Chiral Recognition of L- and D- Amino Acid by Porphyrin Supramolecular Aggregates
Abstract
:1. Introduction
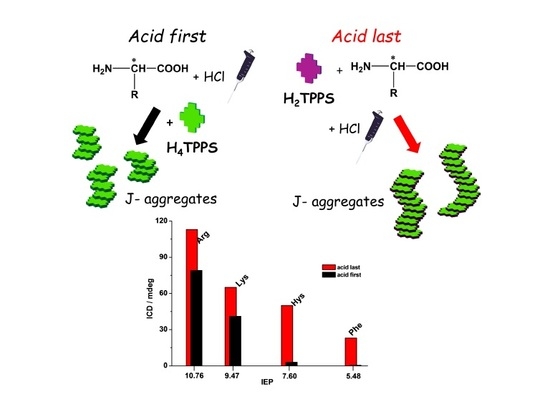
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Whitesides, G.M.; Grzybowski, B. Self-assembly at all scales. Science 2002, 295, 2418–2421. [Google Scholar] [CrossRef] [PubMed]
- Lehn, J.M. Supramolecular Chemistry; VCH: Weinheim, Germany, 1995; p. 263. [Google Scholar]
- Barron, L.D. Chirality and life. Space Sci. Rev. 2008, 135, 187–201. [Google Scholar] [CrossRef]
- Brückner, H.; Becker, D.; Lüpke, M. Chirality of amino acids of microorganisms used in food biotechnology. Chirality 1993, 5, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Corradini, R.; Sforza, S.; Tedeschi, T.; Marchelli, R. Chirality as a tool in nucleic acid recognition: Principles and relevance in biotechnology and in medicinal chemistry. Chirality 2007, 19, 269–294. [Google Scholar] [CrossRef]
- Liu, C.; Yang, D.; Jin, Q.; Zhang, L.; Liu, M. Chiroptical logic circuit based on self-assembled soft materials containing amphiphilic spiropyran. Adv. Mater. 2016, 28, 1644–1649. [Google Scholar] [CrossRef]
- Green, D.W.; Lee, J.M.; Kim, E.J.; Lee, D.J.; Jung, H.S. Chiral biomaterials: From molecular design to regenerative medicine. Adv. Mater. Interfaces 2016, 3, 1500411. [Google Scholar] [CrossRef]
- Caricato, M.; Delforge, A.; Bonifazi, D.; Dondi, D.; Mazzanti, A.; Pasini, D. Chiral nanostructuring of multivalent macrocycles in solution and on surfaces. Org. Biomol. Chem. 2015, 13, 3593–3601. [Google Scholar] [CrossRef] [Green Version]
- Caricato, M.; Jordana, N.; Kinkini Roy, L.; Dondi, D.; Gattuso, G.; Shimizu, L.S.; Vander Griend, D.A.; Pasini, D. A Chiroptical probe for sensing metal ions in water. Eur. J. Org. Chem. 2013, 27, 6078–6083. [Google Scholar] [CrossRef]
- Agnes, M.; Nitti, A.; Vander Griend, D.A.; Dondi, D.; Merlia, D.; Pasini, D. A chiroptical molecular sensor for ferrocene. Chem. Commun. 2016, 52, 11492–11495. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Zhang, L.; Wang, T. Supramolecular chirality in self-assembled systems. Chem. Rev. 2015, 115, 7304–7397. [Google Scholar] [CrossRef]
- Randazzo, R.; Mammana, A.; D’Urso, A.; Lauceri, R.; Purrello, R. Reversible “chiral memory” in ruthenium tris(phenanthroline)–anionic porphyrin complexes. Angew. Chem. Int. Ed. 2008, 47, 9879–9882. [Google Scholar] [CrossRef] [PubMed]
- Rananaware, A.; La, D.D.; Al Kobaisi, M.; Bhosale, R.S.; Bhosale, S.V. Controlled chiral supramolecular assemblies of water soluble achiral porphyrins induced by chiral counterions. Chem. Commun. 2016, 52, 10253–10256. [Google Scholar]
- Ribò, J.M.; Crusats, J.; Farrera, J.A.; Valero, M.L. Aggregation in water solutions of tetrasodium diprotonated meso-tetrakis(4-sulfonatophenyl)porphyrin. J. Chem. Soc. Chem. Commun. 1994, 6, 681–682. [Google Scholar]
- Akins, D.L.; Zhu, H.R.; Guo, C. Aggregation of tetraaryl-substituted porphyrins in homogeneous solution. J. Phys. Chem. 1996, 100, 5420–5425. [Google Scholar] [CrossRef]
- Ohno, O.; Kaizu, Y.; Kobayashi, H. J-aggregate formation of a water-soluble porphyrin in acidic aqueous media. J. Chem. Phys. 1993, 99, 4128–4139. [Google Scholar] [CrossRef]
- Koti, A.S.R.; Taneja, J.; Periasamy, N. Control of coherence length and aggregate size in the J-aggregate of porphyrin. Chem. Phys. Lett. 2003, 375, 171–176. [Google Scholar] [CrossRef]
- Micali, N.; Mallamace, F.; Romeo, A.; Purrello, R.; Monsù Scolaro, L. Fractal structures in homo- and heteroaggregated water soluble porphyrins. J. Phys. Chem. B 2000, 104, 5897–5904. [Google Scholar] [CrossRef]
- Kano, H.; Saito, T.; Kobayashi, T. Dynamic intensity borrowing in porphyrin J-aggregates revealed by sub-5-fs spectroscopy. J. Phys. Chem. B 2001, 105, 413–419. [Google Scholar] [CrossRef]
- Schwab, A.D.; Smith, D.E.; Rich, C.S.; Young, E.R.; Smith, W.F.; de Paula, J.C. Porphyrin nanorods. J. Phys. Chem. B 2003, 107, 11339–11345. [Google Scholar] [CrossRef]
- Romeo, A.; Castriciano, M.A.; Occhiuto, I.; Zagami, R.; Pasternack, R.F.; Monsù Scolaro, L. Kinetic control of chirality in porphyrin J-aggregates. J. Am. Chem. Soc. 2014, 136, 40–43. [Google Scholar] [CrossRef] [PubMed]
- Short, J.M.; Berriman, J.A.; Kubel, C.; El-Hachemi, Z.; Naubron, J.-V.; Balaban, T.S. Electron cryo-microscopy of TPPS4·2HCl tubes reveals a helical organisation explaining the origin of their chirality. ChemPhysChem 2013, 14, 3209–3214. [Google Scholar] [CrossRef] [PubMed]
- El-Hachemi, Z.; Escudero, C.; Arteaga, O.; Canillas, A.; Crusats, J.; Mancini, G.; Purrello, R.; Sorrenti, A.; D’Urso, A.; Ribo, J.M. Chiral sign selection on the J-aggregates of diprotonated tetrakis-(4-sulfonatophenyl)porphyrin by traces of unidentified chiral contaminants present in the ultra-pure water used as solvent. Chirality 2009, 21, 408–412. [Google Scholar] [CrossRef] [PubMed]
- Purrello, R.; Bellacchio, E.; Gurrieri, S.; Lauceri, R.; Raudino, A.; Monsù Scolaro, L.; Santoro, A.M. pH modulation of porphyrins self-assembly onto polylysine. J. Phys. Chem. B 1998, 102, 8852–8857. [Google Scholar] [CrossRef]
- Gaeta, M.; Raciti, D.; Randazzo, R.; Gangemi, C.M.A.; Raudino, A.; D’urso, A.; Fragalà, M.E.; Purrello, R. Chirality enhancement of porphyrin supramolecular assembly driven by template pre-organization effect. Angew. Chem. Int. Ed. 2018, 57, 10656–10660. [Google Scholar] [CrossRef]
- Koti, A.S.R.; Periasamy, N. Self-assembly of template-directed J-aggregates of porphyrin. Chem. Mater. 2003, 15, 369–371. [Google Scholar] [CrossRef]
- Onouchi, H.; Miyagawa, T.; Morino, K.; Yashima, E. Assisted formation of chiral porphyrin homoaggregates by an induced helical poly(phenylacetylene) template and their chiral memory. Angew. Chem. Int. Ed. 2006, 45, 2381–2384. [Google Scholar] [CrossRef]
- Randazzo, R.; Lauceri, R.; Mammana, A.; D’urso, A.; Purrello, R. Interactions of Λ and Δ enantiomers of ruthenium(II) cationic complexes with achiral anionic porphyrins. Chirality 2009, 21, 92–96. [Google Scholar] [CrossRef]
- Castriciano, M.A.; Romeo, A.; Zagami, R.; Micali, N.; Monsù Scolaro, L. Kinetic effects of tartaric acid on the growth of chiral J-aggregates of tetrakis(4-sulfonatophenyl)porphyrin. Chem. Commun. 2012, 48, 4872–4874. [Google Scholar] [CrossRef]
- Dorđević, L.; Arcudi, F.; D’Urso, A.; Cacioppo, M.; Micali, N.; Bürgi, T.; Purrello, R.; Prato, M. Design principles of chiral carbon nanodots help convey chirality from molecular to nanoscale level. Nat. Commun. 2018, 9, 3442. [Google Scholar] [CrossRef]
- D’Urso, A.; Randazzo, R.; Lo Faro, L.; Purrello, R. Vortexes and nanoscale chirality. Angew. Chem. Int. Ed. 2010, 49, 108–112. [Google Scholar] [CrossRef]
- Mineo, P.; Villari, V.; Scamporrino, E.; Micali, N. Supramolecular chirality induced by a weak thermal force. Soft Matter 2014, 10, 44–47. [Google Scholar] [CrossRef] [PubMed]
- Ribo, J.M.; Crusats, J.; Sague, F.; Claret, J.; Rubires, R. Chiral sign induction by vortices during the formation of mesophases in stirred solutions. Science 2001, 292, 2063–2066. [Google Scholar] [CrossRef]
- Micali, N.; Engelkamp, H.; van Rhee, P.G.; Christianen, P.C.M.; Monsù Scolaro, L.; Maan, J.C. Selection of supramolecular chirality by application of rotational and magnetic forces. Nat. Chem. 2012, 4, 201–216. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, J.; Kim, W.Y.; Kim, H.; Lee, S.; Lee, H.C.; Lee, Y.S.; Seo, M.; Kim, S.Y. Induction and control of supramolecular chirality by light in self-assembled helical nanostructures. Nat. Commun. 2015, 6, 6959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charalambidis, G.; Georgilis, E.; Panda, M.K.; Anson, C.E.; Powell, A.K.; Doyle, S.; Moss, D.; Jochum, T.; Horton, P.N.; Coles, S.J.; et al. A switchable self-assembling and disassembling chiral system based on a porphyrin-substituted phenylalanine–phenylalanine motif. Nat. Commun. 2016, 7, 12675. [Google Scholar] [CrossRef] [PubMed]
- Cantonetti, V.; Monti, D.; Venanzi, M.; Bombelli, C.; Ceccacci, F.; Mancini, G. Interaction of a chirally functionalised porphyrin derivative with chiral micellar aggregates. Construction of a system with stereoselective cytochrome-P450 biomimetic activity. Tetrahedron Asymmetry 2004, 15, 1969–1977. [Google Scholar] [CrossRef]
- Monti, D.; Cantonetti, V.; Venanzi, M.; Ceccacci, F.; Bombelli, C.; Mancini, G. Interaction of a chirally functionalised porphyrinderivative with chiral micellar aggregates. Chem. Commun. 2004, 8, 972–973. [Google Scholar] [CrossRef] [PubMed]
- Jintoku, H.; Shimoda, S.; Takafuji, M.; Sagawa, T.; Ihara, H. Tuning of molecular orientation of porphyrin assembly according to monitoring the chiroptical signals. Mol. Cryst. Liq. Cryst. 2011, 539, 63–67. [Google Scholar] [CrossRef]
- Shu-Jun, W.; Wen-Juan, R.; Xiao-Jing, Z.; Dai-Bing, L.; Zhi-Ang, Z. Molecular recognition of chiral zinc porphyrin with amino acid esters. Chin. J. Chem. 2005, 23, 44–49. [Google Scholar]
- Monti, D.; Venanzi, M.; Stefanelli, M.; Sorrenti, A.; Mancini, G.; Di Natale, C.; Paolesse, R. Chiral amplification of chiral porphyrin derivatives by templated heteroaggregation. J. Am. Chem. Soc. 2007, 129, 6688–6689. [Google Scholar] [CrossRef]
- Jintoku, H.; Sagawa, T.; Takafuji, M.; Ihara, H. Chirally self-assembled porphyrin nanowires assisted by L-glutamide-derived lipid for excitation energy transfer. Org. Biomol. Chem. 2009, 7, 2430–2434. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Chen, Y.; Ma, P.; Lu, J.; Zhang, X.; Jiang, J. Morphology and chirality controlled self-assembled nanostructures of porphyrin–pentapeptide conjugate: Effect of the peptide secondary conformation. J. Mater. Chem. 2011, 21, 8057–8065. [Google Scholar] [CrossRef]
- Lauceri, R.; Raudino, A.; Monsù Scolaro, L.; Micali, N.; Purrello, R. From achiral porphyrins to template-imprinted chiral aggregates and further. self-replication of chiral memory from scratch. J. Am. Chem. Soc. 2002, 124, 894–895. [Google Scholar] [CrossRef] [PubMed]
- Uemori, Y.; Munakata, H.; Kitazawa, S.; Osada, S.; Imai, H. Optically active J-aggregate formed from water-soluble porphyrin with phenylalanine. J. Porphyrins Phthalocyanines 2012, 16, 1285–1292. [Google Scholar] [CrossRef]
- Zeng, L.; He, Y.; Dai, Z.; Wang, J.; Cao, Q.; Zhang, Y. Chiral induction, memory, and amplification in porphyrin homoaggregates based on electrostatic interactions. Chem. Phys. Chem. 2009, 10, 954–962. [Google Scholar] [CrossRef] [PubMed]
- Maiti, N.C.; Mazumdar, S.; Periasamy, N. J- and H-aggregates of porphyrin−surfactant complexes: time-resolved fluorescence and other spectroscopic studies. J. Phys. Chem. B 1998, 102, 1528–1538. [Google Scholar] [CrossRef]
- Jiang, S.; Zhang, L.; Liu, M. Photo-triggered J-aggregation and chiral symmetry breaking of an anionic porphyrin (TPPS) in mixed organic solvent. Chem. Commun. 2009, 41, 6252–6254. [Google Scholar] [CrossRef]
- Elemans, J.A.A.W.; Rowan, A.E.; Nolte, R.J.M. Mastering molecular matter. Supramolecular architectures by hierarchical self-assembly. J. Mater. Chem. 2003, 13, 2661–2670. [Google Scholar] [CrossRef]
- Escudero, C.; D’Urso, A.; Lauceri, R.; Bonaccorso, C.; Sciotto, D.; Di Bella, S.; El-Hachemi, Z.; Crusatsa, J.; Ribó, J.M.; Purrello, R. Hierarchical dependence of porphyrin self-aggregation: Controlling and exploiting the complexity. J. Porphyrins Phthalocyanines 2010, 14, 708–712. [Google Scholar] [CrossRef]








© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Randazzo, R.; Gaeta, M.; Gangemi, C.M.A.; Fragalà, M.E.; Purrello, R.; D’Urso, A. Chiral Recognition of L- and D- Amino Acid by Porphyrin Supramolecular Aggregates. Molecules 2019, 24, 84. https://doi.org/10.3390/molecules24010084
Randazzo R, Gaeta M, Gangemi CMA, Fragalà ME, Purrello R, D’Urso A. Chiral Recognition of L- and D- Amino Acid by Porphyrin Supramolecular Aggregates. Molecules. 2019; 24(1):84. https://doi.org/10.3390/molecules24010084
Chicago/Turabian StyleRandazzo, Rosalba, Massimiliano Gaeta, Chiara Maria Antonietta Gangemi, Maria Elena Fragalà, Roberto Purrello, and Alessandro D’Urso. 2019. "Chiral Recognition of L- and D- Amino Acid by Porphyrin Supramolecular Aggregates" Molecules 24, no. 1: 84. https://doi.org/10.3390/molecules24010084