * | PhSH/Et3N/DMF(1:1.4:2) | 300 µL/4 h |
| Cleavage from solid support | Gaseous ammonia at 0.7 MPa | Gas chamber/16 h |
| Procedure | Reagents | Volume/Time |
|---|---|---|
| H-Phosphonate/H-Phosphinate Condensation Step | ||
| Deblocking | 3% DCA in DCM | Flow through the column 6 mL/2 min |
| DCA washout | 5% pyridine in methanol | |
| Condensation | 0.1M monomer in ACN/Py (1:1) | 120 µL/10 min |
| 0.3M DMOCP in ACN/Py (95:5) | ||
| Oxidation or Sulfurization | Ox-C or Ox-E | 220 µL/30 min |
| Capping | Ac2O/Py/THF (1:1:1:17) | 2 × 150 µL/3 min |
| MeIm/THF (1:9) | ||
| Phosphoramidite Condensation Step | ||
| Deblocking | 3% DCA in DCM | Flow through the column 6 mL/2 min |
| DCA washout | 5% pyridine in methanol | |
| Condensation | 0.1M monomer in ACN | 120 µL/5 min |
| 0.25M ETT in ACN | ||
| Oxidation or Sulfurization | CSO, tBuOOH or DDTT | 220 µL/3.5 min |
| End of Synthetic Cycle | ||
| S-cyanoethyl-ester cleavage * | 1M DBU in ACN | 220 µL/3 min |
| Cleavage from solid support | Ammonia gas at 0.7 MPa | Gas chamber/16 h |
| Procedure | Reagents | Volume/Time |
|---|---|---|
| H-Phosphonate/H-Phosphinate Condensation Steps | ||
| Deblocking | 3% DCA in DCM | Flow through the column 6 mL/2 min |
| Condensation | 0.1 M monomer in ACN/Py (1:1) | 120 µL/10 min |
| 0.3 M DMOCP in ACN/Py (95:5) | ||
| Phosphoramidite Condensation Step | ||
| Deblocking | 3% DCA in DCM | Flow through the column 6 mL/2 min |
| DCA washout | 5% pyridine in methanol | |
| Condensation | 0.1 M monomer in ACN | 120 µL/5 min |
| 0.25 M ETT in ACN | ||
| Oxidation | CSO | 220 µL/3.5 min |
| End of Synthetic Cycle | ||
| Amidation of P–H bond | Ox-F | 220 µL/180 min |
| Cleavage from solid support | Ammonia gas at 0.7 MPa | Gas chamber/16 h |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostov, O.; Liboska, R.; Páv, O.; Novák, P.; Rosenberg, I. Solid-Phase Synthesis of Phosphorothioate/Phosphonothioate and Phosphoramidate/Phosphonamidate Oligonucleotides. Molecules 2019, 24, 1872. https://doi.org/10.3390/molecules24101872
Kostov O, Liboska R, Páv O, Novák P, Rosenberg I. Solid-Phase Synthesis of Phosphorothioate/Phosphonothioate and Phosphoramidate/Phosphonamidate Oligonucleotides. Molecules. 2019; 24(10):1872. https://doi.org/10.3390/molecules24101872
Chicago/Turabian StyleKostov, Ondřej, Radek Liboska, Ondřej Páv, Pavel Novák, and Ivan Rosenberg. 2019. "Solid-Phase Synthesis of Phosphorothioate/Phosphonothioate and Phosphoramidate/Phosphonamidate Oligonucleotides" Molecules 24, no. 10: 1872. https://doi.org/10.3390/molecules24101872