Sol-Gel Immobilisation of Lipases: Towards Active and Stable Biocatalysts for the Esterification of Valeric Acid
Abstract
:1. Introduction
2. Results and Discussion
2.1. Protein Concentration and Enzymatic Activity
2.2. Determination of Optimal Conditions
2.2.1. Presence of Water during Esterification Reaction
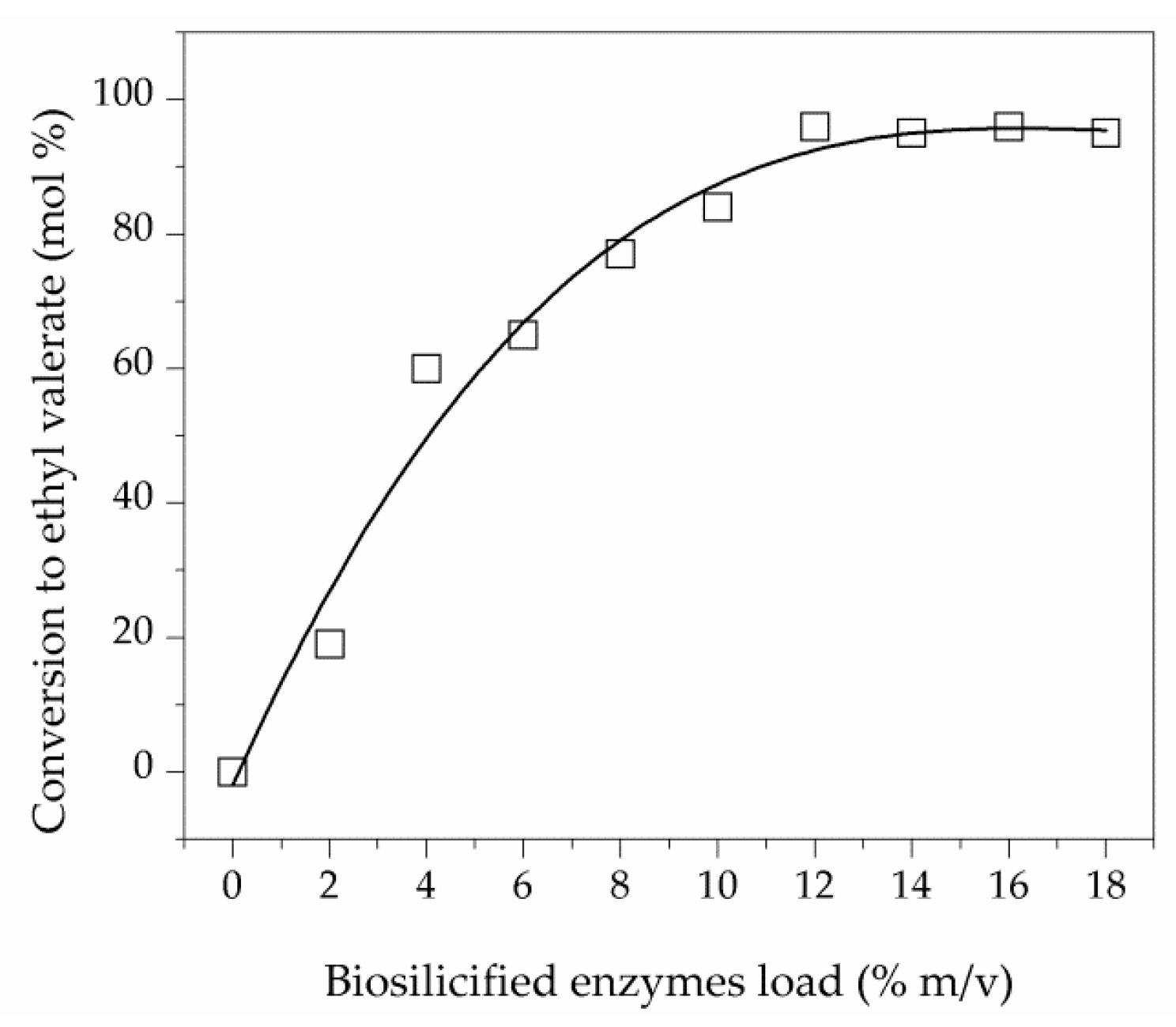
2.2.2. Influence of Biocatalyst Load
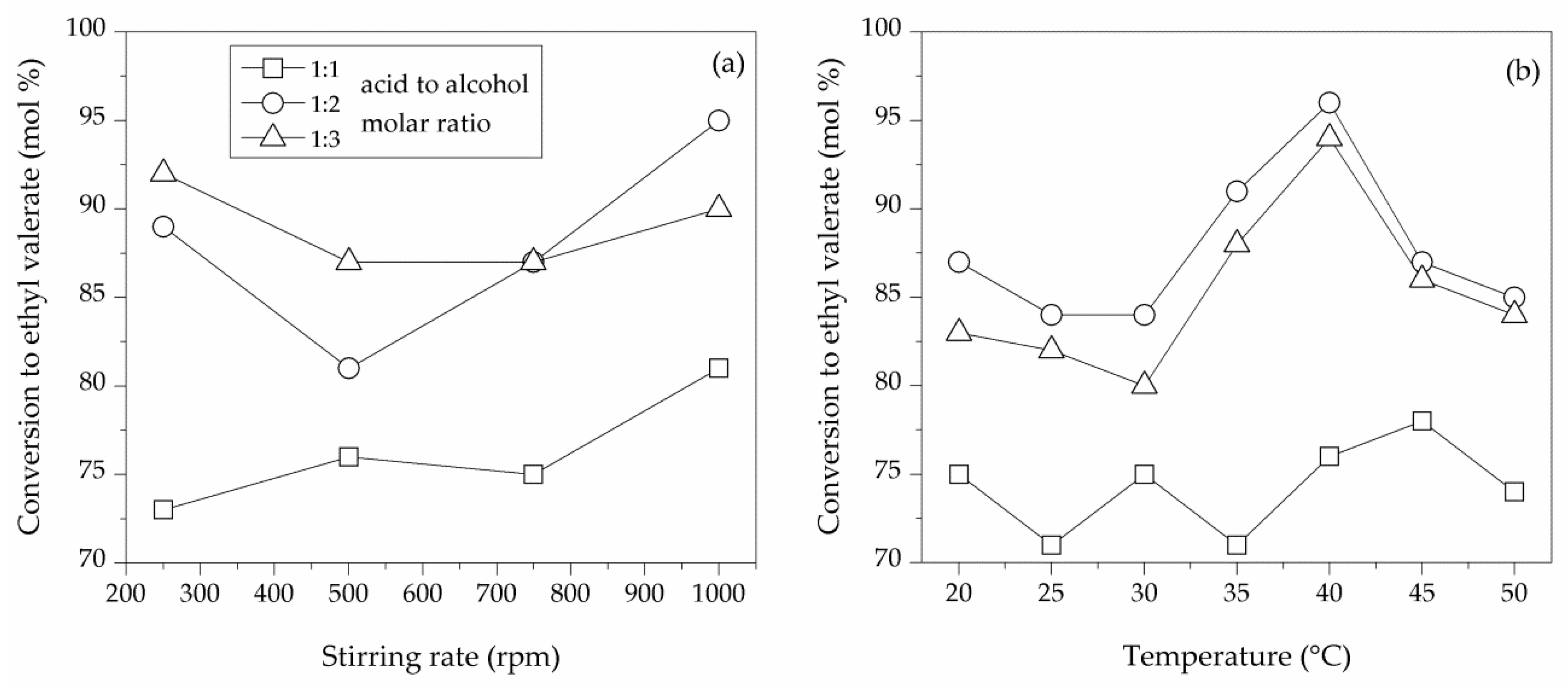
2.2.3. Influence of Stirring Rate
2.2.4. Influence of Reaction Temperature
2.3. Effect of Alcohol Type on Lipase Catalysed Esterification of Valeric Acid
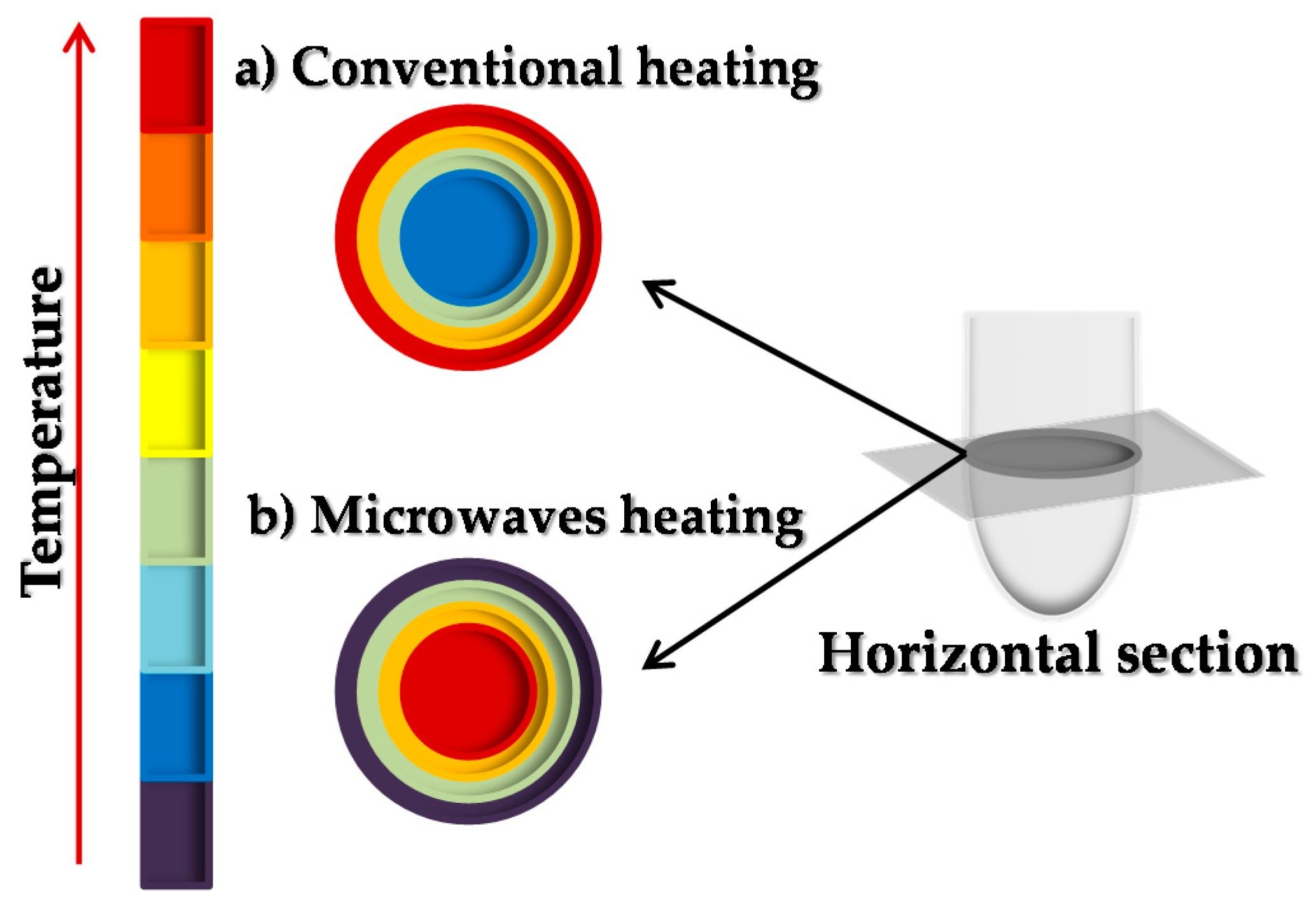
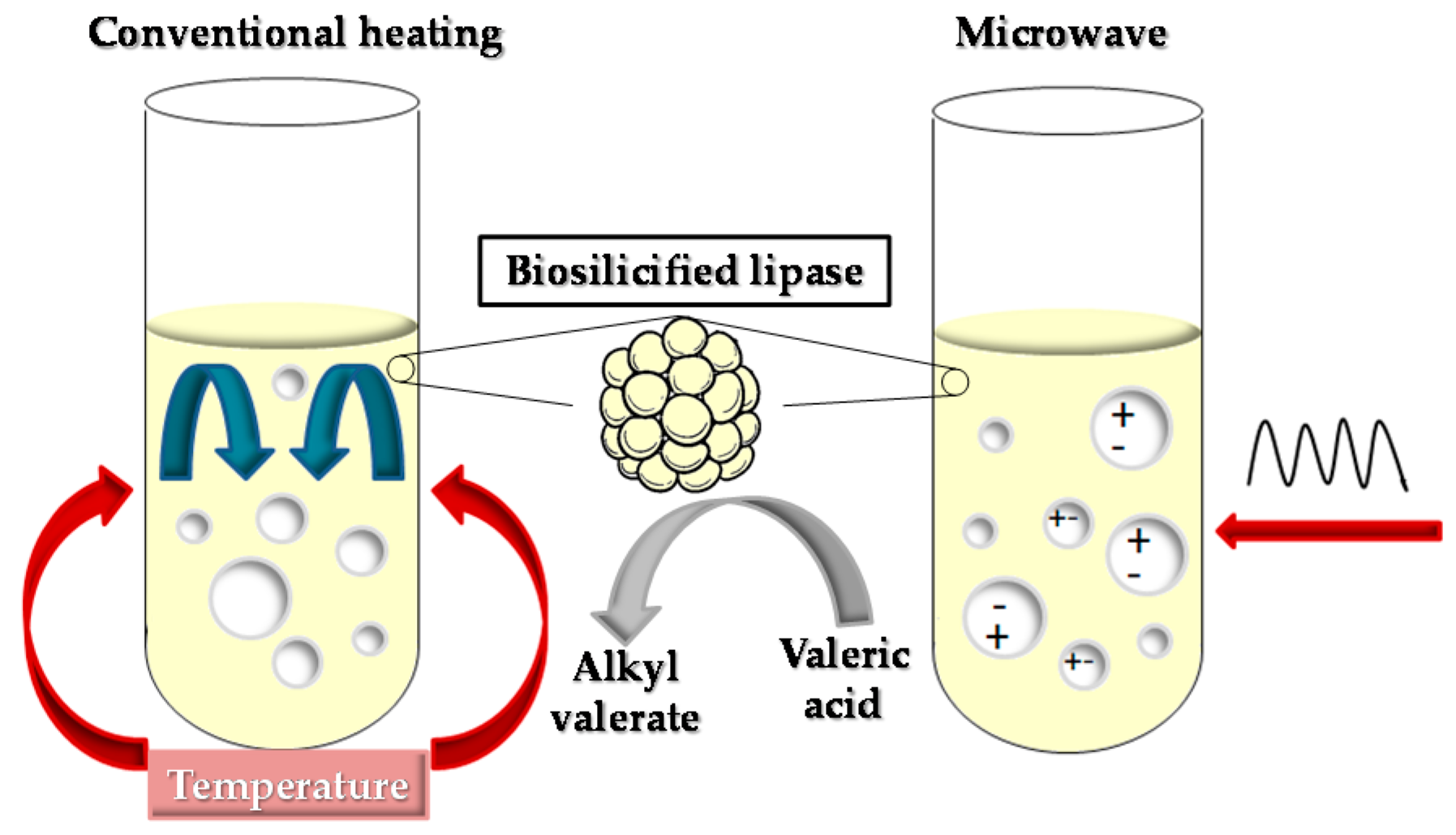
2.3.1. Production of Ethyl Valerate by Conventional Heating (CH) Procedures
2.3.2. Production of Ethyl Valerate Using Microwave (MW) Irradiation
3. Materials and Methods
3.1. Materials
3.2. Enzyme Biosilicification
3.3. Protein Concentration and Enzymatic Activity
3.4. Immobilization Efficiency
3.5. Biocatalytic Esterification Activity
3.6. Evaluation of Biocatalytic Esterification Behaviour
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Klass, D.L. Natural Biochemical Liquefaction. In Biomass for Renewable Energy, Fuels, and Chemicals; Academic Press: San Diego, CA, USA, 1998; pp. 333–382. ISBN 978-0-12-410950-6. [Google Scholar]
- Victor, K.; Sivaramakrishnan, V.; Nalini, R.; Sekar, T.; Premalatha, M.; Subramanian, P. A review on gasification of biomass. Renew. Sustain. Energy Rev. 2009, 13, 179–186. [Google Scholar]
- Dhake, K.P.; Thakare, D.D.; Bhanage, B.M. Lipase: A potential biocatalyst for the synthesis of valuable flavour and fragrance ester compounds. Flavour Fragr. J. 2013, 28, 71–83. [Google Scholar] [CrossRef]
- Padilha, G.S.; de Barros, M.; Alegre, R.M.; Tambourgi, E.B. Production of Ethyl Valerate from Burkholderia cepacia Lipase Immobilized in Alginate. Chem. Eng. Trans. 2013, 32, 1974–9791. [Google Scholar]
- Brault, G.; Shareck, F.; Hurtubise, Y.; Lépine, F.; Doucet, N. Short-Chain Flavor Ester Synthesis in Organic Media by an E. coli Whole-Cell Biocatalyst Expressing a Newly Characterized Heterologous Lipase. PLoS ONE 2014, 9, e91872. [Google Scholar] [CrossRef] [PubMed]
- Ben Akacha, N. Microbial and Enzymatic Technologies used for the Production of Natural Aroma Compounds: Synthesis, Recovery Modeling, and Bioprocesses. Food Bioprod. Process. 2014, 94, 675–706. [Google Scholar] [CrossRef]
- Adlercreutz, P. Immobilisation and application of lipases in organic media. Chem. Soc. Rev. 2013, 42, 6406–6436. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.; Deshpande, M.; Madamwar, D. Increasing esterification efficiency by double immobilization of lipase-ZnO bioconjugate into sodium bis (2-ethylhexyl) sulfosuccinate (AOT)-reverse micelles and microemulsion based organogels. Biocatal. Agric. Biotechnol. 2017, 10, 182–188. [Google Scholar] [CrossRef]
- Itabaiana, I.; Sutili, F.K.; Leite, S.G.F.; Goncalves, K.M.; Cordeiro, Y.; Leal, I.C.R.; Miranda, L.S.M.; Ojeda, M.; Luque, R.; de Souza, R.O.M.A. Continuous flow valorization of fatty acid waste using silica-immobilized lipases. Green Chem. 2013, 15, 518–524. [Google Scholar] [CrossRef]
- Corradini, M.C.C.; Costa, B.M.; Bressani, A.P.P.; Garcia, K.C.A.; Pereira, E.B.; Mendes, A.A. Improvement of the enzymatic synthesis of ethyl valerate by esterification reaction in a solvent system. Prep. Biochem. Biotechnol. 2017, 47, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Pistone, L.; Ottolina, G.; De, S.; Romero, A.A.; Martins, L.O.; Luque, R. Encapsulated Laccases for the Room-Temperature Oxidation of Aromatics: Towards Synthetic Low-Molecular-Weight Lignins. ChemSusChem 2016, 9, 756–762. [Google Scholar] [CrossRef] [PubMed]
- Shinde, S.D.; Yadav, G.D. Insight into microwave assisted immobilized Candida antarctica lipase B catalyzed kinetic resolution of RS-(±)-ketorolac. Process. Biochem. 2015, 50, 230–236. [Google Scholar] [CrossRef]
- Yadav, G.D.; Thorat, P.A. Microwave assisted lipase catalyzed synthesis of isoamyl myristate in solvent-free system. J. Mol. Catal. B 2012, 83, 16–22. [Google Scholar] [CrossRef]
- Qiu, Z.; Zhao, L.; Weatherley, L. Process intensification technologies in continuous biodiesel production. Chem. Eng. Process. 2010, 49, 323–330. [Google Scholar] [CrossRef]
- Gupta, S.M.; Kamble, M.P.; Yadav, G.D. Insight into microwave assisted enzyme catalysis in process intensification of reaction and selectivity: Kinetic resolution of (R,S)-flurbiprofen with alcohols. Mol. Catal. 2017, 440, 50–56. [Google Scholar] [CrossRef]
- Bougrin, K.; Loupy, A.; Soufiaoui, M. Microwave-assisted solvent-free heterocyclic synthesis. J. Photochem. Photobiol. C 2005, 6, 139–167. [Google Scholar] [CrossRef]
- Newman, S.G.; Jensen, K.F. The role of flow in green chemistry and engineering. Green Chem. 2013, 15, 1456–1472. [Google Scholar] [CrossRef]
- Reichardt, C.; Welton, T. Solvents and Solvent Effects in Organic Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2011; ISBN 3527642137. [Google Scholar]
- Ngaosuwan, K.; Goodwin, J.G., Jr.; Prasertdham, P. A green sulfonated carbon-based catalyst derived from coffee residue for esterification. Renew. Energy 2016, 86, 262–269. [Google Scholar] [CrossRef]
- Cebrián-García, S.; Balu, A.M.; Luque, R. Ultrasound-Assisted Esterification of Valeric Acid to Alkyl Valerates Promoted by Biosilicified Lipases. Front. Chem. 2018, 6, 197. [Google Scholar] [CrossRef] [PubMed]
- Karra Chaabouni, M.; Ghamgui, H.; Sofiane, B.; Rekik, A.; Gargouri, Y. Production of flavour esters by immobilized Staphylococcus simulans lipase in a solvent-free system. Process Biochem. 2006, 41, 1692–1698. [Google Scholar] [CrossRef]
- Ajmal, M.; Fieg, G.; Keil, F. Analysis of process intensification in enzyme catalyzed reactions using ultrasound. Chem. Eng. Process. 2016, 110, 106–113. [Google Scholar] [CrossRef]
- Rosa, M.; Roberts, C.J.; Rodrigues, M.A. Connecting high-temperature and low-temperature protein stability and aggregation. PLoS ONE 2017, 12, e0176748. [Google Scholar] [CrossRef] [PubMed]
- Eskandarloo, H.; Abbaspourrad, A. Production of galacto-oligosaccharides from whey permeate using β-galactosidase immobilized on functionalized glass beads. Food Chem. 2018, 251, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Henriquez, C.; Junior, I.; de Souza, R.; Luque, R. Novel nanoparticle/enzyme biosilicified nanohybrids for advanced heterogeneously catalyzed protocols. Catal. Sci. Technol. 2014, 5, 1840–1846. [Google Scholar]
- Lotti, M.; Pleiss, J.; Valero, F.; Ferrer, P. Effects of methanol on lipases: Molecular, kinetic and process issues in the production of biodiesel. Biotechnol. J. 2015, 10, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Dhar, K.; Kanwar, S.S.; Arora, P.K. Lipase catalysis in organic solvents: Advantages and applications. Biol. Proced. Online 2016, 18, 2. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-T.; Liao, J.C. Frontiers in microbial 1-butanol and isobutanol production. FEMS Microbiol. Lett. 2016, 363, fnw020. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Garcia-Galan, C.; Barbosa, O.; Hernandez, K.; dos Santos, J.C.S.; Rodrigues, R.C.; Fernandez-Lafuente, R. Evaluation of styrene-divinylbenzene beads as a support to immobilize lipases. Molecules 2014, 19, 7629–7645. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
| System | Enzymatic Activity (U/genzyme) | Protein Loading Ratio (%) | Activity Yield (%) |
|---|---|---|---|
| Support SiO2 | - | - | - |
| Free enzyme | 656 ± 14 | 100 | 100 |
| Biosilicified lipase (R0) | 592 ± 13 | 86 | 90 |
| Distilled Water (DW) % m/v | Conversion to Ethyl Valerate (mol %) | Specific Activity (U/gbiocatalyst) |
|---|---|---|
| 5 | 87 | 269 ± 10 |
| 10 | 85 | 301 ± 9 |
| 20 | 84 | 364 ± 11 |
| Acid-to-Alcohol Ratio | Conversion to Ethyl Valerate (mol %) | Specific Activity (U/gbiocatalyst) | ||||||
|---|---|---|---|---|---|---|---|---|
| 250 rpm | 500 rpm | 750 rpm | 1000 rpm | 250 rpm | 500 rpm | 750 rpm | 1000 rpm | |
| 1:3 | 73 | 76 | 75 | 81 | 478 ± 10 | 490 ± 11 | 497 ± 9 | 501 ± 13 |
| 1:2 | 89 | 81 | 87 | 95 | ||||
| 1:1 | 92 | 87 | 87 | 90 | ||||
| Acid-to-Alcohol Ratio | Conversion to Ethyl Valerate (mol %) | * Specific Activity (U/gbiocatalyst) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 50 °C | 45 °C | 40 °C | 35 °C | 30 °C | 25 °C | 20 °C | 50 °C | 40 °C | 30 °C | 20 °C | |
| 1:3 | 84 | 86 | 94 | 88 | 80 | 82 | 83 | 234 ± 11 | 485 ± 17 | 623 ± 14 | 630 ± 13 |
| 1:2 | 85 | 87 | 96 | 91 | 84 | 84 | 87 | ||||
| 1:1 | 74 | 78 | 76 | 71 | 75 | 71 | 75 | ||||
| Entry | System | Alcohol | Acid-to-Alcohol Ratio | Conversion to Alkyl Valerate (mol %) | * Specific Activity (U/gbiocatalyst) |
|---|---|---|---|---|---|
| 1 2 3 4 | Blank | MeOH EtOH iPrOH BuOH | All | No conversion | - |
| 5 6 7 8 | Support SiO2 | MeOH EtOH iPrOH BuOH | All | No conversion | - |
| 9 10 11 | Free enzyme | EtOH | 1:1 1:2 1:3 | 82 82 87 | 600 ± 12 |
| 12 | Biosilicified enzyme | MeOH | All | No conversion | - |
| 13 14 15 | Biosilicified enzyme | EtOH | 1:1 1:2 1:3 | 81 95 90 | 640 ± 17 |
| 16 17 18 | Biosilicified enzyme | iPrOH | 1:1 1:2 1:3 | 57 92 75 | 194 ± 15 |
| 19 20 21 | Biosilicified enzyme | BuOH | 1:1 1:2 1:3 | 60 89 77 | 291 ± 10 |
| Reuse | Conversion to Ethyl Valerate (mol%) | Specific Activity (U/gbiocatalyst) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MeOH | EtOH | IPrOH | BuOH | Free Enzyme | MeOH | EtOH | iPrOH | BuOH | Free Enzyme | |
| R0 | - | 95 ± 8 | 92 ± 7 | 89 ± 8 | 82 ± 6 | 418 ± 14 | 506 ± 16 | 508 ± 14 | 427 ± 12 | 352 ± 12 |
| R1 | - | 88 ± 7 | 42 ± 7 | 80 ± 9 | 75 ± 8 | 207 ± 12 | 505 ± 12 | 400 ± 19 | 307 ± 14 | 307 ± 16 |
| R2 | - | 94 ± 6 | - | 70 ± 7 | 68 ± 5 | - | 415 ± 18 | 367 ± 15 | 270 ± 12 | 301 ± 15 |
| R3 | - | 92 ± 8 | - | 69 ± 9 | 60 ± 8 | - | 400 ± 14 | - | 254 ± 17 | 272 ± 9 |
| R4 | - | 93 ± 6 | - | 60 ± 7 | 61 ± 6 | - | 403 ± 11 | - | 207 ± 16 | 260 ± 13 |
| Entry | System | Alcohol | Acid-to-Alcohol Ratio | Conversion to Alkyl Valerate (mol %) | Specific Activity (U/gbiocatalyst) |
|---|---|---|---|---|---|
| 1 2 3 4 | Blank | MeOH EtOH iPrOH BuOH | All | No conversion | - |
| 5 6 7 8 | Support SiO2 | MeOH EtOH iPrOH BuOH | All | No conversion | - |
| 9 10 11 | Free enzyme | EtOH | 1:1 1:2 1:3 | 70 75 71 | 270 ± 13 |
| 12 | Biosilicified enzyme | MeOH | All | No conversion | - |
| 13 14 15 | Biosilicified enzyme | EtOH | 1:1 1:2 1:3 | 58 82 73 | 376 ± 7 |
| 16 17 18 | Biosilicified enzyme | IPrOH | 1:1 1:2 1:3 | 39 40 37 | 300 ± 13 |
| 19 20 21 | Biosilicified enzyme | BuOH | 1:1 1:2 1:3 | 79 81 80 | 337 ± 5 |
| Methodology | Conversion to Ethyl Valerate (mol %) | Specific Activity (U/gbiocatalyst) | ||||
|---|---|---|---|---|---|---|
| 15 min | 30 min | 60 min | 15 min | 30 min | 60 min | |
| MW | 80 | 80 | 82 | 394 ± 18 | 384 ± 16 | 357 ± 10 |
| CH | 86 | 90 | 90 | 545 ± 14 | 495 ± 11 | 460 ± 11 |
| Reuses | Conversion to Ethyl Valerate (mol %) | Specific Activity (U/gbiocatalyst) |
|---|---|---|
| R0 | 82 ± 9 | 357 ± 14 |
| R1 | 66 ± 4 | 310 ± 9 |
| R2 | 51 ± 6 | 285 ± 11 |
| R3 | 35 ± 8 | 210 ± 12 |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cebrián-García, S.; Balu, A.M.; García, A.; Luque, R. Sol-Gel Immobilisation of Lipases: Towards Active and Stable Biocatalysts for the Esterification of Valeric Acid. Molecules 2018, 23, 2283. https://doi.org/10.3390/molecules23092283
Cebrián-García S, Balu AM, García A, Luque R. Sol-Gel Immobilisation of Lipases: Towards Active and Stable Biocatalysts for the Esterification of Valeric Acid. Molecules. 2018; 23(9):2283. https://doi.org/10.3390/molecules23092283
Chicago/Turabian StyleCebrián-García, Soledad, Alina M. Balu, Araceli García, and Rafael Luque. 2018. "Sol-Gel Immobilisation of Lipases: Towards Active and Stable Biocatalysts for the Esterification of Valeric Acid" Molecules 23, no. 9: 2283. https://doi.org/10.3390/molecules23092283





