Phenethyl Isothiocyanate Inhibits In Vivo Growth of Xenograft Tumors of Human Glioblastoma Cells
Abstract
:1. Introduction
2. Results
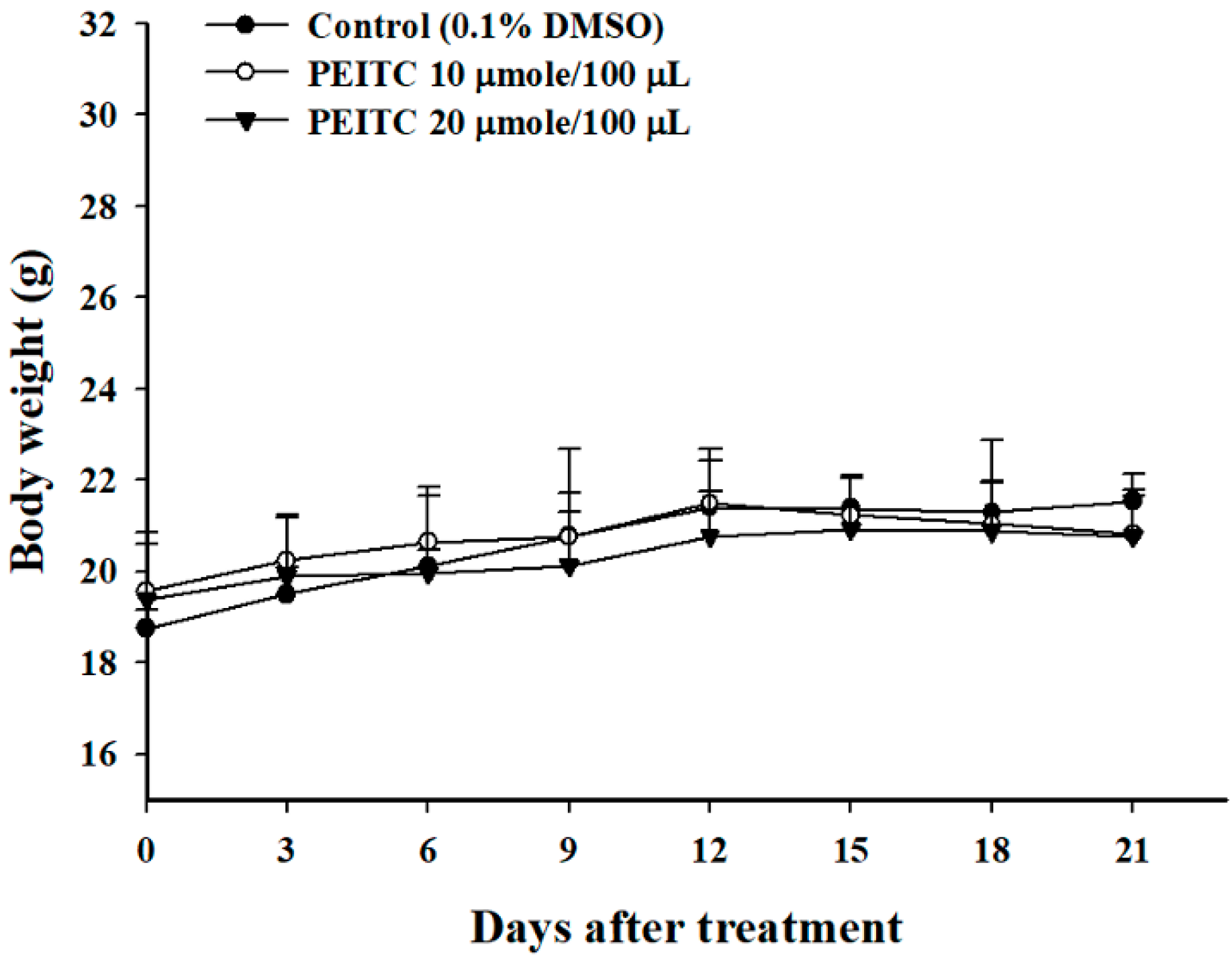
2.1. PEITC Did Not Affect the Body Weights in Xenograft GBM 8401/luc2 Cells-Bearing Animal Models
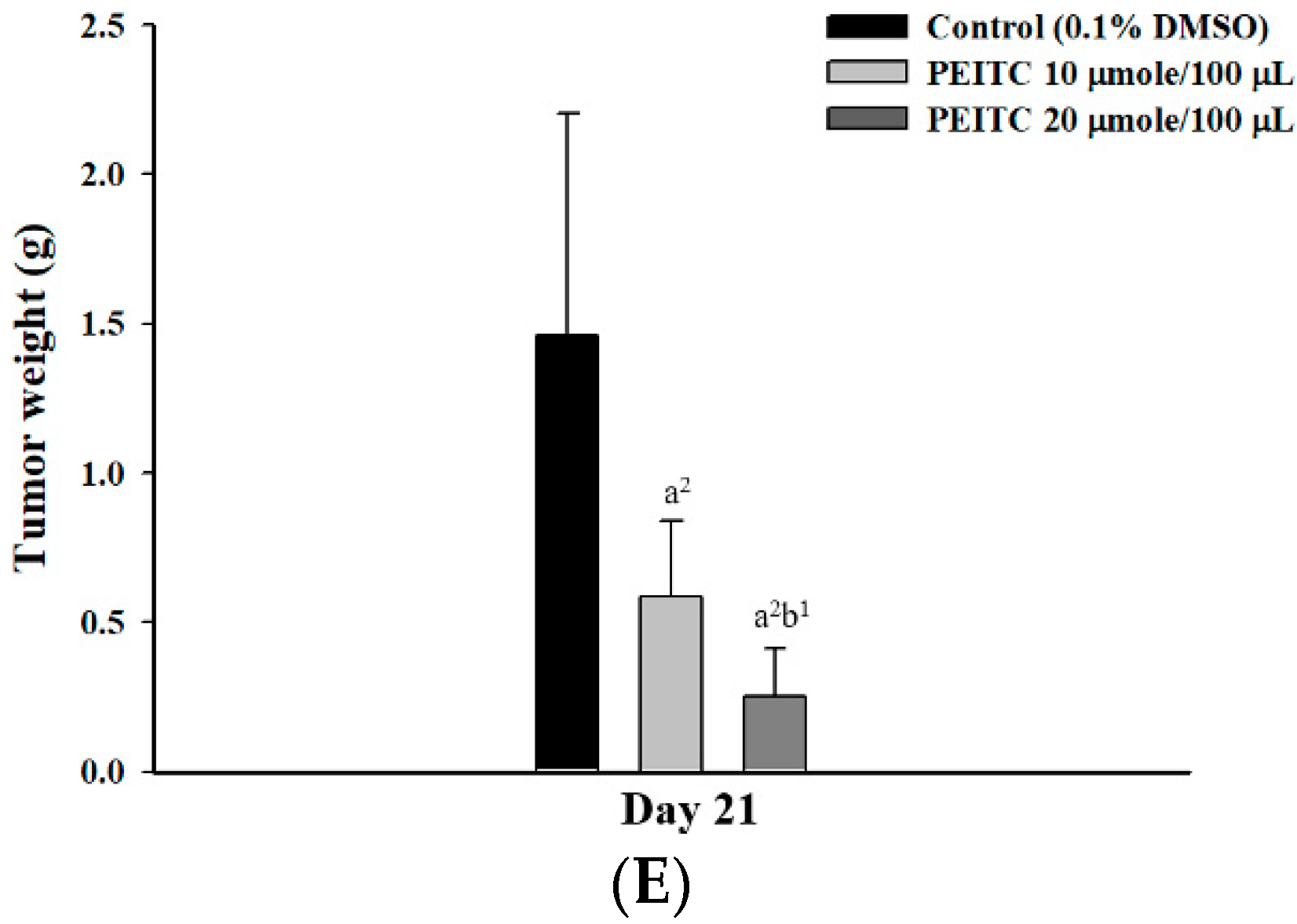
2.2. PEITC Inhibited Xenograft Tumor Growth of GBM 8401/luc2 Cells
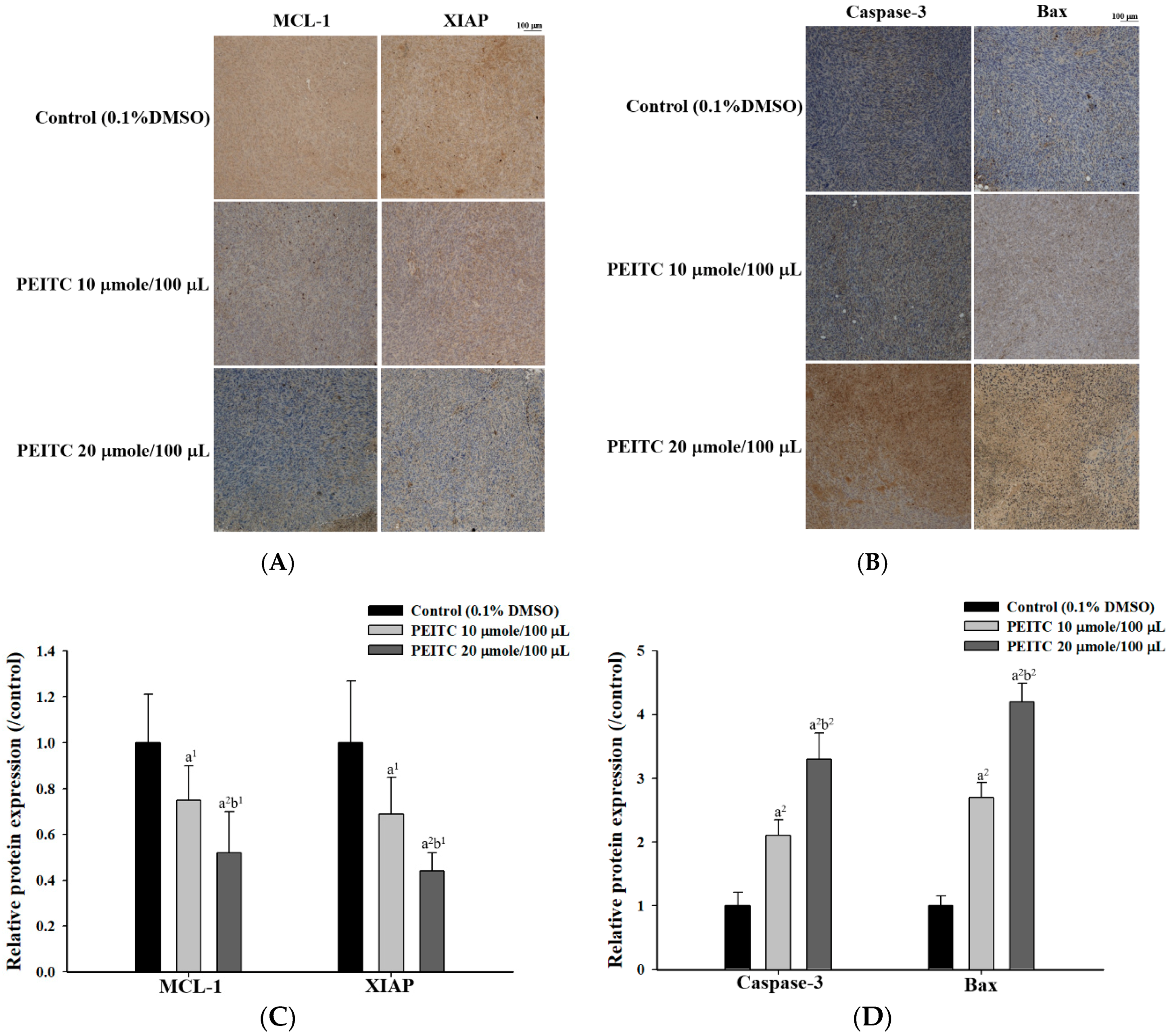
2.3. PEITC Altered Apoptosis Associated Proteins Signaling in Xenograft Tumor of GBM 8401/luc2 Cells
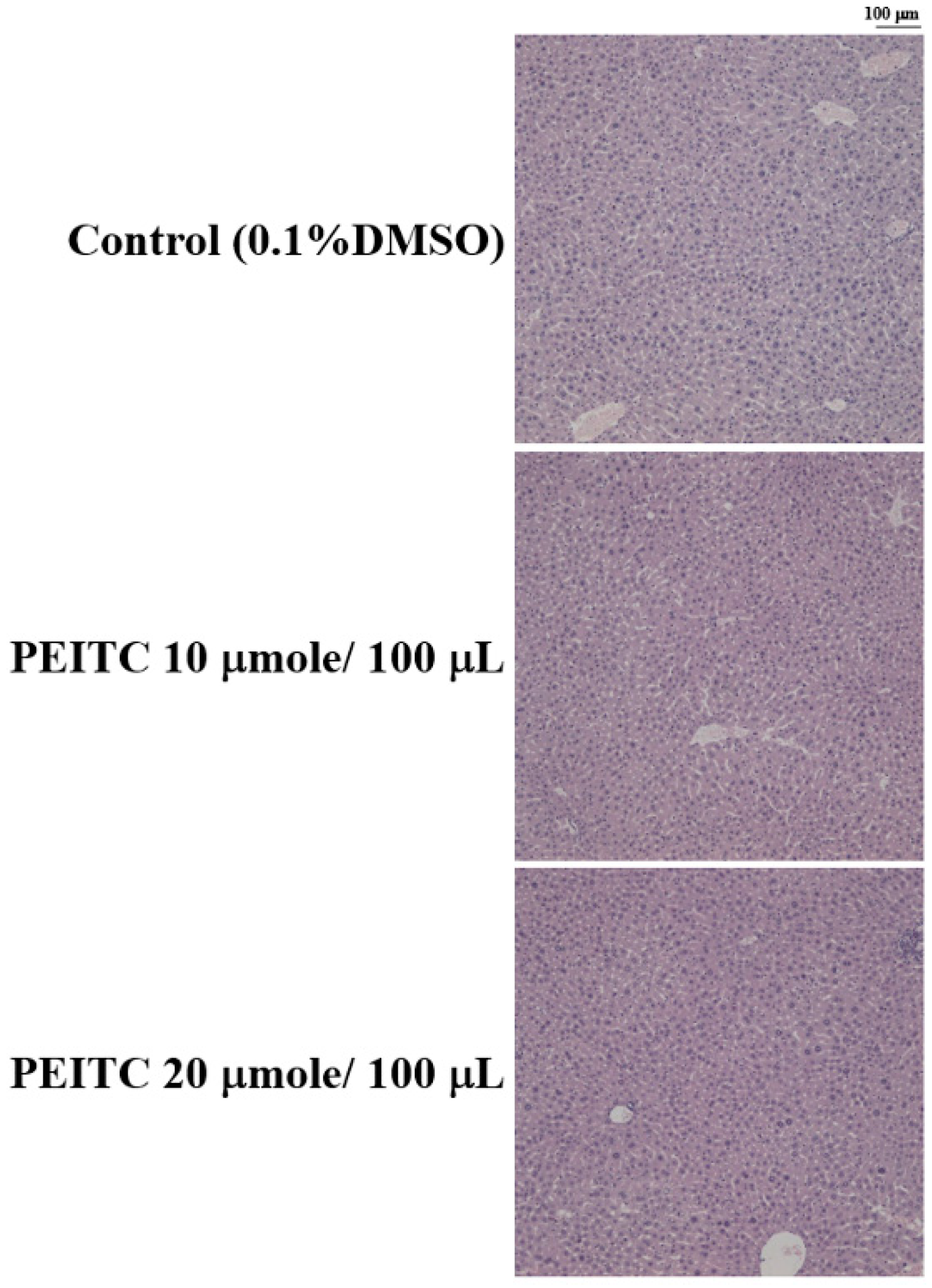
2.4. Effects of PEITC on the Hepatic Histopathological Change in GBM 8401/luc2 Cell Xenograft Animal Model
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Cell Culture
4.3. Transfection and Stable Clone Selection
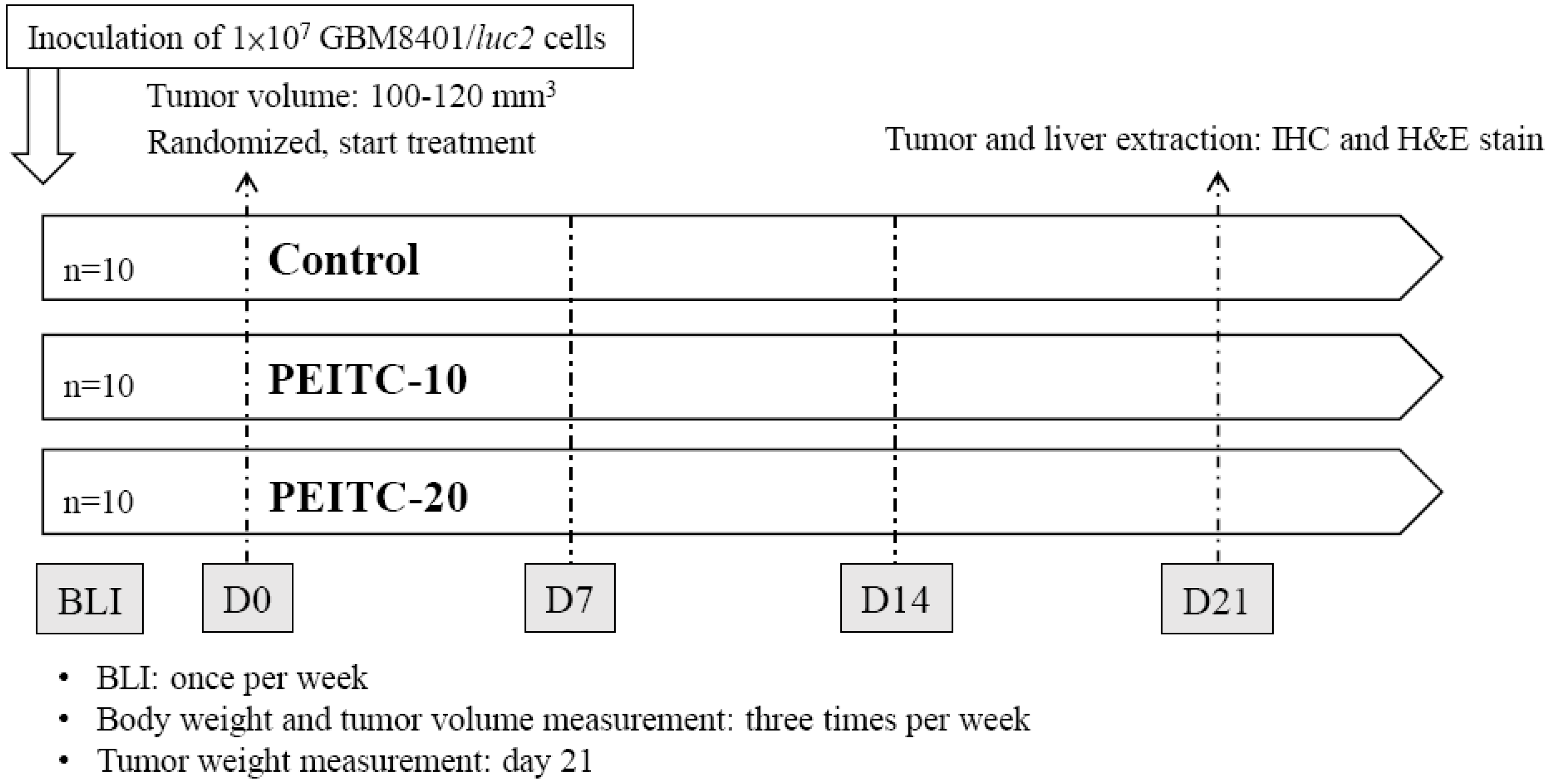
4.4. Animals and Treatments
4.5. In Vivo Bioluminescent Imaging (BLI)
4.6. IHC Staining and Pathological Examination
4.7. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nassiri, F.; Aldape, K.; Zadeh, G. The multiforme of glioblastoma. Neuro-Oncology 2018, 20, 437–438. [Google Scholar] [CrossRef] [PubMed]
- Moon, Y.J.; Brazeau, D.A.; Morris, M.E. Dietary phenethyl isothiocyanate alters gene expression in human breast cancer cells. Evid. Based Complement. Alternat. Med. 2011. [Google Scholar] [CrossRef] [PubMed]
- Palliyaguru, D.L.; Yuan, J.M.; Kensler, T.W.; Fahey, J.W. Isothiocyanates: Translating the Power of Plants to People. Mol. Nutr. Food Res. 2018. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, S.E.; Romero-Oliva, F.A. Epigenetic changes: A common theme in acute myelogenous leukemogenesis. J. Hematol. Oncol. 2013, 6, 57. [Google Scholar] [CrossRef] [PubMed]
- Cang, S.; Ma, Y.; Chiao, J.W.; Liu, D. Phenethyl isothiocyanate and paclitaxel synergistically enhanced apoptosis and alpha-tubulin hyperacetylation in breast cancer cells. Exp. Hematol. Oncol. 2014, 3, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cang, S.; Xu, X.; Ma, Y.; Liu, D.; Chiao, J.W. Hypoacetylation, hypomethylation, and dephosphorylation of H2B histones and excessive histone deacetylase activity in DU-145 prostate cancer cells. J. Hematol. Oncol. 2016, 9, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, S.; Kim, Y.J.; Lee, J.; Jeon, S.; Hong, T.; Park, G.J.; Yoon, J.H.; Yahng, S.A.; Shin, S.H.; Lee, S.E.; et al. Model-based adaptive phase I trial design of post-transplant decitabine maintenance in myelodysplastic syndrome. J. Hematol. Oncol. 2015, 8, 118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, P.; Wright, S.E.; Kim, S.H.; Srivastava, S.K. Phenethyl isothiocyanate: A comprehensive review of anti-cancer mechanisms. Biochim. Biophys. Acta 2014, 1846, 405–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chou, Y.C.; Chang, M.Y.; Wang, M.J.; Harnod, T.; Hung, C.H.; Lee, H.T.; Shen, C.C.; Chung, J.G. PEITC induces apoptosis of Human Brain Glioblastoma GBM8401 Cells through the extrinsic- and intrinsic -signaling pathways. Neurochem. Int. 2015, 81, 32–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chou, Y.C.; Chang, M.Y.; Wang, M.J.; Yu, F.S.; Liu, H.C.; Harnod, T.; Hung, C.H.; Lee, H.T.; Chung, J.G. PEITC inhibits human brain glioblastoma GBM 8401 cell migration and invasion through the inhibition of uPA, Rho, A., and Ras with inhibition of MMP-2, -7 and -9 gene expression. Oncol. Rep. 2015, 34, 2489–2496. [Google Scholar] [CrossRef] [PubMed]
- Chou, Y.C.; Chang, M.Y.; Wang, M.J.; Liu, H.C.; Chang, S.J.; Harnod, T.; Hung, C.H.; Lee, H.T.; Shen, C.C.; Chung, J.G. Phenethyl isothiocyanate alters the gene expression and the levels of protein associated with cell cycle regulation in human glioblastoma GBM 8401 cells. Environ. Toxicol. 2017, 32, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Ni, W.Y.; Lu, H.F.; Hsu, S.C.; Hsiao, Y.P.; Liu, K.C.; Liu, J.Y.; Ji, B.C.; Hsueh, S.C.; Hung, F.M.; Shang, H.S.; et al. Phenethyl isothiocyanate inhibits in vivo growth of subcutaneous xenograft tumors of human malignant melanoma A375.S2 cells. In Vivo 2014, 28, 891–894. [Google Scholar] [PubMed]
- Fuentes, F.; Paredes-Gonzalez, X.; Kong, A.N. Dietary glucosinolates sulforaphane, phenethyl isothiocyanate, indole-3-carbinol/3,3′-diindolylmethane: Anti-oxidative stress/inflammation, Nrf2, epigenetics/epigenomics and in vivo cancer chemopreventive efficacy. Curr. Pharmacol. Rep. 2015, 1, 179–196. [Google Scholar] [CrossRef] [PubMed]
- Ramalho-Carvalho, J.; Graca, I.; Gomez, A.; Oliveira, J.; Henrique, R.; Esteller, M.; Jeronimo, C. Downregulation of miR-130b~301b cluster is mediated by aberrant promoter methylation and impairs cellular senescence in prostate cancer. J. Hematol. Oncol. 2017, 10, 43. [Google Scholar] [CrossRef] [PubMed]
- Izzotti, A.; Calin, G.A.; Steele, V.E.; Cartiglia, C.; Longobardi, M.; Croce, C.M.; De Flora, S. Chemoprevention of cigarette smoke-induced alterations of MicroRNA expression in rat lungs. Cancer Prev. Res. 2010, 3, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Cheung, K.L.; Khor, T.O.; Huang, M.T.; Kong, A.N. Differential in vivo mechanism of chemoprevention of tumor formation in azoxymethane/dextran sodium sulfate mice by PEITC and DBM. Carcinogenesis 2010, 31, 880–885. [Google Scholar] [CrossRef] [PubMed]
- Leverson, J.D.; Zhang, H.; Chen, J.; Tahir, S.K.; Phillips, D.C.; Xue, J.; Nimmer, P.; Jin, S.; Smith, M.; Xiao, Y.; et al. Potent and selective small-molecule MCL-1 inhibitors demonstrate on-target cancer cell killing activity as single agents and in combination with ABT-263 (navitoclax). Cell Death Dis. 2015. [Google Scholar] [CrossRef] [PubMed]
- Szegezdi, E.; Macdonald, D.C.; Ni Chonghaile, T.; Gupta, S.; Samali, A. Bcl-2 family on guard at the ER. Am. J. Physiol. Cell Physiol. 2009, 296, 941–953. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ishida, C.T.; Ishida, W.; Lo, S.L.; Zhao, J.; Shu, C.; Bianchetti, E.; Kleiner, G.; Sanchez-Quintero, M.; Quinzii, C.M.; et al. Combined HDAC and bromodomain protein inhibition reprograms tumor cell metabolism and elicits synthetic lethality in glioblastoma. Clin. Cancer Res. 2018, 24, 3941–3954. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Wu, F.; Wu, J. Targeting histone methylation for cancer therapy: Enzymes, inhibitors, biological activity and perspectives. J. Hematol. Oncol. 2016, 9, 49. [Google Scholar] [CrossRef] [PubMed]
- Karpel-Massler, G.; Ishida, C.T.; Bianchetti, E.; Shu, C.; Perez-Lorenzo, R.; Horst, B.; Banu, M.; Roth, K.A.; Bruce, J.N.; Canoll, P.; et al. Inhibition of mitochondrial matrix chaperones and antiapoptotic Bcl-2 family proteins empower antitumor therapeutic responses. Cancer Res. 2017, 77, 3513–3526. [Google Scholar] [CrossRef] [PubMed]
- Ghobrial, I.M.; Witzig, T.E.; Adjei, A.A. Targeting apoptosis pathways in cancer therapy. CA A Cancer J. Clin. 2005, 55, 178–194. [Google Scholar] [CrossRef]
- Fulda, S.; Vucic, D. Targeting IAP proteins for therapeutic intervention in cancer. Nat. Rev. Drug Discov. 2012, 11, 109–124. [Google Scholar] [CrossRef] [PubMed]
- Zappavigna, S.; Scuotto, M.; Cossu, A.M.; Ingrosso, D.; De Rosa, M.; Schiraldi, C.; Filosa, R.; Caraglia, M. The 1,4 benzoquinone-featured 5-lipoxygenase inhibitor RF-Id induces apoptotic death through downregulation of IAPs in human glioblastoma cells. J. Exp. Clin. Cancer Res. 2016, 35, 167. [Google Scholar] [CrossRef] [PubMed]
- Tchoghandjian, A.; Souberan, A.; Tabouret, E.; Colin, C.; Denicolai, E.; Jiguet-Jiglaire, C.; El-Battari, A.; Villard, C.; Baeza-Kallee, N.; Figarella-Branger, D. Inhibitor of apoptosis protein expression in glioblastomas and their in vitro and in vivo targeting by SMAC mimetic GDC-0152. Cell Death Dis. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; El-Deiry, W.S. Overview of cell death signaling pathways. Cancer Biol. Ther. 2005, 4, 139–163. [Google Scholar] [CrossRef] [PubMed]
- McIlwain, D.R.; Berger, T.; Mak, T.W. Caspase functions in cell death and disease. Cold Spring Harb. Perspect. Biol. 2013, 5. [Google Scholar] [CrossRef] [PubMed]
- Zhong, D.; Zhao, S.; He, G.; Li, J.; Lang, Y.; Ye, W.; Li, Y.; Jiang, C.; Li, X. Stable knockdown of LRG1 by RNA interference inhibits growth and promotes apoptosis of glioblastoma cells in vitro and in vivo. Tumour Biol. 2015, 36, 4271–4278. [Google Scholar] [CrossRef] [PubMed]
- Seo, K.W.; Kim, J.G.; Park, M.; Kim, T.W.; Kim, H.J. Effects of phenethylisothiocyanate on the expression of glutathione S-transferases and hepatotoxicity induced by acetaminophen. Xenobiotica 2000, 30, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.Y.; Yeh, C.C.; Lee, J.H.; Hung, C.F.; Chung, J.G. Berberine inhibited arylamine N-acetyltransferase activity and gene expression and DNA adduct formation in human malignant astrocytoma (G9T/VGH) and brain glioblastoma multiforms (GBM 8401) cells. Neurochem. Res. 2002, 27, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.J.; Pan, P.J.; Hsu, F.T. Regorafenib induces extrinsic and intrinsic apoptosis through inhibition of ERK/NF-kappaB activation in hepatocellular carcinoma cells. Oncol. Rep. 2017, 37, 1036–1044. [Google Scholar] [CrossRef] [PubMed]
- Hsu, F.T.; Liu, Y.C.; Chiang, I.T.; Liu, R.S.; Wang, H.E.; Lin, W.J.; Hwang, J.J. Sorafenib increases efficacy of vorinostat against human hepatocellular carcinoma through transduction inhibition of vorinostat-induced ERK/NF-kappaB signaling. Int. J. Oncol. 2014, 45, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Della Peruta, M.; Badar, A.; Rosales, C.; Chokshi, S.; Kia, A.; Nathwani, D.; Galante, E.; Yan, R.; Arstad, E.; Davidoff, A.M.; et al. Preferential targeting of disseminated liver tumors using a recombinant adeno-associated viral vector. Hum. Gene. Ther. 2015, 26, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Workman, P.; Aboagye, E.O.; Balkwill, F.; Balmain, A.; Bruder, G.; Chaplin, D.J.; Double, J.A.; Everitt, J.; Farningham, D.A.; Glennie, M.J.; et al. Guidelines for the welfare and use of animals in cancer research. Br. J. Cancer 2010, 102, 1555–1577. [Google Scholar] [CrossRef] [PubMed]
- Hsu, F.T.; Chang, B.; Chen, J.C.; Chiang, I.T.; Liu, Y.C.; Kwang, W.K.; Hwang, J.J. Synergistic effect of sorafenib and radiation on human oral carcinoma in vivo. Sci. Rep. 2015, 5, 15391. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.J.; Hsu, F.T.; Pan, P.J.; Chen, C.W.; Kuo, Y.C. Amentoflavone enhances the therapeutic efficacy of sorafenib by inhibiting anti-apoptotic potential and potentiating apoptosis in hepatocellular carcinoma in vivo. Anticancer Res. 2018, 38, 2119–2125. [Google Scholar] [PubMed]
Sample Availability: Samples of the compounds phenethyl isothiocyanate (PEITC) are available from the authors. |

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chou, Y.-C.; Chang, M.-Y.; Lee, H.-T.; Shen, C.-C.; Harnod, T.; Liang, Y.-J.; Wu, R.S.-C.; Lai, K.-C.; Hsu, F.-T.; Chung, J.-G. Phenethyl Isothiocyanate Inhibits In Vivo Growth of Xenograft Tumors of Human Glioblastoma Cells. Molecules 2018, 23, 2305. https://doi.org/10.3390/molecules23092305
Chou Y-C, Chang M-Y, Lee H-T, Shen C-C, Harnod T, Liang Y-J, Wu RS-C, Lai K-C, Hsu F-T, Chung J-G. Phenethyl Isothiocyanate Inhibits In Vivo Growth of Xenograft Tumors of Human Glioblastoma Cells. Molecules. 2018; 23(9):2305. https://doi.org/10.3390/molecules23092305
Chicago/Turabian StyleChou, Yu-Cheng, Meng-Ya Chang, Hsu-Tung Lee, Chiung-Chyi Shen, Tomor Harnod, Yea-Jiuan Liang, Rick Sai-Chuen Wu, Kuang-Chi Lai, Fei-Ting Hsu, and Jing-Gung Chung. 2018. "Phenethyl Isothiocyanate Inhibits In Vivo Growth of Xenograft Tumors of Human Glioblastoma Cells" Molecules 23, no. 9: 2305. https://doi.org/10.3390/molecules23092305
APA StyleChou, Y.-C., Chang, M.-Y., Lee, H.-T., Shen, C.-C., Harnod, T., Liang, Y.-J., Wu, R. S.-C., Lai, K.-C., Hsu, F.-T., & Chung, J.-G. (2018). Phenethyl Isothiocyanate Inhibits In Vivo Growth of Xenograft Tumors of Human Glioblastoma Cells. Molecules, 23(9), 2305. https://doi.org/10.3390/molecules23092305





