Saponins from Quillaja saponaria and Quillaja brasiliensis: Particular Chemical Characteristics and Biological Activities
Abstract
:1. Introduction
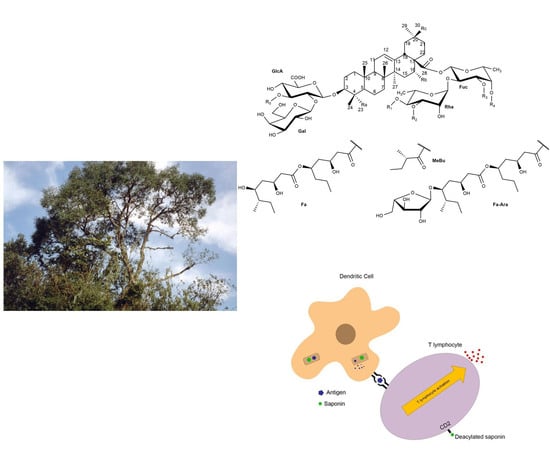
2. Chemical Structure/Characterization
3. Biological Activities
3.1. Antimicrobial Activities of Quillaja Saponins
3.1.1. Antibacterial Activity
3.1.2. Antiviral Activity
3.1.3. Antifungal Activity
3.2. Antiparasitic Activity
3.3. Antitumor Activity of Quillaja Saponins
3.4. Hepatoprotective Activity of Quillaja Saponins
3.5. Immunoadjuvant Activity of Quillaja Saponins
4. Toxicity of Quillaja Saponins
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sparg, S.G.; Light, M.E.; Van Staden, J. Biological activities and distribution of plant saponins. J. Ethnopharmacol. 2004, 94, 219–243. [Google Scholar] [CrossRef]
- Augustin, J.M.; Kuzina, V.; Andersen, S.B.; Bak, S. Molecular activities, biosynthesis and evolution of triterpenoid saponins. Phytochemistry 2011, 72, 435–457. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, R.; Tokimitsu, Y.; Komori, T. An acylated triterpenoid saponin from Quillaja saponaria. Phytochemistry 1988, 27, 1165–1168. [Google Scholar] [CrossRef]
- Nord, L.I.; Kenne, L. Novel acetylated triterpenoid saponins in a chromatographic fraction from Quillaja saponaria Molina. Carbohydr. Res. 2000, 329, 817–829. [Google Scholar] [CrossRef]
- Sun, H.-X.; Xie, Y.; Ye, Y.-P. Advances in saponin-based adjuvants. Vaccine 2009, 27, 1787–1796. [Google Scholar] [CrossRef]
- Codex Alimentarius International Food Standards; Food and Agriculture Organization of United Nations World Health Organization: Rome, Italy, 2018; Volume 49.
- Wojciechowski, K. Surface activity of saponin from Quillaja bark at the air/water and oil/water interfaces. Colloids Surfaces B Biointerfaces 2013, 108, 95–102. [Google Scholar] [CrossRef] [PubMed]
- European Commission Database-CosIng. Available online: http://ec.europa.eu/growth/sectors/cosmetics/cosing_en (accessed on 12 December 2018).
- Ozturk, B.; McClements, D.J. Progress in natural emul sifiers for utilization in food emulsions. Curr. Opin. Food Sci. 2016, 7, 1–6. [Google Scholar] [CrossRef]
- de Faria, J.T.; de Oliveira, E.B.; Minim, V.P.R.; Minim, L.A. Performance of Quillaja bark saponin and β-lactoglobulin mixtures on emulsion formation and stability. Food Hydrocoll. 2017, 67, 178–188. [Google Scholar] [CrossRef]
- Roner, M.R.; Sprayberry, J.; Spinks, M.; Dhanji, S. Antiviral activity obtained from aqueous extracts of the Chilean soapbark tree (Quillaja saponaria Molina). J. Gen. Virol. 2007, 88, 275–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tam, K.I.; Roner, M.R. Characterization of in vivo anti-rotavirus activities of saponin extracts from Quillaja saponaria Molina. Antiviral Res. 2011, 90, 231–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roner, M.R.; Tam, K.I.; Barrager, M.K. Prevention of rotavirus infections in vitro with aqueous extracts of Quillaja saponaria Molina. Futur. Med Chem 2010, 14, 384–399. [Google Scholar] [CrossRef]
- Dixit, V.; Tewari, J.; Obendorf, S.K. Fungal Growth Inhibition of Regenerated Cellulose Nanofibrous Membranes Containing Quillaja Saponin. Arch. Environ. Con. Tox. 2010, 59, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Holtshausen, L.; Chaves, A.V.; Beauchemin, K.A.; McGinn, S.M.; McAllister, T.A.; Odongo, N.E.; Cheeke, P.R.; Benchaar, C. Feeding saponin-containing Yucca schidigera and Quillaja saponaria to decrease enteric methane production in dairy cows. J. Dairy Sci. 2009, 92, 2809–2821. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Makkar, H.P.S.; Muetzel, S.; Becker, K. Effect of Quillaja saponaria saponins and Yucca schidigera plant extract on growth of Escherichia coli. Lett. Appl. Microbiol. 1998, 27, 35–38. [Google Scholar] [CrossRef] [PubMed]
- Pen, B.; Sar, C.; Mwenya, B.; Kuwaki, K.; Morikawa, R.; Takahashi, J. Effects of Yucca schidigera and Quillaja saponaria extracts on in vitro ruminal fermentation and methane emission. Anim. Feed Sci. Technol. 2006, 129, 175–186. [Google Scholar] [CrossRef]
- Rajput, Z.I.; Hu, S.; Xiao, C.; Arijo, A.G. Adjuvant effects of saponins on animal immune responses. J. Zhejiang Univ. Sci. B 2007, 8, 153–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sjölander, A.; Drane, D.; Maraskovsky, E.; Scheerlinck, J.P.; Suhrbier, A.; Tennent, J.; Pearse, M. Immune responses to ISCOM® formulations in animal and primate models. Vaccine 2001, 19, 2661–2665. [Google Scholar] [CrossRef]
- Paula Barbosa, A. de Saponins as immunoadjuvant agent: A review. African J. Pharm. Pharmacol. 2014, 8, 1049–1057. [Google Scholar] [CrossRef]
- Marty-Roix, R.; Vladimer, G.I.; Pouliot, K.; Weng, D.; Buglione-Corbett, R.; West, K.; MacMicking, J.D.; Chee, J.D.; Wang, S.; Lu, S.; et al. Identification of QS-21 as an Inflammasome-activating Molecular Component of Saponin Adjuvants. J. Biol. Chem. 2016, 291, 1123–1136. [Google Scholar] [CrossRef] [PubMed]
- Demana, P.H.; Davies, N.M.; Vosgerau, U.; Rades, T. Pseudo-ternary phase diagrams of aqueous mixtures of Quil A, cholesterol and phospholipid prepared by the lipid-film hydration method. Int. J. Pharm. 2004, 270, 229–239. [Google Scholar] [CrossRef]
- Myschik, J.; Lendemans, D.G.; McBurney, W.T.; Demana, P.H.; Hook, S.; Rades, T. On the preparation, microscopic investigation and application of ISCOMs. Micron. 2006, 37, 724–734. [Google Scholar] [CrossRef] [PubMed]
- De Groot, C.; Müller-Goymann, C.C. Saponin Interactions with Model Membrane Systems-Langmuir Monolayer Studies, Hemolysis and Formation of ISCOMs. Planta Med. 2016, 82, 1496–1512. [Google Scholar] [CrossRef] [PubMed]
- Cibulski, S.P.; Mourglia-Ettlin, G.; Teixeira, T.F.; Quirici, L.; Roehe, P.M.; Ferreira, F.; Silveira, F. Novel ISCOMs from Quillaja brasiliensis saponins induce mucosal and systemic antibody production, T-cell responses and improved antigen uptake. Vaccine 2016, 34, 1162–1171. [Google Scholar] [CrossRef]
- De Costa, F.; Yendo, A.C.A.; Cibulski, S.P.; Fleck, J.D.; Roehe, P.M.; Spilki, F.R.; Gosmann, G.; Fett-Neto, A.G. Alternative inactivated poliovirus vaccines adjuvanted with Quillaja brasiliensis or Quil-A saponins are equally effective in inducing specific immune responses. PLoS ONE 2014, 9, e105374. [Google Scholar] [CrossRef] [PubMed]
- Silveira, F.; Cibulski, S.P.; Varela, A.P.; Marqués, J.M.; Chabalgoity, A.; de Costa, F.; Yendo, A.C.A.; Gosmann, G.; Roehe, P.M.; Fernández, C.; Ferreira, F. Quillaja brasiliensis saponins are less toxic than Quil A and have similar properties when used as an adjuvant for a viral antigen preparation. Vaccine 2011, 29, 9177–9182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fleck, J.D.; Kauffmann, C.; Spilki, F.; Lencina, C.L.; Roehe, P.M.; Gosmann, G. Adjuvant activity of Quillaja brasiliensis saponins on the immune responses to bovine herpesvirus type 1 in mice. Vaccine 2006, 24, 7129–7134. [Google Scholar] [CrossRef]
- Wallace, F.; Bennadji, Z.; Ferreira, F.; Olivaro, C. Analysis of an immunoadjuvant saponin fraction from Quillaja brasiliensis leaves by electrospray ionization ion trap multiple-stage mass spectrometry. Phytochem. Lett. 2017, 20, 228–233. [Google Scholar] [CrossRef]
- Kite, G.C.; Howes, M.J.R.; Simmonds, M.S.J. Metabolomic analysis of saponins in crude extracts of Quillaja saponaria by liquid chromatography/mass spectrometry for product authentication. Rapid Commun. Mass Spectrom. 2004, 18, 2859–2870. [Google Scholar] [CrossRef]
- Jacobsen, N.E.; Fairbrother, W.J.; Kensil, C.R.; Lim, A.; Wheeler, D.A.; Powell, M.F. Structure of the saponin adjuvant QS-21 and its base-catalyzed isomerization product by1H and natural abundance13C-NMR spectroscopy. Carbohydr. Res. 1996, 280, 1–14. [Google Scholar] [CrossRef]
- Kauffmann, C.; Machado, A.M.; Fleck, J.D.; Provensi, G.; Pires, V.S.; Guillaume, D.; Sonnet, P.; Reginatto, F.H.; Schenkel, E.P.; Gosmann, G. Constituents from leaves of Quillaja brasiliensis. Nat. Prod. Res. 2004, 18, 153–157. [Google Scholar] [CrossRef]
- Guo, S.; Lennart, K.; Lundgren, L.N.; Rönnberg, B.; Sundquist, B.G. Triterpenoid saponins from Quillaja saponaria. Phytochemistry 1998, 48, 175–180. [Google Scholar] [CrossRef]
- Guo, S.; Kenne, L. Structural studies of triterpenoid saponins with new acyl components from Quillaja saponaria Molina. Phytochemistry 2000, 55, 419–428. [Google Scholar] [CrossRef]
- Guo, S.; Kenne, L. Characterization of some O-acetylated saponins from Quillaja saponaria Molina. Phytochemistry 2000, 54, 615–623. [Google Scholar] [CrossRef]
- Nyberg, N.T.; Kenne, L.; Rönnberg, B.; Sundquist, B.G. Separation and structural analysis of some saponins from Quillaja saponaria Molina. Carbohydr. Res. 1999, 323, 87–97. [Google Scholar] [CrossRef]
- Nyberg, N.T.; Baumann, H.; Kenne, L. Solid-phase extraction NMR studies of chromatographic fractions of saponins from Quillaja saponaria. Anal. Chem. 2003, 75, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.M.; Byrd, J.A.; Cartwright, A.L.; Bailey, C.A. Hemolytic and Antimicrobial Activities Differ Among Saponin-rich Extracts From Guar, Quillaja, Yucca, and Soybean. Appl. Biochem. Biotechnol. 2010, 162, 1008–1017. [Google Scholar] [CrossRef] [PubMed]
- Makkar, H.P.S.; Sen, S.; Blümmel, M.; Becker, K. Effects of fractions containing saponins from Yucca schidigera, Quillaja saponaria, and Acacia auriculoformis on rumen fermentation. J. Agric. Food Chem. 1998, 46, 4324–4328. [Google Scholar] [CrossRef]
- Wallace, R.J.; Arthaud, L.; Newbold, C.J. Influence of Yucca shidigera extract on ruminal ammonia concentrations and ruminal microorganisms. Appl. Environ. Microbiol. 1994, 60, 1762–1767. [Google Scholar]
- Bangham, A.D.; Horne, R.W. Action of saponin on biological cell membranes. Nature 1962, 196, 952–955. [Google Scholar] [CrossRef]
- Oda, K.; Matsuda, H.; Murakami, T.; Katayama, S.; Ohgitani, T.; Yoshikawa, M. Adjuvant and haemolytic activities of 47 saponins derived from medicinal and food plants. Biol. Chem. 2000, 381, 67–74. [Google Scholar] [CrossRef]
- Baumann, E.; Stoya, G.; Vo, A.; Richter, W.; Lemke, C.; Linss, W. Hemolysis of human erythrocytes with saponin affects the membrane structure. Acta Histochem. 2000, 35. [Google Scholar] [CrossRef] [PubMed]
- Walther, R.U.; Padilla, L.; González, J.; Otero, R. Quillaja saponaria wood extract Refined processing and forestry management guarantee sustainability and ecological benefits. Compend. Deterg. 2011, 2, 3–4. [Google Scholar]
- Bachran, C.; Sutherland, M.; Heisler, I.; Hebestreit, P.; Melzig, M.F.; Fuchs, H. The saponin-mediated enhanced uptake of targeted saporin-based drugs is strongly dependent on the saponin structure. Exp. Biol. Med. 2006, 231, 412–420. [Google Scholar] [CrossRef]
- Arabski, M.; We, A.; Czerwonka, G.; Lankoff, A.; Kaca, W. Effects of Saponins against Clinical E. coli Strains and Eukariotic Cell Line. J. Biomed. Biotechnol. 2012, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Sewlikar, S.; D’Souza, D.H. Antimicrobial Effects of Quillaja saponaria Extract Against Escherichia coli O157:H7 and the Emerging Non-O157 Shiga Toxin-Producing E. coli. J. Food Sci. 2017, 82, 1171–1177. [Google Scholar] [CrossRef] [PubMed]
- Antolak, H.; Mizerska, U.; Berlowska, J.; Otlewska, A.; Krȩgiel, D. Quillaja saponaria Saponins with potential to enhance the effectiveness of disinfection processes in the beverage industry. Appl. Sci. 2018, 8, 368. [Google Scholar] [CrossRef]
- Yoshiki, Y.; Kudou, S.; Okubo, K. Relationship between Chemical Structures and Biological Activities of Triterpenoid Saponins from Soybean. Biosci. Biotechnol. Biochem 1998, 62, 2291–2299. [Google Scholar] [CrossRef] [Green Version]
- Patra, A.K.; Saxena, J. The effect and mode of action of saponins on the microbial populations and fermentation in the rumen and ruminant production. Nutr. Res. Rev. 2009, 22, 204–219. [Google Scholar] [CrossRef]
- Bei, L.; Hu, T.; Qian, Z.M.; Shen, X. Extracellular Ca2+ regulates the respiratory burst of human neutrophils. Biochim. Biophys. Acta 1998, 1404, 475–483. [Google Scholar] [CrossRef]
- Chapagain, B.P.; Wiesman, Z.; Tsror (Lahkim) L., L. In vitro study of the antifungal activity of saponin-rich extracts against prevalent phytopathogenic fungi. Ind. Crops Prod. 2007, 26, 109–115. [Google Scholar] [CrossRef]
- Grabensteiner, E.; Arshad, N.; Hess, M. Differences in the in vitro susceptibility of mono-eukaryotic cultures of Histomonas meleagridis, Tetratrichomonas gallinarum and Blastocystis sp. to natural organic compounds. Parasitol. Res. 2007, 101, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Rocha, T.D.; De Brum Vieira, P.; Gnoatto, S.C.B.; Tasca, T.; Gosmann, G. Anti-Trichomonas vaginalis activity of saponins from Quillaja, Passiflora, and Ilex species. Parasitol. Res. 2012, 110, 2551–2556. [Google Scholar] [CrossRef] [PubMed]
- Francis, G.; Kerem, Z.; Makkar, H.P.S.; Becker, K. The biological action of saponins in animal systems: A review. Br. J. Nutr. 2002, 88, 587–605. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-P. Saponins as Anticancer Agent. United States Pat. Appl. Publ. 2005, 2005/01756, 1–10. [Google Scholar]
- Ebbesen, P.; Dalsgaard, K. Prolonged survival of AKR mice treated with the Saponin adjuvant Quil A. Acta Path. Microbiol 1976, 84, 358–360. [Google Scholar] [CrossRef]
- Hu, K.; Berenjian, S.; Larsson, R.; Gullbo, J.; Nygren, P.; Lövgren, T.; Morein, B. Nanoparticulate Quillaja saponin induces apoptosis in human leukemia cell lines with a high therapeutic index. Int. J. Nanomedicine 2010, 5, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Ahmed Abdel-Reheim, M.; Messiha, B.A.S.; Abo-Saif, A.A. Quillaja saponaria bark saponin protects Wistar rats against ferrous sulphate-induced oxidative and inflammatory liver damage. Pharm. Biol. 2017, 55, 1972–1983. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.J.; Choi, J.H.; Kim, H.G.; Han, E.H.; Hwang, Y.P.; Lee, Y.C.; Chung, Y.C.; Jeong, H.G. Protective effect of saponins derived from the roots of Platycodon grandiflorum against carbon tetrachloride induced hepatotoxicity in mice. Food Chem. Toxicol. 2008, 46, 1778–1785. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Zheng, L.; Yin, L.; Xu, L.; Qi, Y.; Han, X.; Xu, Y.; Liu, K.; Peng, J. Protective effects of the total saponins from Dioscorea nipponica Makino against carbon tetrachloride-induced liver injury in mice through suppression of apoptosis and inflammation. Int. Immunopharmacol. 2014, 19, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Rao, D.; Gurfinkel, D. The bioactivity of saponins: Triterpenoid and steroidal glycosides. Drug Metab. Drug Interact 2000, 17, 211–236. [Google Scholar]
- Bomford, R.; Stapleton, M.; Winsor, S.; Beesley, J.E.; Jessup, E.A.; Price, K.R.; Fenwick, G.R. Adjuvanticity and ISCOM formation by structurally diverse saponins. Vaccine 1992, 10, 572–577. [Google Scholar] [CrossRef]
- van Setten, D.C.; van de Werken, G. Molecular Structures of Saponins from Quillaja saponaria Molina. In Saponins Used in Traditional and Modern Medicine; Waller, G.R., Yamasaki, K., Eds.; Springer: Boston, MA, USA, 1996; pp. 185–193. ISBN 978-1-4899-1367-8. [Google Scholar]
- Ríos, J.-L. Effects of triterpenes on the immune system. J. Ethnopharmacol. 2010, 128, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Callaway, J.B.; Ting, J.P.-Y. Inflammasomes: Mechanism of action, role in disease, and therapeutics. Nat. Med. 2015, 21, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Coffman, R.L.; Sher, A.; Seder, R.A. Vaccine Adjuvants: Putting Innate Immunity to Work. Immunity 2010, 33, 492–503. [Google Scholar] [CrossRef] [Green Version]
- Ahlberg, V.; Hjertner, B.; Wallgren, P.; Hellman, S.; Lövgren Bengtsson, K.; Fossum, C. Innate immune responses induced by the saponin adjuvant Matrix-M in specific pathogen free pigs. Vet. Res. 2017, 48, 30. [Google Scholar] [CrossRef] [PubMed]
- Podolak, I.; Galanty, A.; Sobolewska, D. Saponins as cytotoxic agents: A review. Phytochem. Rev. 2010, 9, 425–474. [Google Scholar] [CrossRef] [PubMed]
- Fernando, G.J.P.; Stewart, T.J.; Tindle, R.W.; Frazer, I.H. Vaccine-induced Th1-type responses are dominant over Th2-type responses in the short term whereas pre-existing Th2 responses are dominant in the longer term. Scand. J. Immunol. 1998, 47, 459–465. [Google Scholar] [CrossRef]
- Katayama, S.; Mine, Y. Quillaja saponin can modulate ovalbumin-induced IgE allergic responses through regulation of Th1/Th2 balance in a murine model. J. Agric. Food Chem. 2006, 54, 3271–3276. [Google Scholar] [CrossRef]
- Newman, M.J.; Wu, J.Y.; Gardner, B.H.; Anderson, C.A.; Kensil, C.R.; Recchia, J.; Coughlin, R.T.; Powell, M.F. Induction of cross-reactive cytotoxic T-lymphocyte responses specific for HIV-1 gp120 using saponin adjuvant (QS-21) supplemented subunit vaccine formulations. Vaccine 1997, 15, 1001–1007. [Google Scholar] [CrossRef]
- Marciani, D.J. Elucidating the Mechanisms of Action of Saponin-Derived Adjuvants. Trends Pharmacol. Sci. 2018, 39, 573–585. [Google Scholar] [CrossRef]
- Stills, H.F. Adjuvants and Antibody Production: Dispelling the Myths Associated with Freund’s Complete and Other Adjuvants. ILAR J. 2005, 46, 280–293. [Google Scholar] [CrossRef] [PubMed]
- Awate, S.; Babiuk, L.A.; Mutwiri, G. Mechanisms of action of adjuvants. Front. Immunol. 2013, 4, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Barr, I.G.; Sjölander, A.; Cox, J.C. ISCOMs and other saponin based adjuvants. Adv. Drug Deliv. Rev. 1998, 32, 247–271. [Google Scholar] [CrossRef]
- Garcia, A.; Lema, D. An Updated Review of ISCOMSTM and ISCOMATRIXTM Vaccines. Curr. Pharm. Des. 2016, 22, 6294–6299. [Google Scholar] [CrossRef]
- Sjölander, A.; Cox, J.C.; Barr, I.G. ISCOMs: An adjuvant with multiple functions. J. Leukoc. Biol. 1998, 64, 713–723. [Google Scholar] [CrossRef]
- Karandikar, S.; Mirani, A.; Waybhase, V.; Patravale, V.B.; Patankar, S. Nanovaccines for Oral Delivery-Formulation Strategies and Challenges; Elsevier Inc.: London, UK, 2017; ISBN 9780323477208. [Google Scholar]
- Garçon, N.; Chomez, P.; Van Mechelen, M. GlaxoSmithKline Adjuvant Systems in vaccines: Concepts, achievements and perspectives. Expert Rev. Vaccines 2007, 6, 723–739. [Google Scholar] [CrossRef]
- Garg, R.; Babiuk, L.; van Drunen Littel-van den Hurk, S.; Gerdts, V. A novel combination adjuvant platform for human and animal vaccines. Vaccine 2017, 35, 4486–4489. [Google Scholar] [CrossRef]
- Lacaille-Dubois, M.A.; Wagner, H. A review of the biological and pharmacological activities of saponins. Phytomedicine 1996, 2, 363–386. [Google Scholar] [CrossRef]
- Slovin, S.F.; Ragupathi, G.; Fernandez, C.; Jefferson, M.P.; Diani, M.; Wilton, A.S.; Powell, S.; Spassova, M.; Reis, C.; Clausen, H.; et al. A bivalent conjugate vaccine in the treatment of biochemically relapsed prostate cancer: A study of glycosylated MUC-2-KLH and Globo H-KLH conjugate vaccines given with the new semi-synthetic saponin immunological adjuvant GPI-0100 OR QS-21. Vaccine 2005, 23, 3114–3122. [Google Scholar] [CrossRef]
- Jansen, C.; Kuipers, B.; Van Der Biezen, J.; De Cock, H.; Van Der Ley, P.; Tommassen, J. Immunogenicity of in vitro folded outer membrane protein PorA of Neisseria meningitidis. FEMS Immunol. Med. Microbiol. 2000, 27, 227–233. [Google Scholar] [CrossRef] [Green Version]
- Shu, Q.; Bird, S.H.; Gill, H.S.; Duan, E.; Xu, Y.; Hillard, M.A.; Rowe, J.B.; Industry, B.; Health, H.; North, P.; et al. Antibody Response in Sheep Following Immunization with Streptococcus bovis in Different Adjuvants. Vet. Res. Commun. 2001, 25, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Da Fonseca, D.P.A.J.; Frerichs, J.; Singh, M.; Snippe, H.; Verheul, A.F.M. Induction of antibody and T-cell responses by immunization with ISCOMS containing the 38-kilodalton protein of Mycobacterium tuberculosis. Vaccine 2000, 19, 122–131. [Google Scholar] [CrossRef]
- Boyaka, P.N.; Marinaro, M.; Jackson, R.J.; van Ginkel, F.W.; Cormet-Boyaka, E.; Kirk, K.L.; Kensil, C.R.; McGhee, J.R. Oral QS-21 requires early IL-4 help for induction of mucosal and systemic immunity. J. Immunol. 2001, 166, 2283–2290. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Malhotra, D.V.; Dhar, S.; Nichani, A.K. Vaccination of donkeys against Babesia equi using killed merozoite immunogen. Vet. Parasitol. 2002, 106, 19–33. [Google Scholar] [CrossRef]
- Liu, G.; Anderson, C.; Scaltreto, H.; Barbon, J.; Kensil, C.R. QS-21 structure/function studies: Effect of acylation on adjuvant activity. Vaccine 2002, 20, 2808–2815. [Google Scholar] [CrossRef]
- Borja-Cabrera, G.P.; Correia Pontes, N.N.; Da Silva, V.O.; Paraguai De Souza, E.; Santos, W.R.; Gomes, E.M.; Luz, K.G.; Palatnik, M.; Palatnik De Sousa, C.B. Long lasting protection against canine kala-azar using the FML-QuilA saponin vaccine in an endemic area of Brazil (São Gonçalo do Amarante, RN). Vaccine 2002, 20, 3277–3284. [Google Scholar] [CrossRef]
- Stittelaar, K.J.; Vos, H.W.; Van Amerongen, G.; Kersten, G.F.A.; Osterhaus, A.D.M.E.; De Swart, R.L. Longevity of neutralizing antibody levels in macaques vaccinated with Quil A-adjuvanted measles vaccine candidates. Vaccine 2002, 21, 155–157. [Google Scholar] [CrossRef]
- Marciani, D.J.; Ptak, R.G.; Voss, T.G.; Reynolds, R.C.; Pathak, A.K.; Chamblin, T.L.; Scholl, D.R.; May, R.D. Corrigendum to “Degradation of Quillaja saponaria Molina saponins: Loss of the protective effects of a herpes simplex virus 1 subunit vaccine” [International Immunopharmacology 2/12 (2002) 1703–1711]. Int. Immunopharmacol. 2005, 5, 1658. [Google Scholar] [CrossRef]
- De Jonge, M.I.; Vidarsson, G.; Van Dijken, H.H.; Hoogerhout, P.; Van Alphen, L.; Dankert, J.; Van der Ley, P. Functional activity of antibodies against the recombinant OpaJ protein from Neisseria meningitidis. Infect. Immun. 2003, 71, 2331–2340. [Google Scholar] [CrossRef]
- Zhang, P.; Yang, Q.B.; Marciani, D.J.; Martin, M.; Clements, J.D.; Michalek, S.M.; Katz, J. Effectiveness of the quillaja saponin semi-synthetic analog GPI-0100 in potentiating mucosal and systemic responses to recombinant HagB from Porphyromonas gingivalis. Vaccine 2003, 21, 4459–4471. [Google Scholar] [CrossRef]
- Borja-Cabrera, G.P.; Mendes, A.C.; Paraguai De Souza, E.; Okada, L.Y.H.; Trivellato, F.A.D.A.; Kawasaki, J.K.A.; Costa, A.C.; Reis, A.B.; Genaro, O.; Batista, L.M.M.; et al. Effective immunotherapy against canine visceral leishmaniasis with the FML-vaccine. Vaccine 2004, 22, 2234–2243. [Google Scholar] [CrossRef] [PubMed]
- Regner, M.; Culley, F.; Fontannaz, P.; Hu, K.; Morein, B.; Lambert, P.H.; Openshaw, P.; Siegrist, C.A. Safety and efficacy of immune-stimulating complex-based antigen delivery systems for neonatal immunisation against respiratory syncytial virus infection. Microbes Infect. 2004, 6, 666–675. [Google Scholar] [CrossRef] [PubMed]
- Palatnik De Sousa, C.B.; Santos, W.R.; Casas, C.P.; Paraguai De Souza, E.; Tinoco, L.W.; Da Silva, B.P.; Palatnik, M.; Parente, J.P. Protective vaccination against murine visceral leishmaniasis using aldehyde-containing Quillaja saponaria sapogenins. Vaccine 2004, 22, 2470–2479. [Google Scholar] [CrossRef] [PubMed]
- Demana, P.H.; Fehske, C.; White, K.; Rades, T.; Hook, S. Effect of incorporation of the adjuvant Quil A on structure and immune stimulatory capacity of liposomes. Immunol. Cell Biol. 2004, 82, 547–554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skeiky, Y.A.W.; Alderson, M.R.; Ovendale, P.J.; Guderian, J.A.; Brandt, L.; Dillon, D.C.; Campos-Neto, A.; Lobet, Y.; Dalemans, W.; Orme, I.M.; et al. Differential Immune Responses and Protective Efficacy Induced by Components of a Tuberculosis Polyprotein Vaccine, Mtb72F, Delivered as Naked DNA or Recombinant Protein. J. Immunol. 2004, 172, 7618–7628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meraldi, V.; Romero, J.F.; Kensil, C.; Corradin, G. A strong CD8+T cell response is elicited using the synthetic polypeptide from the C-terminus of the circumsporozoite protein of Plasmodium berghei together with the adjuvant QS-21: Quantitative and phenotypic comparison with the vaccine model of irradiate. Vaccine 2005, 23, 2801–2812. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.F.; Regner, M.; Siegrist, C.A.; Lambert, P.; Chen, M.; Bengtsson, K.L.; Morein, B. The immunomodulating properties of human respiratory syncytial virus and immunostimulating complexes containing Quillaja saponin components QH-A, QH-C and ISCOPREPTM703. FEMS Immunol. Med. Microbiol. 2005, 43, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Cristillo, A.D.; Wang, S.; Caskey, M.S.; Unangst, T.; Hocker, L.; He, L.; Hudacik, L.; Whitney, S.; Keen, T.; Chou, T.H.W.; et al. Preclinical evaluation of cellular immune responses elicited by a polyvalent DNA prime/protein boost HIV-1 vaccine. Virology 2006, 346, 151–168. [Google Scholar] [CrossRef]
- Oliveira-Freitas, E.; Casas, C.P.; Borja-Cabrera, G.P.; Santos, F.N.; Nico, D.; Souza, L.O.P.; Tinoco, L.W.; da Silva, B.P.; Palatnik, M.; Parente, J.P.; et al. Acylated and deacylated saponins of Quillaja saponaria mixture as adjuvants for the FML-vaccine against visceral leishmaniasis. Vaccine 2006, 24, 3909–3920. [Google Scholar] [CrossRef]
- Pickering, R.J.; Smith, S.D.; Strugnell, R.A.; Wesselingh, S.L.; Webster, D.E. Crude saponins improve the immune response to an oral plant-made measles vaccine. Vaccine 2006, 24, 144–150. [Google Scholar] [CrossRef]
- López-Abán, J.; Casanueva, P.; Nogal, J.; Arias, M.; Morrondo, P.; Diez-Baños, P.; Hillyer, G.V.; Martínez-Fernández, A.R.; Muro, A. Progress in the development of Fasciola hepatica vaccine using recombinant fatty acid binding protein with the adjuvant adaptation system ADAD. Vet. Parasitol. 2007, 145, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Parra, L.E.; Borja-Cabrera, G.P.; Santos, F.N.; Souza, L.O.P.; Palatnik-de-Sousa, C.B.; Menz, I. Safety trial using the Leishmune®vaccine against canine visceral leishmaniasis in Brazil. Vaccine 2007, 25, 2180–2186. [Google Scholar] [CrossRef] [PubMed]
- Quenelle, D.C.; Collins, D.J.; Marciani, D.J.; Kern, E.R. Effect of immunization with herpes simplex virus type-1 (HSV-1) glycoprotein D (gD) plus the immune enhancer GPI-0100 on infection with HSV-1 or HSV-2. Vaccine 2006, 24, 1515–1522. [Google Scholar] [CrossRef]
- Deng, K.; Adams, M.M.; Damani, P.; Livingston, P.O.; Ragupathi, G.; Gin, D.Y. Synthesis of QS-21-xylose: Establishment of the immunopotentiating activity of synthetic QS-21 adjuvant with a melanoma vaccine. Angew. Chemie Int. Ed. 2008, 47, 6395–6398. [Google Scholar] [CrossRef] [PubMed]
- Kaba, S.A.; Price, A.; Zhou, Z.; Sundaram, V.; Schnake, P.; Goldman, I.F.; Lal, A.A.; Udhayakumar, V.; Todd, C.W. Immune responses of mice with different genetic backgrounds to improved multiepitope, multitarget malaria vaccine candidate antigen FALVAC-1A. Clin. Vaccine Immunol. 2008, 15, 1674–1683. [Google Scholar] [CrossRef] [PubMed]
- Borja-Cabrera, G.P.; Santos, F.N.; Bauer, F.S.; Parra, L.E.; Menz, I.; Morgado, A.A.; Soares, I.S.; Batista, L.M.M.; Palatnik-de-Sousa, C.B. Immunogenicity assay of the Leishmune®vaccine against canine visceral leishmaniasis in Brazil. Vaccine 2008, 26, 4991–4997. [Google Scholar] [CrossRef] [PubMed]
- Quenelle, D.C.; Collins, D.J.; Rice, T.L.; Prichard, M.N.; Marciani, D.J.; Kern, E.R. Effect of an immune enhancer, GPI-0100, on vaccination with live attenuated herpes simplex virus (HSV) type 2 or glycoprotein D on genital HSV-2 infections of guinea pigs. Antiviral Res. 2008, 80, 223–224. [Google Scholar] [CrossRef] [Green Version]
- Radošević, K.; Rodriguez, A.; Mintardjo, R.; Tax, D.; Bengtsson, K.L.; Thompson, C.; Zambon, M.; Weverling, G.J.; UytdeHaag, F.; Goudsmit, J. Antibody and T-cell responses to a virosomal adjuvanted H9N2 avian influenza vaccine: Impact of distinct additional adjuvants. Vaccine 2008, 26, 3640–3646. [Google Scholar] [CrossRef]
- Skene, C.D.; Doidge, C.; Sutton, P. Evaluation of ISCOMATRIXTM and ISCOMTM vaccines for immunisation against Helicobacter pylori. Vaccine 2008, 26, 3880–3884. [Google Scholar] [CrossRef]
- Karanam, B.; Gambhira, R.; Peng, S.; Jagu, S.; Kim, D.-J.; Ketner, G.W.; Stern, P.L.; Adams, R.J.; Roden, R.B.S. Vaccination with HPV16 L2E6E7 fusion protein in GPI-0100 adjuvant elicits protective humoral and cell-mediated immunity. Vaccine 2009, 27, 1040–1049. [Google Scholar] [CrossRef] [Green Version]
- Buendía, A.J.; Ortega, N.; Caro, M.R.; Del Río, L.; Gallego, M.C.; Sánchez, J.; Navarro, J.A.; Cuello, F.; Salinas, J. B cells are essential for moderating the inflammatory response and controlling bacterial multiplication in a mouse model of vaccination against Chlamydophila abortus infection. Infect. Immun. 2009, 77, 4868–4876. [Google Scholar] [CrossRef] [PubMed]
- Ragupathi, G.; Damani, P.; Srivastava, G.; Srivastava, O.; Sucheck, S.J.; Ichikawa, Y.; Livingston, P.O. Synthesis of sialyl Lewisa (sLea, CA19-9) and construction of an immunogenic sLea vaccine. Cancer Immunol. Immunother. 2009, 58, 1397–1405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tafaghodi, M.; Rastegar, S. Preparation and in vivo study of dry powder microspheres for nasal immunization. J. Drug Target. 2010, 18, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Duewell, P.; Kisser, U.; Heckelsmiller, K.; Hoves, S.; Stoitzner, P.; Koernig, S.; Morelli, A.B.; Clausen, B.E.; Dauer, M.; Eigler, A.; et al. ISCOMATRIX Adjuvant Combines Immune Activation with Antigen Delivery to Dendritic Cells In Vivo Leading to Effective Cross-Priming of CD8+ T Cells. J. Immunol. 2011, 187, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Mohaghegh, M.; Tafaghodi, M. Dextran microspheres could enhance immune responses against PLGA nanospheres encapsulated with tetanus toxoid and Quillaja saponins after nasal immunization in rabbit. Pharm. Dev. Technol. 2011, 16, 36–43. [Google Scholar] [CrossRef]
- Tafaghodi, M.; Khamesipour, A.; Jaafari, M.R. Immunization against leishmaniasis by PLGA nanospheres encapsulated with autoclaved Leishmania major (ALM) and CpG-ODN. Parasitol. Res. 2011, 108, 1265–1273. [Google Scholar] [CrossRef]
- Ahmed, F.K.; Clark, B.E.; Burton, D.R.; Pantophlet, R. An engineered mutant of HIV-1 gp120 formulated with adjuvant Quil A promotes elicitation of antibody responses overlapping the CD4-binding site. Vaccine 2012, 30, 922–930. [Google Scholar] [CrossRef] [Green Version]
- Ariaee, F.M.; Tafaghodia, M. Mucosal adjuvant potential of Quillaja saponins and cross-linked dextran microspheres, co-administered with liposomes encapsulated with tetanus toxoid. Iran. J. Pharm. Res. 2012, 11, 723–732. [Google Scholar]
- Reimer, J.M.; Karlsson, K.H.; Lövgren-Bengtsson, K.; Magnusson, S.E.; Fuentes, A.; Stertman, L. Matrix-mTM adjuvant induces local recruitment, activation and maturation of central immune cells in absence of antigen. PLoS ONE 2012, 7, e41451. [Google Scholar] [CrossRef]
- da Cunha, I.A.L.; Zulpo, D.L.; Bogado, A.L.G.; de Barros, L.D.; Taroda, A.; Igarashi, M.; Navarro, I.T.; Garcia, J.L. Humoral and cellular immune responses in pigs immunized intranasally with crude rhoptry proteins of Toxoplasma gondii plus Quil-A. Vet. Parasitol. 2012, 186, 216–221. [Google Scholar] [CrossRef] [Green Version]
- Barhate, G.; Gautam, M.; Gairola, S.; Jadhav, S.; Pokharkar, V. Quillaja saponaria extract as mucosal adjuvant with chitosan functionalized gold nanoparticles for mucosal vaccine delivery: Stability and immunoefficiency studies. Int. J. Pharm. 2013, 441, 636–642. [Google Scholar] [CrossRef]
- Buglione-Corbett, R.; Pouliot, K.; Marty-Roix, R.; West, K.; Wang, S.; Lien, E.; Lu, S. Serum Cytokine Profiles Associated with Specific Adjuvants Used in a DNA Prime-Protein Boost Vaccination Strategy. PLoS ONE 2013, 8, e74820. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Tejada, A.; Chea, E.K.; George, C.; Gardner, J.R.; Livingston, P.O.; Ragupathi, G.; Tan, D.S.; Gin, D.Y. Design, synthesis, and immunologic evaluation of vaccine adjuvant conjugates based on QS-21 and tucaresol. Bioorg. Med. Chem. 2014, 22, 5917–5923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Didierlaurent, A.M.; Collignon, C.; Bourguignon, P.; Wouters, S.; Fierens, K.; Fochesato, M.; Dendouga, N.; Langlet, C.; Malissen, B.; Lambrecht, B.N.; et al. Enhancement of Adaptive Immunity by the Human Vaccine Adjuvant AS01 Depends on Activated Dendritic Cells. J. Immunol. 2014, 193, 1920–1930. [Google Scholar] [CrossRef] [Green Version]
- Selenica, M.L.B.; Davtyan, H.; Housley, S.B.; Blair, L.J.; Gillies, A.; Nordhues, B.A.; Zhang, B.; Liu, J.; Gestwicki, J.E.; Lee, D.C.; et al. Epitope analysis following active immunization with tau proteins reveals immunogens implicated in tau pathogenesis. J. Neuroinflammation 2014, 11, 1–12. [Google Scholar] [CrossRef]
- Dye, J.M.; Warfield, K.L.; Wells, J.B.; Unfer, R.C.; Shulenin, S.; Vu, H.; Nichols, D.K.; Aman, M.J.; Bavari, S. Virus-like particle vaccination protects nonhuman primates from lethal aerosol exposure with marburgvirus (VLP vaccination protects macaques against aerosol challenges). Viruses 2016, 8, 94. [Google Scholar] [CrossRef] [PubMed]
- Konduru, K.; Shurtleff, A.C.; Bradfute, S.B.; Nakamura, S.; Bavari, S.; Kaplan, G. Ebolavirus Glycoprotein Fc Fusion Protein Protects Guinea Pigs against Lethal Challenge. PLoS ONE 2016, 11, e0162446. [Google Scholar] [CrossRef] [PubMed]
- Ng, H.I.; Fernando, G.J.P.; Depelsenaire, A.C.I.; Kendall, M.A.F. Potent response of QS-21 as a vaccine adjuvant in the skin when delivered with the Nanopatch, resulted in adjuvant dose sparing. Sci. Rep. 2016, 6, 29368. [Google Scholar] [CrossRef] [PubMed]
- Detienne, S.; Welsby, I.; Collignon, C.; Wouters, S.; Coccia, M.; Delhaye, S.; Van Maele, L.; Thomas, S.; Swertvaegher, M.; Detavernier, A.; et al. Central role of CD169+lymph node resident macrophages in the adjuvanticity of the QS-21 component of AS01. Sci. Rep. 2016, 6, 1–14. [Google Scholar] [CrossRef]
- Rivera, F.; Espino, A.M. Adjuvant-enhanced antibody and cellular responses to inclusion bodies expressing FhSAP2 correlates with protection of mice to Fasciola hepatica. Exp. Parasitol. 2016, 160, 31–38. [Google Scholar] [CrossRef] [Green Version]
- Cibulski, S.P.; Silveira, F.; Mourglia-Ettlin, G.; Teixeira, T.F.; dos Santos, H.F.; Yendo, A.C.; de Costa, F.; Fett-Neto, A.G.; Gosmann, G.; Roehe, P.M. Quillaja brasiliensis saponins induce robust humoral and cellular responses in a bovine viral diarrhea virus vaccine in mice. Comp. Immunol. Microbiol. Infect. Dis. 2016, 45, 1–8. [Google Scholar] [CrossRef]
- Lambracht-Washington, D.; Fu, M.; Frost, P.; Rosenberg, R.N. Evaluation of a DNA Aβ42 vaccine in adult rhesus monkeys (Macaca mulatta): Antibody kinetics and immune profile after intradermal immunization with full-length DNA Aβ42 trimer. Alzheimer’s Res. Ther. 2017, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Welsby, I.; Detienne, S.; N’Kuli, F.; Thomas, S.; Wouters, S.; Bechtold, V.; De Wit, D.; Gineste, R.; Reinheckel, T.; Elouahabi, A.; et al. Lysosome-dependent activation of human dendritic cells by the vaccine adjuvant QS-21. Front. Immunol. 2017, 7, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Genito, C.J.; Beck, Z.; Phares, T.W.; Kalle, F.; Limbach, K.J.; Stefaniak, M.E.; Patterson, N.B.; Bergmann-Leitner, E.S.; Waters, N.C.; Matyas, G.R.; et al. Liposomes containing monophosphoryl lipid A and QS-21 serve as an effective adjuvant for soluble circumsporozoite protein malaria vaccine FMP013. Vaccine 2017, 35, 3865–3874. [Google Scholar] [CrossRef] [PubMed]
- Poirier, D.; Renaud, F.; Dewar, V.; Strodiot, L.; Wauters, F.; Janimak, J.; Shimada, T.; Nomura, T.; Kabata, K.; Kuruma, K.; et al. Hepatitis B surface antigen incorporated in dissolvable microneedle array patch is antigenic and thermostable. Biomaterials 2017, 145, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Cibulski, S.P.; Rivera-Patron, M.; Mourglia-Ettlin, G.; Casaravilla, C.; Yendo, A.C.A.; Fett-Neto, A.G.; Chabalgoity, J.A.; Moreno, M.; Roehe, P.M.; Silveira, F. Quillaja brasiliensis saponin-based nanoparticulate adjuvants are capable of triggering early immune responses. Sci. Rep. 2018, 8, 13582. [Google Scholar] [CrossRef]
- Wenbin Tuo, D.Z. QS-21: A Potent Vaccine Adjuvant. Nat. Prod. Chem. Res. 2015, 03. [Google Scholar] [CrossRef]
- Lacaille-Dubois, M.A.; Wagner, H. New perspectives for natural triterpene glycosides as potential adjuvants. Phytomedicine 2017, 37, 49–57. [Google Scholar] [CrossRef]
- Cibulski, S.; Rivera-Patron, M.; Suárez, N.; Pirez, M.; Rossi, S.; Yendo, A.C.; de Costa, F.; Gosmann, G.; Fett-Neto, A.; Roehe, P.M.; Silveira, F. Leaf saponins of Quillaja brasiliensis enhance long-term specific immune responses and promote dose-sparing effect in BVDV experimental vaccines. Vaccine 2018, 36, 55–65. [Google Scholar] [CrossRef]
- Naknukool, S.; Horinouchi, I.; Hatta, H. Stimulating Macrophage Activity in Mice and Humans by Oral Administration of Quillaja Saponin. Biosci. Biotechnol. Biochem. 2011, 75, 1889–1893. [Google Scholar] [CrossRef] [Green Version]
- Gilewski, T.; Ragupathi, G.; Bhuta, S.; Williams, L.J.; Musselli, C.; Zhang, X.-F.; Bencsath, K.P.; Panageas, K.S.; Chin, J.; Hudis, C.A.; et al. Immunization of metastatic breast cancer patients with a fully synthetic globo H conjugate: A phase I trial. Proc. Natl. Acad. Sci. 2001, 98, 3270–3275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krug, L.M.; Ragupathi, G.; Ng, K.K.; Hood, C.; Jennings, H.J.; Guo, Z.; Kris, M.G.; Miller, V.; Pizzo, B.; Tyson, L.; et al. Vaccination of Small Cell Lung Cancer Patients with Polysialic Acid or N-Propionylated Polysialic Acid Conjugated to Keyhole Limpet Hemocyanin. Clin. Cancer Res. 2004, 10, 916–923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thera, M.A.; Doumbo, O.K.; Coulibaly, D.; Diallo, D.A.; Sagara, I.; Dicko, A.; Diemert, D.J.; Heppner, D.G.; Stewart, V.A.; Angov, E.; et al. Safety and Allele-Specific Immunogenicity of a Malaria Vaccine in Malian Adults: Results of a Phase I Randomized Trial. PLoS Clin. Trials 2006, 1, e34. [Google Scholar] [CrossRef] [PubMed]
- Mbawuike, I.; Zang, Y.; Couch, R.B. Humoral and cell-mediated immune responses of humans to inactivated influenza vaccine with or without QS21 adjuvant. Vaccine 2007, 25, 3263–3269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, T.G.; McElrath, M.J.; Matthews, T.; Montefiori, D.; Weinhold, K.; Wolff, M.; Keefer, M.C.; Kallas, E.G.; Corey, L.; Gorse, G.J.; et al. QS-21 promotes an adjuvant effect allowing for reduced antigen dose during HIV-1 envelope subunit immmunization in humans. Vaccine 2001, 19, 2080–2091. [Google Scholar] [CrossRef]
- Leroux-Roels, G.; Van Belle, P.; Vandepapeliere, P.; Horsmans, Y.; Janssens, M.; Carletti, I.; Garçon, N.; Wettendorff, M.; Van Mechelen, M. Vaccine Adjuvant Systems containing monophosphoryl lipid A and QS-21 induce strong humoral and cellular immune responses against hepatitis B surface antigen which persist for at least 4 years after vaccination. Vaccine 2015, 33, 1084–1091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krug, L.M.; Ragupathi, G.; Hood, C.; George, C.; Hong, F.; Shen, R.; Abrey, L.; Jennings, H.J.; Kris, M.G.; Livingston, P.O. Immunization with N-propionyl polysialic acid–KLH conjugate in patients with small cell lung cancer is safe and induces IgM antibodies reactive with SCLC cells and bactericidal against group B meningococci. Cancer Immunol. Immunother. 2012, 61, 9–18. [Google Scholar] [CrossRef]
- Kruit, W.H.J.; Suciu, S.; Dreno, B.; Mortier, L.; Robert, C.; Chiarion-Sileni, V.; Maio, M.; Testori, A.; Dorval, T.; Grob, J.J.; et al. Selection of immunostimulant AS15 for active immunization with MAGE-A3 protein: Results of a randomized phase II study of the European organisation for research and treatment of cancer melanoma group in metastatic melanoma. J. Clin. Oncol. 2013, 31, 2413–2420. [Google Scholar] [CrossRef]
- Vandepapelière, P.; Rehermann, B.; Koutsoukos, M.; Moris, P.; Garçon, N.; Wettendorff, M.; Leroux-Roels, G. Potent enhancement of cellular and humoral immune responses against recombinant hepatitis B antigens using AS02A adjuvant in healthy adults. Vaccine 2005, 23, 2591–2601. [Google Scholar] [CrossRef]
- Kester, K.E.; McKinney, D.A.; Tornieporth, N.; Ockenhouse, C.F.; Heppner, D.G.; Hall, T.; Wellde, B.T.; White, K.; Sun, P.; Schwenk, R.; et al. A phase I/IIa safety, immunogenicity, and efficacy bridging randomized study of a two-dose regimen of liquid and lyophilized formulations of the candidate malaria vaccine RTS,S/AS02A in malaria-naïve adults. Vaccine 2007, 25, 5359–5366. [Google Scholar] [CrossRef]
- Roestenberg, M.; Remarque, E.; de Jonge, E.; Hermsen, R.; Blythman, H.; Leroy, O.; Imoukhuede, E.; Jepsen, S.; Ofori-Anyinam, O.; Faber, B.; et al. Safety and immunogenicity of a recombinant Plasmodium falciparum AMA1 malaria vaccine adjuvanted with AlhydrogelTM, Montanide ISA 720 or AS02. PLoS ONE 2008, 3, e3960. [Google Scholar] [CrossRef] [PubMed]
- Sacarlal, J.; Aponte, J.J.; Aide, P.; Mandomando, I.; Bassat, Q.; Guinovart, C.; Leach, A.; Milman, J.; Macete, E.; Espasa, M.; et al. Safety of the RTS,S/AS02A malaria vaccine in Mozambican children during a Phase IIb trial. Vaccine 2008, 26, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Thera, M.A.; Doumbo, O.K.; Coulibaly, D.; Diallo, D.A.; Kone, A.K.; Guindo, A.B.; Traore, K.; Dicko, A.; Sagara, I.; Sissoko, M.S.; et al. Safety and immunogenicity of an AMA-1 malaria vaccine in Malian adults: Results of a phase 1 randomized controlled trial. PLoS ONE 2008, 3, e1465. [Google Scholar] [CrossRef] [PubMed]
- O’Cearbhaill, R.E.; Ragupathi, G.; Zhu, J.; Wan, Q.; Mironov, S.; Yang, G.; Spassova, M.K.; Iasonos, A.; Kravetz, S.; Ouerfelli, O.; et al. A phase i study of unimolecular pentavalent (Globo-H-GM2-sTn-TF-Tn) immunization of patients with epithelial ovarian, fallopian tube, or peritoneal cancer in first remission. Cancers (Basel) 2016, 8, 46. [Google Scholar] [CrossRef]
- Coccia, M.; Collignon, C.; Hervé, C.; Chalon, A.; Welsby, I.; Detienne, S.; Van Helden, M.J.; Dutta, S.; Genito, C.J.; Waters, N.C.; et al. Cellular and molecular synergy in AS01-adjuvanted vaccines results in an early IFNγ response promoting vaccine immunogenicity. npj Vaccines 2017, 2. [Google Scholar] [CrossRef] [PubMed]
- Rönnberg, B.; Fekadu, M.; Morein, B. Adjuvant activity of non-toxic Quillaja saponaria Molina components for use in ISCOM matrix. Vaccine 1995, 13, 1375–1382. [Google Scholar] [CrossRef]
- Marciani, D.J.; Pathak, A.K.; Reynolds, R.C.; Seitz, L.; May, R.D. Altered immunomodulating and toxicological properties of degraded Quillaja saponaria Molina saponins. Int. Immunopharmacol. 2001, 1, 813–818. [Google Scholar] [CrossRef]
- Rönnberg, B.; Fekadu, M.; Behboudi, S.; Kenne, L.; Morein, B. Effects of carbohydrate modification of Quillaja saponaria Molina QH-B fraction on adjuvant activity, cholesterol-binding capacity and toxicity. Vaccine 1997, 15, 1820–1826. [Google Scholar] [CrossRef]
- Abdulla, S.; Oberholzer, R.; Juma, O.; Kubhoja, S.; Machera, F.; Membi, C.; Omari, S.; Urassa, A.; Mshinda, H.; Jumanne, A.; et al. Safety and Immunogenicity of RTS,S/AS02D Malaria Vaccine in Infants. N. Engl. J. Med. 2008, 359, 2533–2544. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.; Cao, Y.; von Gersdorff Jørgensen, L.; Strobel, B.W.; Hansen, H.C.B.; Cedergreen, N. Where does the toxicity come from in saponin extract? Chemosphere 2018, 204, 243–250. [Google Scholar] [CrossRef]
- de Koning, C.; Beekhuijzen, M.; Tobor-Kapłon, M.; de Vries-Buitenweg, S.; Schoutsen, D.; Leeijen, N.; van de Waart, B.; Emmen, H. Visualizing Compound Distribution during Zebrafish Embryo Development: The Effects of Lipophilicity and DMSO. Birth Defects Res. Part B Dev. Reprod. Toxicol. 2015, 104, 253–272. [Google Scholar] [CrossRef] [PubMed]
- Vinay, T.N.; Park, C.S.; Kim, H.Y.; Jung, S.J. Toxicity and dose determination of quillaja saponin, aluminum hydroxide and squalene in olive flounder (Paralichthys olivaceus). Vet. Immunol. Immunopathol. 2014, 158, 73–85. [Google Scholar] [CrossRef] [PubMed]



| Nº | Saponin | Mi | Structure | Ref. | |||||
|---|---|---|---|---|---|---|---|---|---|
| Aglycone | R0 | R1 | R2 | R3 | R4 | ||||
| 1 | DS-1 | 1512.0 | Q | Xyl | Api-Xyl | H | H | [3] | |
| 2 | DS-2 | 1674.0 | Q | Xyl | Api-Xyl | H | Glc | [3] | |
| 3 | * | 824.0 | Q | H | - | - | - | - | [33] |
| 4 | * | 970.0 | Q | Rha | - | - | - | - | [33] |
| 5 | * | 956.0 | Q | Xyl | - | - | - | - | [34] |
| 6 | 4 | 1436.6 | Q | H | Xyl | H | Rha | Ac | [34] |
| 7 | 5 | 1582.7 | Q | Rha | Xyl | H | Rha | Ac | [34] |
| 8 | 6 | 1568.7 | Q | Xyl | Xyl | H | Rha | Ac | [34] |
| 9 | 7 | 1714.7 | Q | Rha | Xyl-Api | H | Rha | Ac | [34] |
| 10 | 8 | 1700.7 | Q | Xyl | Xyl-Api | H | Rha | Ac | [34] |
| 11 | 9 | 1714.7 | Q | Rha | Xyl-Xyl | H | Rha | Ac | [34] |
| 12 | 10 | 1700.7 | Q | Xyl | Xyl-Xyl | H | Rha | Ac | [34] |
| 13 | 11a | 1598.7 | Q | Rha | Xyl | H | Glc | Ac | [35] |
| 14 | 11b | 1584.7 | Q | Xyl | Xyl | H | Glc | Ac | [35] |
| 15 | 12a | 1628.7 | Q | Rha | H | Glc | Glc | Ac | [35] |
| 16 | 12b | 1614.7 | Q | Xyl | H | Glc | Glc | Ac | [35] |
| 17 | 13a | 1730.7 | Q | Rha | Xyl-Api | Glc | H | Ac | [35] |
| 18 | 13b | 1716.7 | Q | Xyl | Xyl-Api | Glc | H | Ac | [35] |
| 19 | 14a | 1760.8 | Q | Rha | Xyl | Glc | Glc | Ac | [35] |
| 20 | 14b | 1746.7 | Q | Xyl | Xyl | Glc | Glc | Ac | [35] |
| 21 | 15a | 1640.7 | Q | Rha | Xyl | H | Glc-Ac | Ac | [35] |
| 22 | 15b | 1626.7 | Q | Xyl | Xyl | H | Glc-Ac | Ac | [35] |
| 23 | 16a | 1802.8 | Q | Rha | Xyl | Glc | Glc-Ac | Ac | [35] |
| 24 | 16b | 1788.7 | Q | Xyl | Xyl | Glc | Glc-Ac | Ac | [35] |
| 25 | 17a | 1744.8 | Q | Rha | Xyl | Glc | Rha | Ac | [35] |
| 26 | 17b | 1730.7 | Q | Xyl | Xyl | Glc | Rha | Ac | [35] |
| 27 | 18a | 1876.8 | Q | Rha | Xyl-Api | Glc | Rha | Ac | [35] |
| 28 | 18b | 1862.8 | Q | Xyl | Xyl-Api | Glc | Rha | Ac | [35] |
| 29 | S1 | 1870.9 | Q | Rha | Xyl | H | H | Fa-Ara | [36] |
| 30 | S2 | 1856.9 | Q | Xyl | Xyl | H | H | Fa-Ara | [36] |
| 31 | S3 | 2002.9 | Q | Rha | Xyl-Xyl | H | H | Fa-Ara | [36] |
| 32 | S4 | 1988.9 | Q | Xyl | Xyl-Xyl | H | H | Fa-Ara | [36] |
| 33 | S5 | 2002.9 | Q | Rha | Xyl-Api | H | H | Fa-Ara | [36] |
| 34 | S6 | 1988.9 | Q | Xyl | Xyl-Api | H | H | Fa-Ara | [36] |
| 35 | S7 | 1912.9 | Q | Rha | Xyl | H | Ac | Fa-Ara | [4] |
| 36 | S8 | 1898.9 | Q | Xyl | Xyl | H | Ac | Fa-Ara | [4] |
| 37 | S9 | 2045.0 | Q | Rha | Xyl-Xyl | H | Ac | Fa-Ara | [4] |
| 38 | S10 | 2030.9 | Q | Xyl | Xyl-Xyl | H | Ac | Fa-Ara | [4] |
| 39 | S11 | 2045.0 | Q | Rha | Xyl-Api | H | Ac | Fa-Ara | [4] |
| 40 | S12 | 2030.9 | Q | Xyl | Xyl-Api | H | Ac | Fa-Ara | [4] |
| 41 | B1 | 2033.0 | Q | Rha | Xyl | Glc | H | Fa-Ara | [36] |
| 42 | B2 | 2018.9 | Q | Xyl | Xyl | Glc | H | Fa-Ara | [36] |
| 43 | B3 | 2165.0 | Q | Rha | Xyl-Api | Glc | H | Fa-Ara | [36] |
| 44 | B4 | 2151.0 | Q | Xyl | Xyl-Api | Glc | H | Fa-Ara | [36] |
| 45 | B5 | 2165.0 | Q | Rha | Xyl-Xyl | Glc | H | Fa-Ara | [37] |
| 46 | B6 | 2151.0 | Q | Xyl | Xyl-Xyl | Glc | H | Fa-Ara | [37] |
| 47 | B7 | 1886.9 | Q | H | Xyl | Glc | H | Fa-Ara | [37] |
| 48 | B8 | 2018.9 | Q | H | Xyl-Api | Glc | H | Fa-Ara | [37] |
| 49 | QS-III | 2297.0 | Q | Xyl | Xyl-Api | Glc | Fa-Ara-Rha | H | [3] |
| 50 | 20a | 1656.7 | Q-OH | Rha | Xyl | Glc | H | MeBu | [34] |
| 51 | 20b | 1642.7 | Q-OH | Xyl | Xyl | Glc | H | MeBu | [34] |
| 52 | 21a | 1788.8 | Q-OH | Rha | Xyl-Api | Glc | H | MeBu | [34] |
| 53 | 21b | 1774.8 | Q-OH | Xyl | Xyl-Api | Glc | H | MeBu | [34] |
| 54 | 22a | 1978.9 | Q-OH | Rha | Xyl-Api | Glc | Rha | OHMeHex | [34] |
| 55 | 22b | 1964.9 | Q-OH | Xyl | Xyl-Api | Glc | Rha | OHMeHex | [34] |
| 56 | 19 | 1392.7 | P | H | H | H | Glc | MeBu | [34] |
| 57 | 23 | 1732.8 | E | Xyl | Xyl | Glc | Glc | Ac | [34] |
| 58 | S13 | 1560.7 | P-Ac | H | H | MeBu | Glc | MeBu | [4] |
| Antigen | Adjuvant | Model | Findings | Ref. |
|---|---|---|---|---|
| PorA P1.6 (membrane porin of Neisseria meningitidis) | Quil-A® | Mice | PorA supplemented with Quil-A® resulted in four to seven times higher antibody titers (IgG1, IgG2a, and IgG2b) when compared with lipopolysaccharide as an adjuvant. | [84] |
| S. bovis and Lactobacillus spp. | Quil-A® | Cattle | Immunization of feedlot steers against S bovis and Lactobacillus spp with vaccines incorporating Freund adjuvant, Quil-A®, dextran, or alum as an adjuvant effectively induced high IgG concentrations when dextran was used as an adjuvant. | [85] |
| 38-kDa mycobacterial protein | ISCOMs | Mice | An increase in antibody titers was observed after a booster injection, with significant levels of IgG2a. The ISCOM formulations induced this in the same way. Regarding the induction of CTL responses, the differences shown comparing various ISCOMS formulations were minimal. | [86] |
| Tetanus toxoid | QS-21 | Mice | Administration of QS-21 p.o. as an adjuvant elicits strong serum IgM and IgG Ab responses. | [87] |
| Babesian equi immunogen | Quil-A® | Donkeys | A mixture of Quil A® and Babesia equi immunogen was optimal in generating a significant immune response and reducing the lethality. | [88] |
| OVA | QS-21 and deacylated QS-21 (DS-1) | Mice | Despite DS-1 requiring a higher dose to induce IgG1 responses, it did not induce IgG2a or CTL responses. Lower doses of QS-21 induced higher IgG titers, including IgG2a and CTL responses. | [89] |
| FML * | Quil-A® | Dogs | The formulation protected dogs against canine kala-azar in the field with robust immunogenicity. | [90] |
| Measles virus | ISCOMs QA-22 | Rats and Macaques | Vaccines induced high levels of antibodies, which showed no decrease during a 2-year follow up. | [91] |
| Herpes virus | Quillaja saponins | Mice | Saponins’ deacylation significantly reduced antibody production and increased mortality rates during viral challenge test. | [92] |
| Opa J from Neisseria meningitidis | Quil-A® | Mice | Quil-A® formulated with OpaJ was highly immunogenic, inducing the production of Opa-specific antibodies. | [93] |
| Non-fimbrial adhesin hemagglutinin B (HagB) | Semi-synthetic saponin of Quillaja GPI-0100 | Mice | GPI-0100 showed better immunoadjuvancy than monophosphoryl lipid A and alum, as a mucosal and systemic adjuvant, in inducing serum anti-HagB. | [94] |
| FML * | Quil-A® | Dogs | The formulation was shown be effective in immunotherapy against visceral leishmaniasis of asymptomatic infected dogs. All animals showed significantly increased CD8 lymphocyte percentages. | [95] |
| Human respiratory syncytial virus (HRSV) | HRSV-ISCOMs prepared with Quillaja saponin fractions: QH-A; QH-C; QH-A + QH-C (QH-AC) | Mice | In general, the ISCOMs tested were well tolerated. However, the combination of QH-A + QH-C ISCOMs was lethal in neonates despite the QH-A or QH-C fractions alone being well tolerated. | [96] |
| FML * | Quillaja saponaria sapogenins containing aldehyde | Mice | Vaccines elicited high levels of antibodies and cellular specific response to FML and IFNγ sera levels and protection against L. donovani murine infection was shown. | [97] |
| Ovalbumin (OVA) | Quil-A® | Mice | Increasing amounts of Quil-A® (20% to 70%) were tested in liposomes. Higher doses of Quil A reduced the particle sizes formed, thus decreasing antigen incorporation and uptake by DC. Liposomes containing 20% Quil-A® were more effective as immunostimulants, and more toxic in cell cultures too, when compared with those containing 70% Quil-A®. | [98] |
| Mtb72F (Mycobacterium tuberculosis) | AS02A | Mice | Increase in both Th1 and Th2 response, with Th1 response being more pronounced. | [99] |
| LSP PbCS 242–310 ** | QS-21 | Mice | The use of LSP PbCS 242–310 combined with QS-21 induced a satisfactory immune response similar to the one generated with the injection of radiation-attenuated sporozoites. | [100] |
| HRSV | ISCOMs formulated with Quillaja saponin fractions | Mice | All three formulations favor Th1 responses to different degrees with IFN-c being produced up to 50 times more than Il-4 and IL-5. The HRSV 703 ISCOMs induced the most pronounced innate and acquired response with the most prominent Th1 profile. | [101] |
| HIV-1 DNA prime/protein–Env and Gag *** | QS-21 | Rhesus macaques and mice | Cellular and humoral responses were observed when Polyvalent DNA prime/protein boost vaccine was administered. CD8+ CTL, CD4+ T-helper cells and Th1 cytokines were involved in cellular immune response. | [102] |
| FML * | Fractions of the Riedel de Haen saponin mixture (QS-21 saponin fraction; two deacylsaponins mixture; a mixture of glucose, rutin, quercetin, and sucrose). | Mice | QS-21 and the deacylsaponins induced the most significant reduction of parasite burden in the liver, demonstrating a promising adjuvant potential in the Riedel de Haen saponin mixture, which contains deacylated saponins that are not toxic and induce robust immunity. | [103] |
| Plant-made measles virus hemagglutinin (MV-H) protein | Cholera toxin (CTB/CT); LT(R192G) ****; Saponin extracted from Quillaja bark (Sigma; S-4521) | Mice | Despite both LT(R192G) and the crude saponins showing strong adjuvant activity, the crude extract had superior immunostimulatory properties. | [104] |
| Fh15 (recombinant 15 kDa Fasciola hepatica protein) | ADAD System ***** | Mice and sheep | Mice immunized with ADAD system with Qs had a survival rate of 50–62.5% while animals without Qs had a survival rate of 40–50%. Sheep immunized with ADAD system with Qs showed less hepatic damage compared to control group. | [105] |
| FML * | QS-21 | Dogs | Despite nonspecific reactions being observed in the immunized animals (similar to other veterinary vaccines), a significant decrease was shown with subsequent doses. | [106] |
| LTB-ESAT-6 ⬪; and Bacillus Calmette-Guérin (BCG) | Quillaja extract | Mice | Mice vaccinated with a combination of plant-made LTB-ESAT-6 fusion BCG oral adjuvant had significantly more IL-10 production when compared with mice vaccinated without adjuvant. However, no protection was shown during the challenge test with M. tuberculosis, in groups treated with oral BCG (with or without saponin). | [107] |
| GD3-KLH | GPI-0100 ⬪⬪; sQS-21 ⬪⬪⬪ | Mice | Both synthetic Quillaja saponin molecules produced antibodies in mice. | [108] |
| FALVAC-1A (different epitopes from Plasmodium falciparum) | AIPO4, QS-21, Montanide ISA-720, or CRL-1005 | Mice | QS-21 was the second adjuvant that induced the most significant levels of antibodies, inducing predominantly IgG2c. QS-21 also induced the highest levels of IL-4 compared to the other adjuvants, indicating Th1 and Th2 responses. | [109] |
| FML * | QS-21 | Dogs | The vaccine induced robust immunogenicity with high levels of FML-seroconversion, as demonstrated by CD8+ and CD4+ T cell populations. | [110] |
| AD-472 ⬪⬪⬪⬪ or HSV-2 glycoprotein D | GPI-0100 ⬪⬪ | Guinea pigs | Both formulations reduced clinical disease; however, GPI-0100 improved the glycoprotein-D formulation only. | [111] |
| H9N2 | Aluminium phosphate; aluminium hydroxide; MF59 ⬪⬪⬪⬪⬪; and MATRIX-M# | Mice | MATRIX-M shifted the immune response to an IgG2a response (towards a Th1 response). On the other hand, the CD8+ T-cell response could be improved using MATRIX-M or MF59. Among all tested molecules, MATRIX-M was the most effective in inducing the immune response, followed by MF59 and aluminium-based adjuvants. | [112] |
| HpaA and catalase | ISCOMATRIXTM; ISCOMTM; Cholera toxin; and aluminium hydroxide | Mice | ISCOMATRIXTM and ISCOMTM vaccines, using two different antigens and different delivery systems (intranasal or subcutaneous), have the same efficacy in reducing H. pylori colonization as the gold standard cholera toxin (CT) adjuvant | [113] |
| TA-CIN ## | GPI-0100 ⬪⬪ | Mice and monkeys | Prophylactic vaccination with adjuvanted TA-CIN protected the mice from viral challenge, whereas vaccination without adjuvant was almost ineffective. Moreover, GPI-0100 boosted IFN-γ secreting CD8+ T cell response in mice compared to the formulation without adjuvant. Vaccination of macaques induced specific T cell responses. | [114] |
| Inactivated Chlamydophila abortus | QS-21 | Mice | The formulation induced the proliferation of B cells, which is important to confer immunity to the bacteria rather than cellular response. | [115] |
| sLEa-KLH (glycolipid/glycoprotein expressed on cancer cell surface) | GP1-0100 | Mice | Immunized animals produced high titers of highly reactive IgM and IgG specific antibodies. | [116] |
| Tetanus toxoid | Quillaja saponins or cross-linked dextran microspheres (CDM) | Rabbits | Formulations with Quillaja saponins did not increase mucosal IgA. On the other hand, the saponins induced the highest systemic IgG titers. | [117] |
| Ovalbumin | ISCOM matrices | Mice | The formulation with ISCOMs induced high specificity CTLs targeting different tumoral cells. | [118] |
| Tetanus toxoid loaded onto PLGA nanospheres | Quillaja saponins, CDM | Rabbits | The combination of Quillaja saponins with CDM in PLGA nanospheres loaded with antigens improved IgA production, suggesting better mucosal protection. The same was observed with systemic IgG. | [119] |
| Inactivated Bovine herpesvirus-5 | Aluminum hydroxide, Quil-A®, or QB-90 (from Quillaja brasiliensis) | Mice | Quil-A® and QB-90 induced similar immunity regarding humoral or cellular responses. | [27] |
| ALM (Leishmania major) | PLGA nanoparticles containing purified saponin extract | Mice | Increase in both Th1 andTh2 response. | [120] |
| HIV-1 gp120 with Q105N mutation | Quil-A® or MPL | Mice | Despite both adjuvants producing specific antibodies, there was no significant neutralizing activity against HIV-1. | [121] |
| Tetanus toxoid | Quillaja saponins | Rabbits | Quillaja formulation was more efficient in conferring mucosal protection due to increased levels of IgA. | [122] |
| No antigen was used | Alhydrogel or MATRIX-MTM formulated with fractions of Q. saponaria | Mice | Besides stimuli caused by MATRIX-MTM during the immune response (increase in cell recruitment and cytokine secretion), it induced the expression of co-stimulatory molecule CD86, participating in early events of the immune response. | [123] |
| Rhoptry Toxoplasma gondii protein | Quil-A® | Swine | The formulation induced high humoral, local, and systemic immune response, partially preventing brains from forming cysts. | [124] |
| Tetanus toxoid loaded onto chitosan functionalized gold nanoparticles | Quillaja saponins | Mice | When administered orally, the nanoparticles can better deliver the formulation, increasing immune response up to 28-fold compared to control. | [125] |
| HIV-1 gp120 | QS-21, aluminum hydroxide, MPLA, or ISCOMATRIXTM | Mice | Each adjuvant stimulated a different cytokine profile secretion, having different properties in the induction of the inflammation. | [126] |
| MUC1-KLH (prostate cancer marker) and/or ovalbumin | QS-21, synthetic QS-21, or other conjugated saponins | Mice | Despite QS-21 inducing robust Th1 and Th2 response, conjugated saponins had improved activity and toxicity profiles relative to QS-21. | [127] |
| Varicella zoster glycoprotein E and ovalbumin | AS01 | Mice | AS01 can improve adaptive humoral response through the generation of a great number of antigen-presenting cells. | [128] |
| Inactivated Poliovirus | Quil-A®, QB-90, and Aqueous Extract from Quillaja brasiliensis | Mice | The humoral enhancements caused by Quil-A® and QB-90 were statistically similar. However, the mucosal immune response was increased with QB-90 by increases in IgA. | [26] |
| Tau antigen (associated with Alzheimer’s disease) | Quil-A® | Mice | Animals that received the formulations with Quil-A® had reduced neuroinflammation and tau pathogenesis. | [129] |
| MARV-VLP (Marburg virus virus-like particle containing glycoprotein, matrix vp40, and nucleoprotein) | QS-21 or polyI:C | Cynomolgus macaques | Formulations prepared with either of the adjuvants provided full protection in the challenge test. QS-21 produced a lower response to antigens compared to polyI:C. | [130] |
| EBOVgp-Fc (Ebola virus glycoprotein fused to Fc fragment of human IgG1) | QS-21, aluminum hydroxide, or poly-ICLC | Guinea pigs | All formulations induced antibody formation, however, despite QS-21 inducing a strong humoral response, in the challenge test only poly-ICLC induced robust protection. | [131] |
| Intanza 2013 (Trivalent Influenza Vaccine, Sanofi Pasteur) | QS-21 | Mice | When administered in the skin as a Nanopatch vaccine, a lower dose of QS-21 and adjuvant was required to enhance humoral response compared to intramuscular administration. | [132] |
| Ovalbumin or HBsAg (Hepatitis B virus surface antigen) | QS-21 liposome | Mice | The immunoadjuvancy of QS-21 relies on macrophages to induce activation of dendritic cells and stimulate the immune system. | [133] |
| FhSAP2-IBs (Fasciola hepatica protein) | QS-21 or Montanide ISA720 | Mice | Increase in both Th1 and Th2 responses with significant increase in Th1 response. | [134] |
| QS-21 or QS-21 formulated with HIV-1 gp120 | QS-21 | Mice | QS-21 activates NLRP3 inflammasome. | [21] |
| Inactivated bovine viral diarrhea virus | Saponins of Q. brasiliensis (QB-90) and Q. brasiliensis aqueous extract (AE) | Mice | In vaccines adjuvanted with QB-90 and AE higher levels of antibody were detected. Animals that received QB-90 adjuvanted vaccine had enhanced cytokines and IFN-γ production by CD4+andCD8+T lymphocytes whereas AE-adjuvanted preparation stimulated humoral response only. | [135] |
| Ovalbumin | ISCOMs of Q. brasiliensis saponin fraction (IQB-90) and Quil-A® ISCOMs | Mice | IQB-90 inoculated subcutaneously induced strong antibody response (IgG1and IgG2a) and increased Th1 response. IQ-90 delivered intranasally induced secretion of serum IgG and IgG1 and mucosal IgA. | [25] |
| No antigen was used | MATRIX-MTM | Swine | MATRIX-MTM formulation induced pro-inflammatory cytokines, suggesting enhancement of innate immune response of specific pathogen free pigs exposed to reared pigs. | [68] |
| Aβ42 (Alzheimer’s disease pathological hallmark) | QS-21 | Rhesus monkeys | The formulation induced a good humoral response with high titers of IgG and IgA. No inflammatory cellular immune response was observed. | [136] |
| Ovalbumin and HBsAg (Hepatitis B virus surface antigen) | QS-21 | In vitro (THP-1 cell) and mice | Human monocyte-derived dendritic cells were directly activated by QS-21, requiring cathepsin B to induce high CD4 and CD8 T cell response. | [137] |
| FMP013 (Falsiparum Malaria Protein-013 from Plasmodium falciparum) | ALF, ALF + aluminum hydroxide, ALFQ + QS-21, or Montanide ISA720 | Mice | The ALF adjuvant conjugated with QS-21 induced the highest antibody titer with the highest IgG2c titers. Also, it augmented the number of activated B-cells compared to Montanide. | [138] |
| HBsAg | QS-21 liposome | Swine | The study demonstrated that the HBsAg formulated with QS-21 liposomes in dissolvable microneedle arraypatches induced similar immunization to the commercial HBsAg formulation. | [139] |
| Inactivated Bovine Viral Diarrhea Virus | QB-90 and IMXQB-90 (ISCOMs and Q. brasiliensis saponins | Mice | The use of Q. brasiliensis saponins in vaccine formulations either formulated in ISCOM structures or not promoted a satisfactory and promising immune response, similar to Quil-A®. | [140] |
| Antigen | Adjuvant | Objective | Findings | Ref. |
|---|---|---|---|---|
| CHO-derived gp120 protein from HIV-1 | QS-21 or Al(OH)3 | Evaluate safety and assess kinetics of immune response | Improved T cell response inducing CTL activation with T helper cells was observed in formulations with QS-21. | [149] |
| Globo H-KLH (carbohydrate antigen found in most breast cancer cells) | QS-21 | Determine the formulation toxicity against cancerous cells, its immune response, and if the conjugation of Globo H with KLH would affect the immune response | Increase in specific antibody production and increased cytotoxicity either from complement system or antibody signaling. | [145] |
| PolySA and NP-polySA both conjugated with KLH (protein that in adults is associated with small cell lung cancer) | QS-21 | Determine the immune response after vaccination and assess the impact of polySA chemical manipulation | NP-polySA vaccination resulted in higher antibody titers with IgM response. The IgM was reactive to small cell lung cancer. | [146] |
| MUC-2G/Globo H-KLH | GPI-0100⬪; GPI-0100-P ⬪⬪; QS-21 | Present the use of GPI-0100 in humans and determine the safety and immunogenicity of a vaccine with different doses of GPI-0100. | GPI-0100 (5000 μg) and QS-21 (100 μg) produced comparable antibody titers. All adjuvanted vaccine doses were well tolerated and antigen-specific antibody titers matched increasing dose levels. | [83] |
| SL * | AS02A ⬪⬪⬪ | Evaluate the potential of an AS02A adjuvanted formulation in healthy individuals and compare it to a non-adjuvanted vaccine. | The adjuvanted vaccine induced more significative humoral and Th1 immune responses compared with the non-adjuvanted formulation. Despite the AS02A recipients reporting local and general reactions more frequently than the non-adjuvanted group, the safety profile was acceptable. | [153] |
| FMP1 ** | AS02A (oil-in-water formulation with mono- phosphoryl lipid A and QS-21 | Evaluate the safety and immunogenicity of malaria vaccine FMP1/AS02A in adults | FMP1 formulated with AS02A was well tolerated and showed high immunogenicity with specific anti-MSP-142 antibody titers boosted and prolonged due to the vaccination. | [147] |
| Influenza | QS-21 | Evaluate QS21 as an adjuvant and compare it with standard trivalent inactivated influenza | Local pain and post vaccination myalgias were greater in individuals that received QS-21. Despite increased serum antibodies, the mean titers for formulations (with or without QS-21) were not different. | [148] |
| RTS,S (recombinant proteins from P. falciparum) | AS02D | Evaluate the immunogenicity of RTS,S/AS02D formulation (liquid and lyophilized) | Lyophilized formulation is as efficient and safe as the liquid formulation, promoting satisfactory immunization. | [154] |
| PfAMA1 *** | AlhydrogelTM, Montanide ISA720 and AS02 Adjuvant System | Evaluate the immunogenicity and safety of PfAMA1 antigen (two different doses with three different adjuvants) | All formulations caused different reactogenicity with no serious reported adverse effects. All formulations tested induced antibody production, with AS02 being the most pronounced. | [155] |
| RTS,S **** | AS02A | Determine the safety of the RTS,S antigen formulated with AS02A adjuvant. | The formulation was well tolerated in children, with a good safety profile within the number of doses. The AS02A formulation caused fewer serious adverse events compared to the control group. | [156] |
| FMP2.1 ***** | AS02A | Evaluate the reactogenicity, safety, and immunogenicity of the malaria vaccine FMP2.1/AS02A in adults. | The formulation was well tolerated and showed good safety. Also, it was highly immunogenic. | [157] |
| No antigen was used | Quillaja saponins | Determine if dietary QS can modify macrophage activity and investigate its effects on liver function and inflammatory response | An increase in chemotactic and phagocytosis activities were observed. Furthermore, no adverse effects were seen since no significant changes in immunoglobulin, transaminase, IL-1α, and TNF- α were observed. | [144] |
| NP-polySA-KLH (Polysialic acid conjugated to keyhole limpet hemocyanin (KLH)) | QS-21 | Confirm the safety profile and determine the optimal dose | The lowest optimal immunogenic dose was 10 µg, which resulted in consistent high-titer antibody responses. | [151] |
| MAGE-A3 (tumor-specific protein usually expressed in melanoma) | AS02B or AS15 | Discover which adjuvant would cause a more robust and persistent immune response | AS15 provided a more robust immune response by activating more dendritic and B cells. | [152] |
| HBsAg (Hepatitis B virus surface antigen) | AS02B, AS02V, or AS01B | Evaluate the duration of humoral and cellular responses | All formulations induced persistent T CD4+ and CD8+ specific response, as well as B-cell response, indicating immunological memory. | [150] |
| Unimolecular conjugated Globo-H, GM2, sTn, TF, and Tf (markers usually expressed on ovarian cancer cell-surface) | QS-21 | Evaluate safety and immunogenicity of the pentavalent synthetic vaccine | 83% of individuals responded to at least three antigens with satisfactory immune response. | [158] |
| HBsAg (surface antigen of Hepatitis B virus), ovalbumin, CSP (P. falciparum circumsporozoite protein), and Varicella zoster glycoprotein E | AS01 | Investigate how combining immune-stimulants results in innate immune response | AS01 triggers innate response, such as NK-cells, and activates CD8 T-cells in the lymph nodes, depending on macrophage, IL-12, and IL-18. | [159] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fleck, J.D.; Betti, A.H.; Da Silva, F.P.; Troian, E.A.; Olivaro, C.; Ferreira, F.; Verza, S.G. Saponins from Quillaja saponaria and Quillaja brasiliensis: Particular Chemical Characteristics and Biological Activities. Molecules 2019, 24, 171. https://doi.org/10.3390/molecules24010171
Fleck JD, Betti AH, Da Silva FP, Troian EA, Olivaro C, Ferreira F, Verza SG. Saponins from Quillaja saponaria and Quillaja brasiliensis: Particular Chemical Characteristics and Biological Activities. Molecules. 2019; 24(1):171. https://doi.org/10.3390/molecules24010171
Chicago/Turabian StyleFleck, Juliane Deise, Andresa Heemann Betti, Francini Pereira Da Silva, Eduardo Artur Troian, Cristina Olivaro, Fernando Ferreira, and Simone Gasparin Verza. 2019. "Saponins from Quillaja saponaria and Quillaja brasiliensis: Particular Chemical Characteristics and Biological Activities" Molecules 24, no. 1: 171. https://doi.org/10.3390/molecules24010171