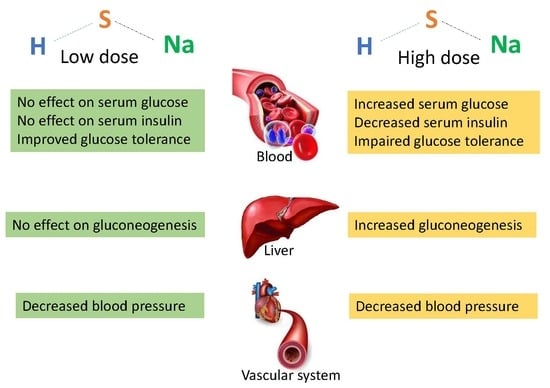
Effects of Hydrogen Sulfide on Carbohydrate Metabolism in Obese Type 2 Diabetic Rats
Abstract
:1. Introduction
2. Results
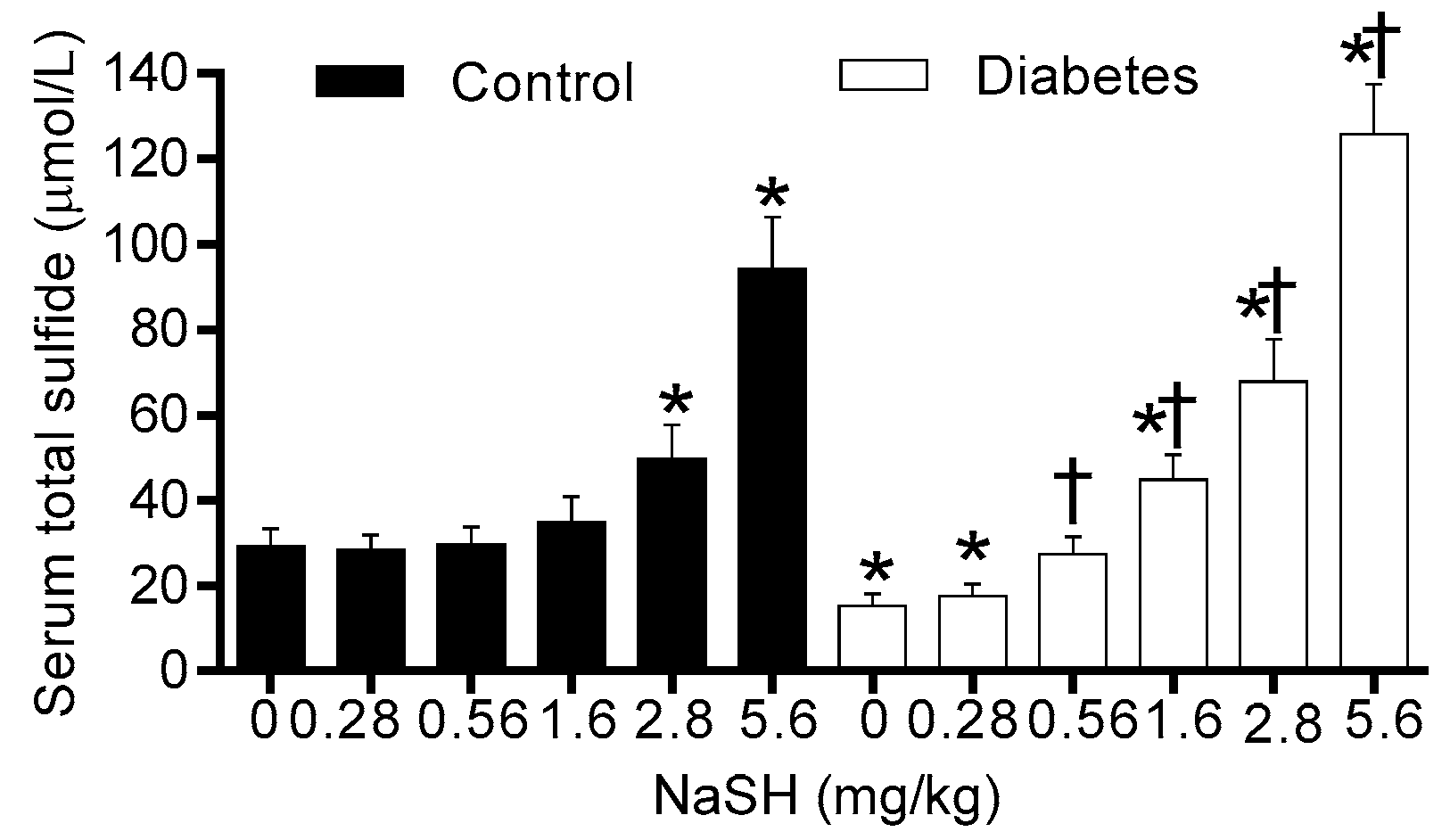
2.1. Effect of NaSH on Serum Total Sulfide Levels
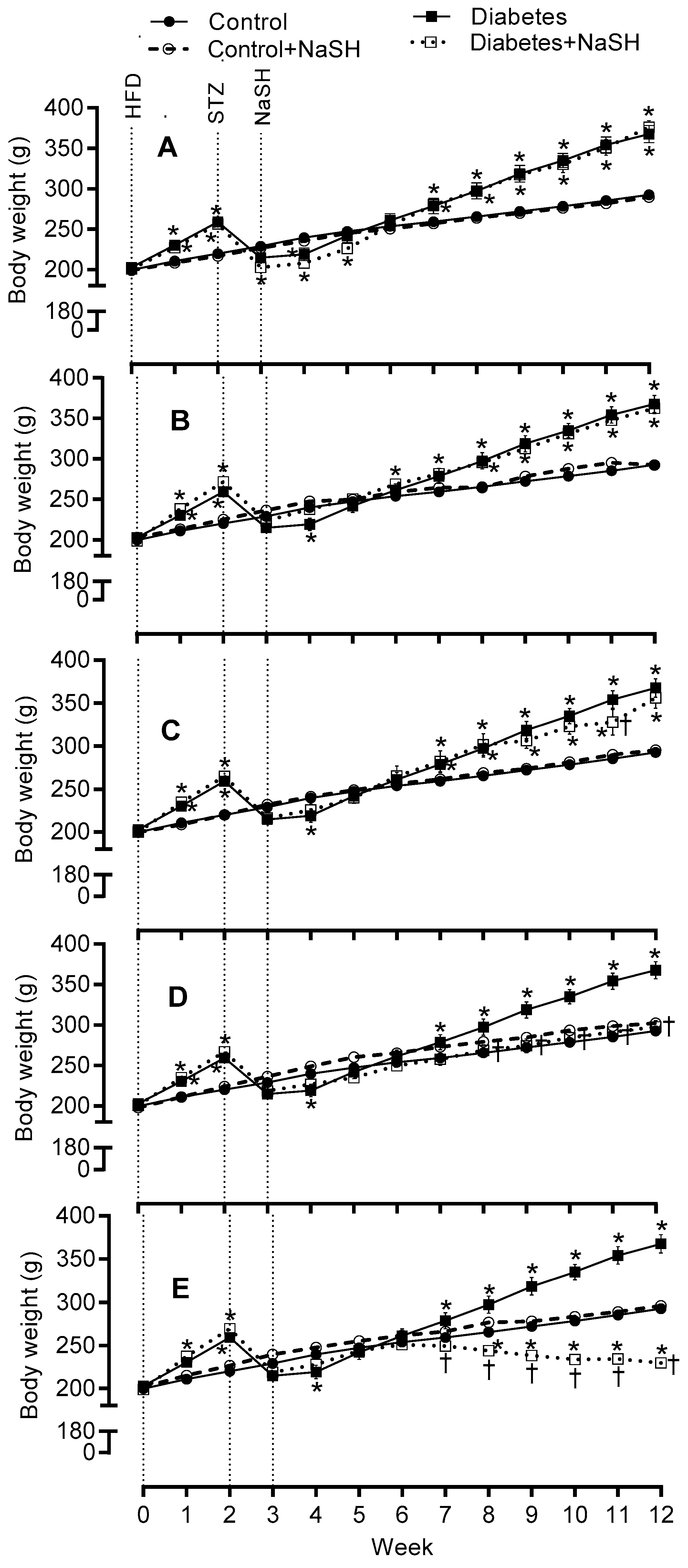
2.2. Effect of NaSH on Body Weight, Water Consumption, and Food Intake
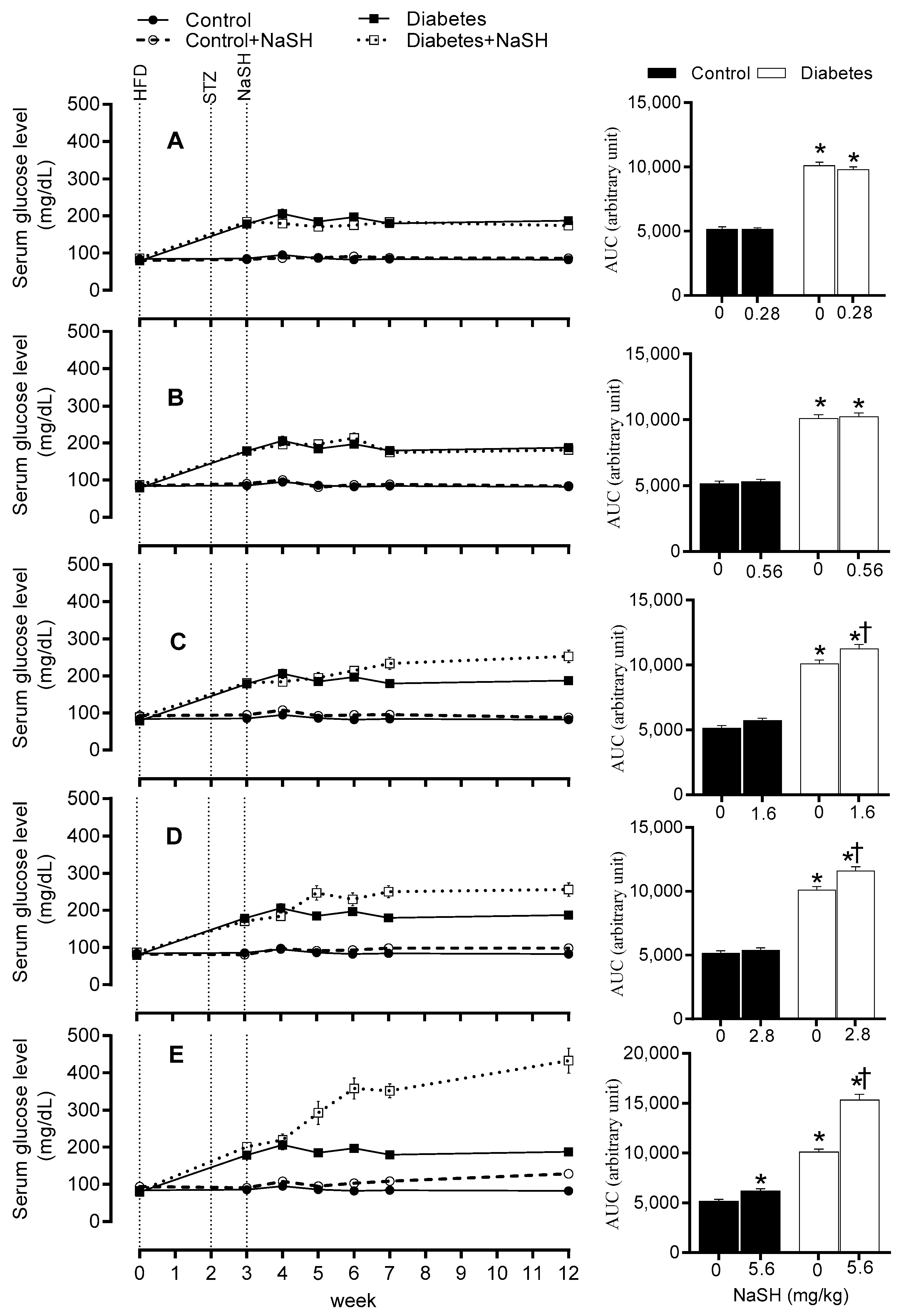
2.3. Effect of NaSH on Serum Glucose Concentration
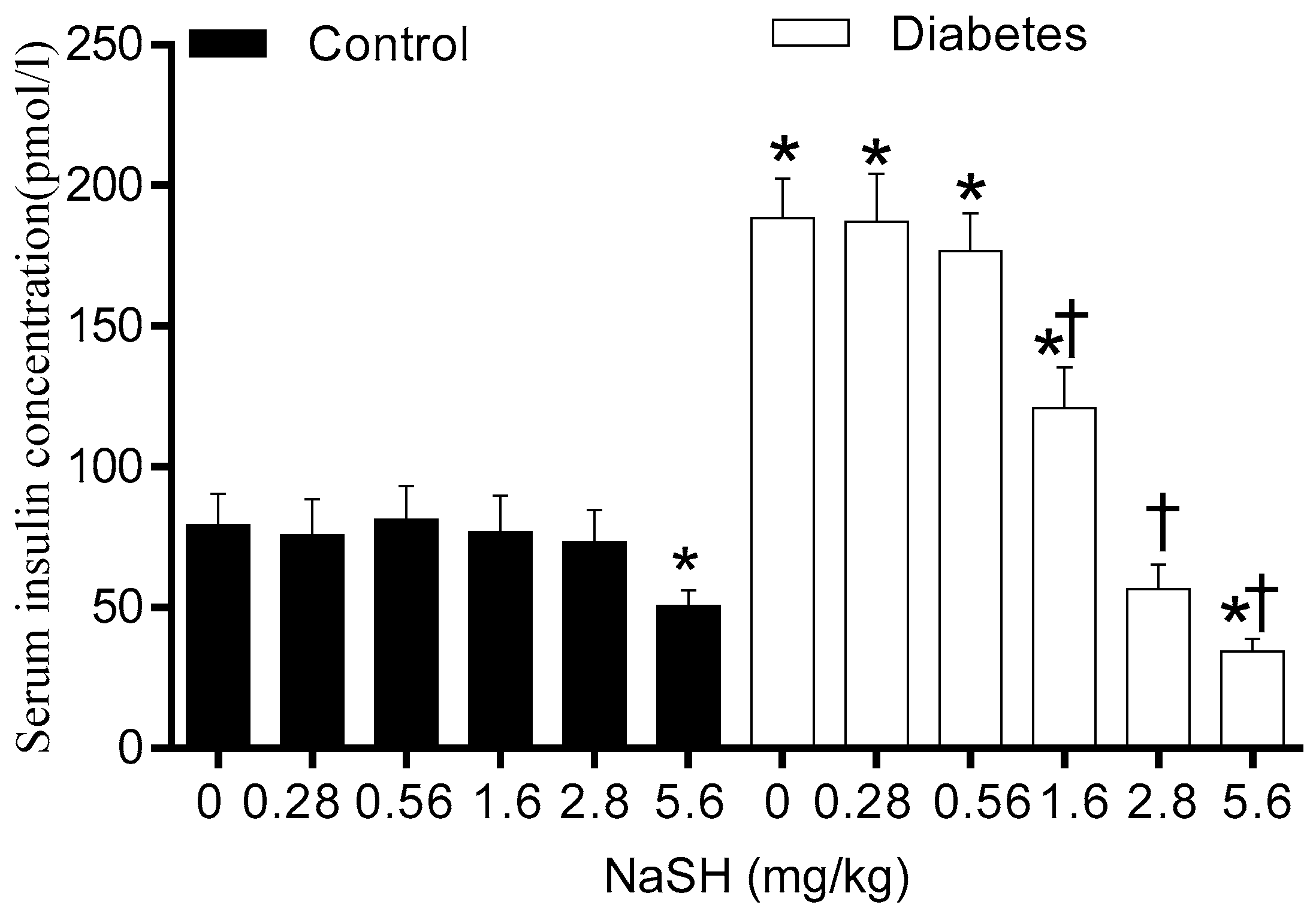
2.4. Effect of NaSH on Serum Insulin Concentrations
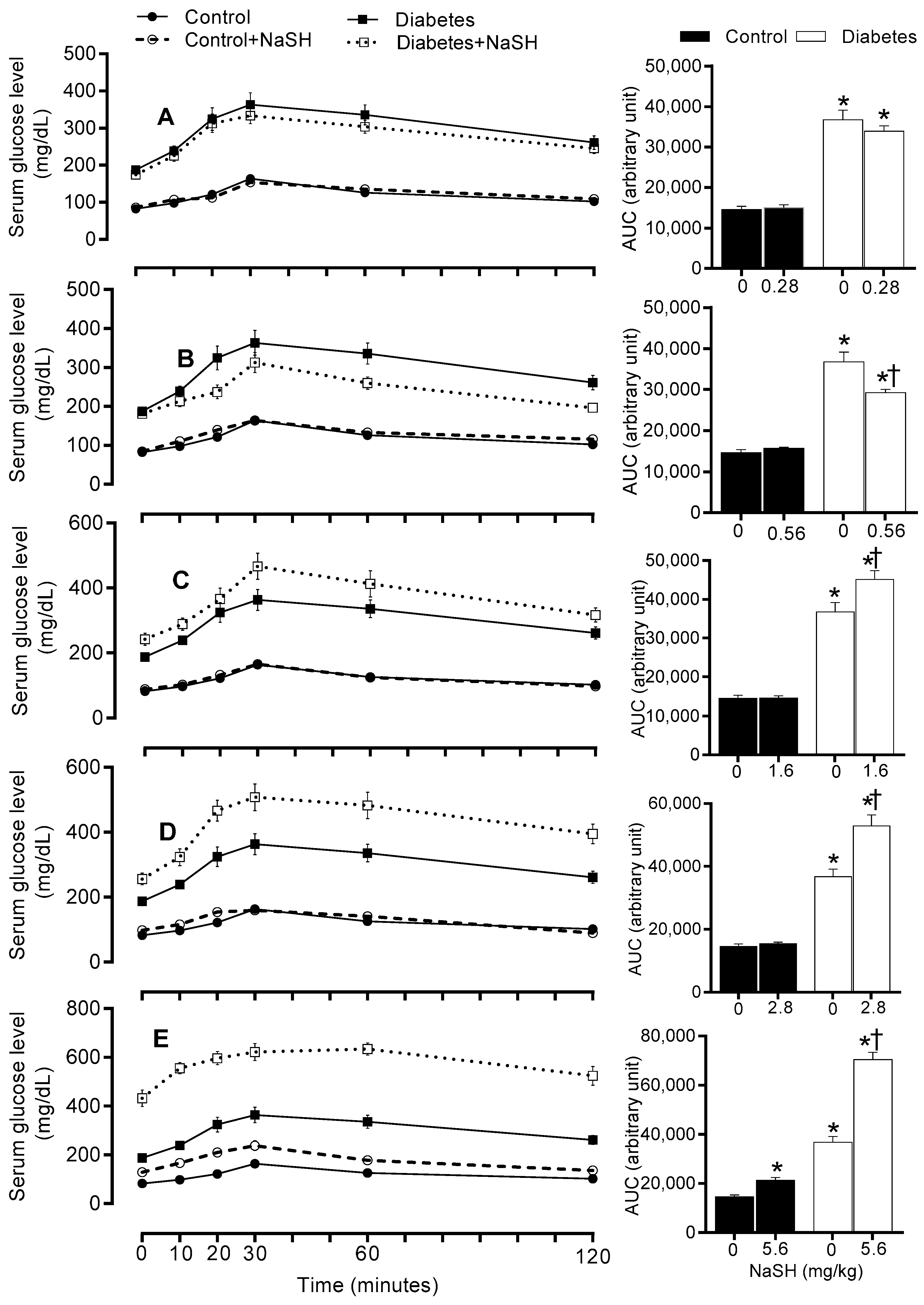
2.5. Effect of NaSH on Glucose Tolerance
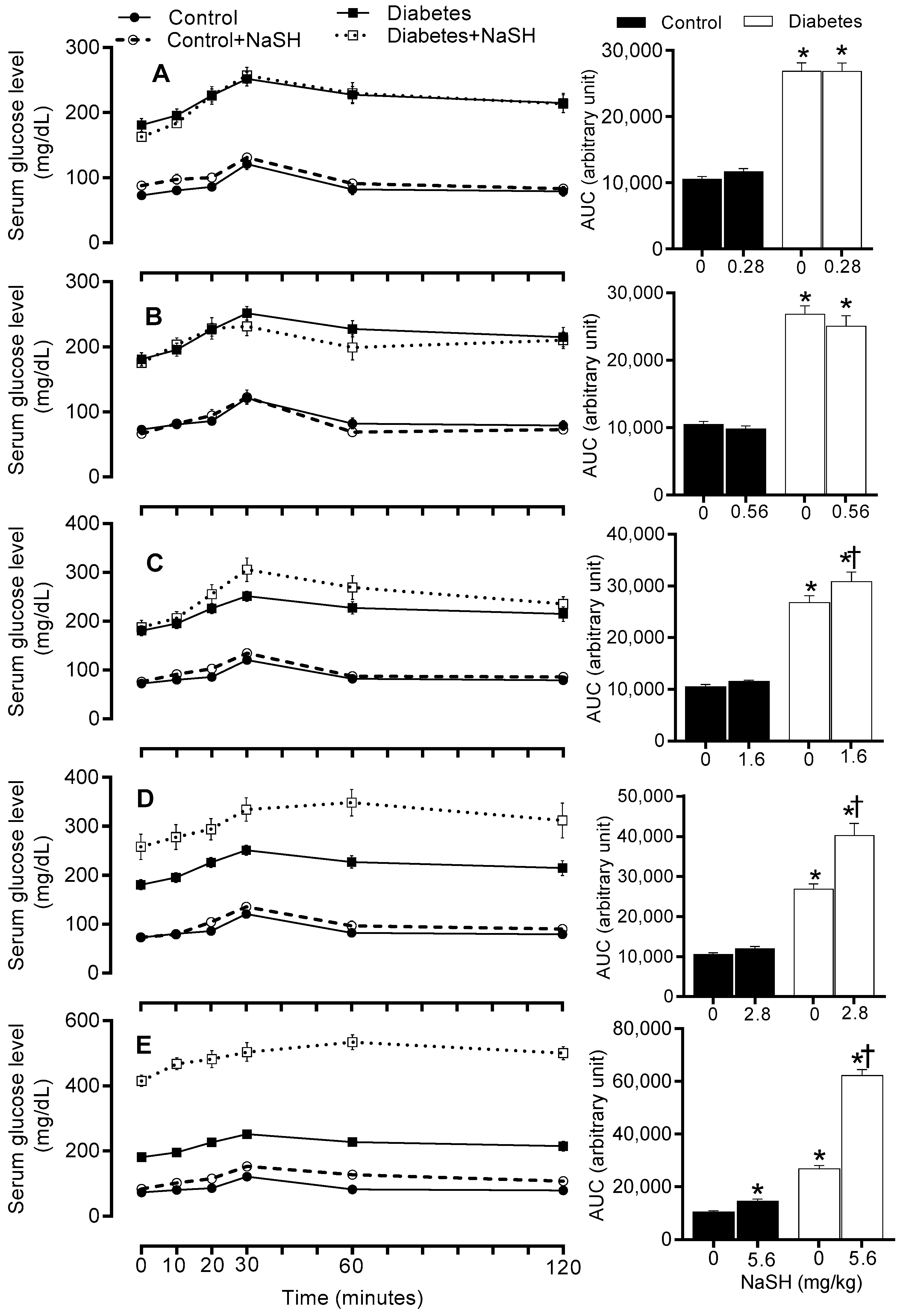
2.6. Effect of NaSH on Gluconeogenesis
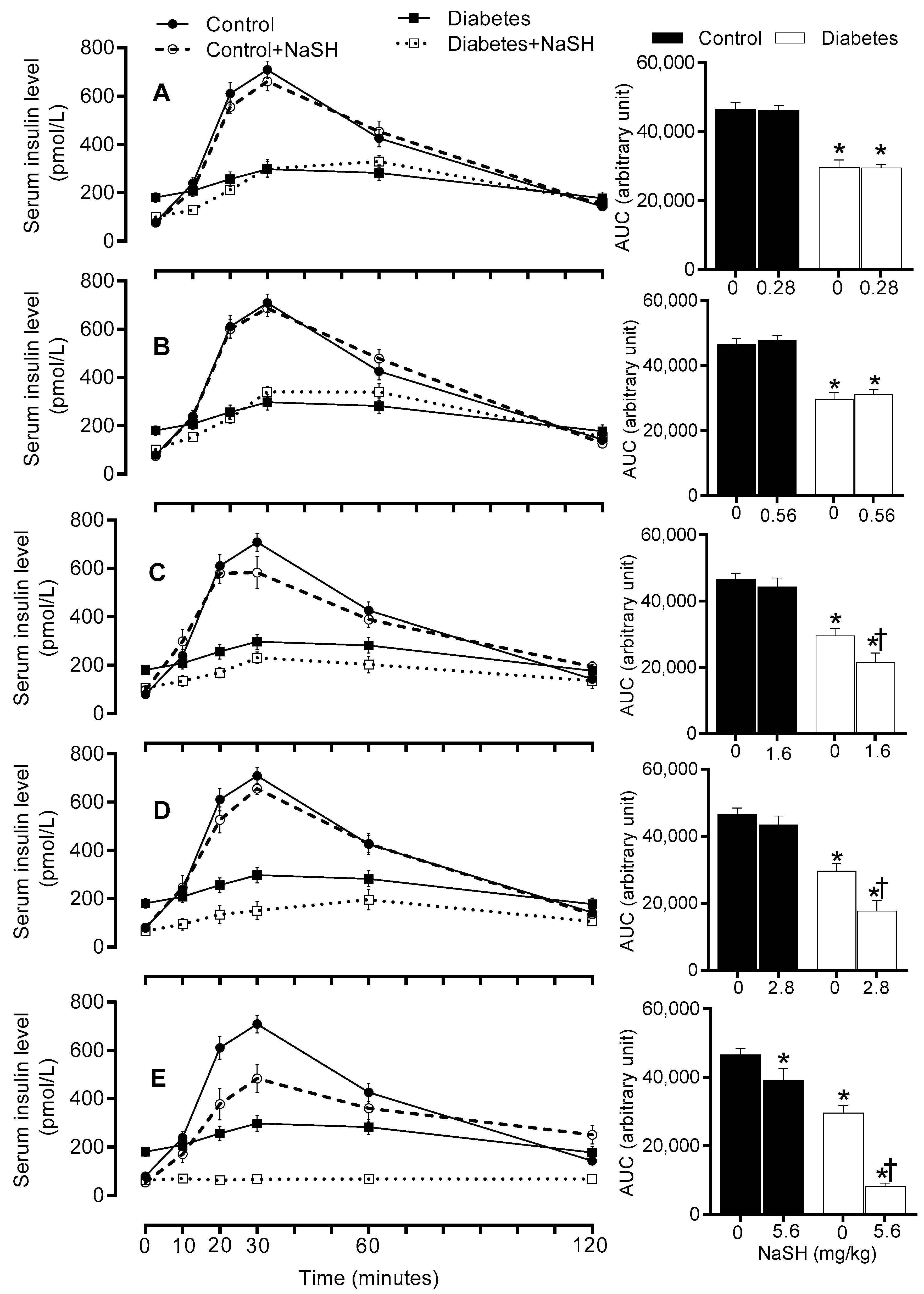
2.7. Effect of NaSH on In Vivo Insulin Secretion
2.8. Effect of NaSH on Blood Pressure
3. Discussion
4. Materials and Methods
4.1. Animals and Induction of Diabetes
4.2. Experimental Design
4.3. Measurement of Serum Glucose and Insulin Levels
4.4. Measurement of Serum Total Sulfide
4.5. Intraperitoneal Glucose Tolerance Test, Pyruvate Tolerance Test, and In Vivo Insulin Secretion
4.6. Measurement of Systolic Blood Pressure
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, L.; Wang, Y.; Li, Y.; Li, L.; Xu, S.; Feng, X.; Liu, S. Hydrogen sulfide (H2S)-releasing compounds: Therapeutic potential in cardiovascular diseases. Front. Pharmacol. 2018, 9, 1066. [Google Scholar] [CrossRef] [PubMed]
- Hosoki, R.; Matsuki, N.; Kimura, H. The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochem. Biophys. Res. Commun. 1997, 237, 527–531. [Google Scholar] [CrossRef] [PubMed]
- Bonfanti, D.H.; Alcazar, L.P.; Arakaki, P.A.; Martins, L.T.; Agustini, B.C.; de Moraes Rego, F.G.; Frigeri, H.R. ATP-dependent potassium channels and type 2 diabetes mellitus. Clin. Biochem. 2015, 48, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Yang, G.; Jia, X.; Wu, L.; Wang, R. Activation of KATP channels by H2S in rat insulin-secreting cells and the underlying mechanisms. J. Physiol. 2005, 569, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.; Zhang, L.; Yang, G.; Wu, L.; Wang, R. Hydrogen sulfide-induced inhibition of L-type Ca2+ channels and insulin secretion in mouse pancreatic beta cells. Diabetologia 2013, 56, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Chen, Y.; Zhao, J.; Tang, C.; Jiang, Z.; Geng, B. Hydrogen sulfide from adipose tissue is a novel insulin resistance regulator. Biochem. Biophys. Res. Commun. 2009, 380, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Yang, W.; Jia, X.; Yang, G.; Duridanova, D.; Cao, K.; Wang, R. Pancreatic islet overproduction of H2S and suppressed insulin release in Zucker diabetic rats. Lab. Invest. 2009, 89, 59–67. [Google Scholar] [CrossRef]
- Szabo, C. Roles of hydrogen sulfide in the pathogenesis of diabetes mellitus and its complications. Antioxid. Redox. Signal. 2012, 17, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Sagara, M.; Aoki, C.; Tanaka, S.; Aso, Y. Clinical implication of plasma hydrogen sulfide levels in Japanese patients with type 2 diabetes. Intern. Med. 2017, 56, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.K.; Bull, R.; Rains, J.L.; Bass, P.F.; Levine, S.N.; Reddy, S.; McVie, R.; Bocchini, J.A. Low levels of hydrogen sulfide in the blood of diabetes patients and streptozotocin-treated rats causes vascular inflammation? Antioxid. Redox. Signal. 2010, 12, 1333–1337. [Google Scholar] [CrossRef]
- Whiteman, M.; Gooding, K.M.; Whatmore, J.L.; Ball, C.I.; Mawson, D.; Skinner, K.; Tooke, J.E.; Shore, A.C. Adiposity is a major determinant of plasma levels of the novel vasodilator hydrogen sulphide. Diabetologia 2010, 53, 1722–1726. [Google Scholar] [CrossRef] [PubMed]
- Durante, W. Hydrogen sulfide therapy in diabetes-accelerated atherosclerosis: A whiff of success. Diabetes 2016, 65, 2832–2834. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, M.; Yamaoka, M.; Takei, M.; Ando, T.; Taniguchi, S.; Ishii, I.; Tohya, K.; Ishizaki, T.; Niki, I.; Kimura, T. Endogenous hydrogen sulfide protects pancreatic beta-cells from a high-fat diet-induced glucotoxicity and prevents the development of type 2 diabetes. Biochem. Biophys. Res. Commun. 2013, 442, 227–233. [Google Scholar] [CrossRef]
- Pichette, J.; Fynn-Sackey, N.; Gagnon, J. Hydrogen sulfide and sulfate prebiotic stimulates the secretion of GLP-1 and improves glycemia in male mice. Endocrinology 2017, 158, 3416–3425. [Google Scholar] [CrossRef] [PubMed]
- Manna, P.; Jain, S.K. Hydrogen sulfide and L-cysteine increase phosphatidylinositol 3,4,5-trisphosphate (PIP3) and glucose utilization by inhibiting phosphatase and tensin homolog (PTEN) protein and activating phosphoinositide 3-kinase (PI3K)/serine/threonine protein kinase (AKT)/protein kinase Czeta/lambda (PKCzeta/lambda) in 3T3l1 adipocytes. J. Biol. Chem. 2011, 286, 39848–39859. [Google Scholar] [CrossRef] [PubMed]
- Manna, P.; Jain, S.K. Vitamin D up-regulates glucose transporter 4 (GLUT4) translocation and glucose utilization mediated by cystathionine-gamma-lyase (CSE) activation and H2S formation in 3T3L1 adipocytes. J. Biol. Chem. 2012, 287, 42324–42332. [Google Scholar] [CrossRef]
- Xue, R.; Hao, D.-D.; Sun, J.-P.; Li, W.-W.; Zhao, M.-M.; Li, X.-H.; Chen, Y.; Zhu, J.-H.; Ding, Y.-J.; Liu, J.; et al. Hydrogen sulfide treatment promotes glucose uptake by increasing insulin receptor sensitivity and ameliorates kidney lesions in type 2 diabetes. Antioxid. Redox. Signal. 2013, 19, 5–23. [Google Scholar] [CrossRef] [PubMed]
- Peake, B.F.; Nicholson, C.K.; Lambert, J.P.; Hood, R.L.; Amin, H.; Amin, S.; Calvert, J.W. Hydrogen sulfide preconditions the db/db diabetic mouse heart against ischemia-reperfusion injury by activating Nrf2 signaling in an Erk-dependent manner. Am. J. Physiol. Heart Circ. Physiol. 2013, 304, 1215–1224. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Tian, Z.; Sun, Y.; Lu, C.; Liu, N.; Gao, Z.; Zhang, L.; Dong, S.; Yang, F.; Zhong, X.; et al. Exogenous H2S facilitating ubiquitin aggregates clearance via autophagy attenuates type 2 diabetes-induced cardiomyopathy. Cell. Death Dis. 2017, 8, 2992. [Google Scholar] [CrossRef]
- Sun, Y.; Tian, Z.; Liu, N.; Zhang, L.; Gao, Z.; Sun, X.; Yu, M.; Wu, J.; Yang, F.; Zhao, Y.; et al. Exogenous H2S switches cardiac energy substrate metabolism by regulating SIRT3 expression in db/db mice. J. Mol. Med. 2018, 96, 281–299. [Google Scholar] [CrossRef]
- Ma, S.; Zhong, D.; Ma, P.; Li, G.; Hua, W.; Sun, Y.; Liu, N.; Zhang, L.; Zhang, W. Exogenous hydrogen sulfide ameliorates diabetes-associated cognitive decline by regulating the mitochondria-mediated apoptotic pathway and IL-23/IL-17 expression in db/db mice. Cell. Physiol. Biochem. 2017, 41, 1838–1850. [Google Scholar] [CrossRef] [PubMed]
- Saha, P.; Banerjee, P.; Pal, P.; Auddya, L.; Sen, S.; Sau, T.J.; Kumar, A.; Biswas, U.K. Enhanced plasma H2S levels associated with fasting blood glucose in type-2 diabetes mellitus. Asian J. Med. Sci. 2015, 6, 5. [Google Scholar] [CrossRef]
- Yusuf, M.; Kwong Huat, B.T.; Hsu, A.; Whiteman, M.; Bhatia, M.; Moore, P.K. Streptozotocin-induced diabetes in the rat is associated with enhanced tissue hydrogen sulfide biosynthesis. Biochem. Biophys. Res. Commun. 2005, 333, 1146–1152. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Feng, S.J.; Zhang, G.Z.; Wang, S.X. Correlation of lower concentrations of hydrogen sulfide with atherosclerosis in chronic hemodialysis patients with diabetic nephropathy. Blood. Purif. 2014, 38, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Ng, H.H.; Yildiz, G.S.; Ku, J.M.; Miller, A.A.; Woodman, O.L.; Hart, J.L. Chronic NaHS treatment decreases oxidative stress and improves endothelial function in diabetic mice. Diab. Vasc. Dis. Res. 2017, 14, 246–253. [Google Scholar] [CrossRef]
- Suzuki, K.; Olah, G.; Modis, K.; Coletta, C.; Kulp, G.; Gero, D.; Szoleczky, P.; Chang, T.; Zhou, Z.; Wu, L.; et al. Hydrogen sulfide replacement therapy protects the vascular endothelium in hyperglycemia by preserving mitochondrial function. Proc. Natl. Acad. Sci. USA 2011, 108, 13829–13834. [Google Scholar] [CrossRef] [Green Version]
- Mani, S.; Yang, G.; Wang, R. A critical life-supporting role for cystathionine gamma-lyase in the absence of dietary cysteine supply. Free Radic. Biol. Med. 2011, 50, 1280–1287. [Google Scholar] [CrossRef]
- Geng, B.; Cai, B.; Liao, F.; Zheng, Y.; Zeng, Q.; Fan, X.; Gong, Y.; Yang, J.; Cui, Q.H.; Tang, C.; et al. Increase or decrease hydrogen sulfide exert opposite lipolysis, but reduce global insulin resistance in high fatty diet induced obese mice. PLoS ONE 2013, 8, 73892. [Google Scholar] [CrossRef]
- Kaneko, Y.; Kimura, Y.; Kimura, H.; Niki, I. L-cysteine inhibits insulin release from the pancreatic beta-cell: Possible involvement of metabolic production of hydrogen sulfide, a novel gasotransmitter. Diabetes 2006, 55, 1391–1397. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, G.; Untereiner, A.; Ju, Y.; Wu, L.; Wang, R. Hydrogen sulfide impairs glucose utilization and increases gluconeogenesis in hepatocytes. Endocrinology 2013, 154, 114–126. [Google Scholar] [CrossRef]
- Picarel-Blanchot, F.; Berthelier, C.; Bailbe, D.; Portha, B. Impaired insulin secretion and excessive hepatic glucose production are both early events in the diabetic GK rat. Am. J. Physiol. 1996, 271, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Warenycia, M.W.; Goodwin, L.R.; Benishin, C.G.; Reiffenstein, R.J.; Francom, D.M.; Taylor, J.D.; Dieken, F.P. Acute hydrogen sulfide poisoning. Demonstration of selective uptake of sulfide by the brainstem by measurement of brain sulfide levels. Biochem. Pharmacol. 1989, 38, 973–981. [Google Scholar] [CrossRef]
- Truong, D.H.; Mihajlovic, A.; Gunness, P.; Hindmarsh, W.; O’Brien, P.J. Prevention of hydrogen sulfide (H2S)-induced mouse lethality and cytotoxicity by hydroxocobalamin (vitamin B(12a)). Toxicology 2007, 242, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Cronican, A.A.; Frawley, K.L.; Ahmed, H.; Pearce, L.L.; Peterson, J. Antagonism of acute sulfide poisoning in mice by nitrite anion without methemoglobinemia. Chemical Res. Toxicol. 2015, 28, 1398–1408. [Google Scholar] [CrossRef] [PubMed]
- Untereiner, A.A.; Wang, R.; Ju, Y.; Wu, L. Decreased gluconeogenesis in the absence of cystathionine gamma-lyase and the underlying mechanisms. Antioxid. Redox. Signal. 2016, 24, 129–140. [Google Scholar] [CrossRef]
- Sun, L.; Jin, H.; Chen, S.; Huang, Y.; Liu, J.; Li, Z.; Zhao, M.; Sun, Y.; Tang, C.; Zhao, B.; et al. Hydrogen sulfide alleviates myocardial collagen remodeling in association with inhibition of TGF-beta/Smad signaling pathway in spontaneously hypertensive rats. Mol. Med. 2015, 20, 503–515. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Chen, S.; Wang, Y.; Liu, J.; Yao, Q.; Huang, Y.; Li, H.; Zhu, M.; Wang, S.; Li, L.; et al. Down-regulated CBS/H2S pathway is involved in high-salt-induced hypertension in Dahl rats. Nitric Oxide 2015, 46, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, F.U.; Sattar, M.A.; Rathore, H.A.; Tan, Y.C.; Akhtar, S.; Jin, O.H.; Pei, Y.P.; Abdullah, N.A.; Johns, E.J. Hydrogen sulphide and tempol treatments improve the blood pressure and renal excretory responses in spontaneously hypertensive rats. Ren. Fail. 2014, 36, 598–605. [Google Scholar] [CrossRef] [Green Version]
- Lu, M.; Liu, Y.H.; Goh, H.S.; Wang, J.J.; Yong, Q.C.; Wang, R.; Bian, J.S. Hydrogen sulfide inhibits plasma renin activity. J. Am. Soc. Nephrol. 2010, 21, 993–1002. [Google Scholar] [CrossRef]
- Shi, Y.X.; Chen, Y.; Zhu, Y.Z.; Huang, G.Y.; Moore, P.K.; Huang, S.H.; Yao, T.; Zhu, Y.C. Chronic sodium hydrosulfide treatment decreases medial thickening of intramyocardial coronary arterioles, interstitial fibrosis, and ROS production in spontaneously hypertensive rats. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, 2093–2100. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, J.; Lu, Y.; Wang, R. The vasorelaxant effect of H(2)S as a novel endogenous gaseous K(ATP) channel opener. EMBO J. 2001, 20, 6008–6016. [Google Scholar] [CrossRef] [PubMed]
- Gheibi, S.; Kashfi, K.; Ghasemi, A. A practical guide for induction of type-2 diabetes in rat: Incorporating a high-fat diet and streptozotocin. Biomed. Pharmacother. 2017, 95, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, M.; Kodela, R.; Duvalsaint, P.L.; Kashfi, K. Gastrointestinal safety, chemotherapeutic potential, and classic pharmacological profile of NOSH-naproxen (AVT-219) a dual NO- and H2S-releasing hybrid. Pharmacol. Res. Perspect. 2016, 4. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Pattillo, C.B.; Pardue, S.; Bir, S.C.; Wang, R.; Kevil, C.G. Measurement of plasma hydrogen sulfide in vivo and in vitro. Free Radic. Biol. Med. 2011, 50, 1021–1031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gheibi, S.; Jeddi, S.; Carlström, M.; Gholami, H.; Ghasemi, A. Effects of long-term nitrate supplementation on carbohydrate metabolism, lipid profiles, oxidative stress, and inflammation in male obese type 2 diabetic rats. Nitric Oxide 2018, 75, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Ghanbari, M.; Jeddi, S.; Bagheripuor, F.; Ghasemi, A. The effect of maternal hypothyroidism on cardiac function and tolerance to ischemia-reperfusion injury in offspring male and female rats. J. Endocrinol. Invest. 2015, 38, 915–922. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available |


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gheibi, S.; Jeddi, S.; Kashfi, K.; Ghasemi, A. Effects of Hydrogen Sulfide on Carbohydrate Metabolism in Obese Type 2 Diabetic Rats. Molecules 2019, 24, 190. https://doi.org/10.3390/molecules24010190
Gheibi S, Jeddi S, Kashfi K, Ghasemi A. Effects of Hydrogen Sulfide on Carbohydrate Metabolism in Obese Type 2 Diabetic Rats. Molecules. 2019; 24(1):190. https://doi.org/10.3390/molecules24010190
Chicago/Turabian StyleGheibi, Sevda, Sajad Jeddi, Khosrow Kashfi, and Asghar Ghasemi. 2019. "Effects of Hydrogen Sulfide on Carbohydrate Metabolism in Obese Type 2 Diabetic Rats" Molecules 24, no. 1: 190. https://doi.org/10.3390/molecules24010190