Synthesis and Characterization of Bis[1]benzothieno[3,2-b:2′,3′-d]pyrroles: Quantitative Effects of Benzannulation on Dithieno[3,2-b:2′,3′-d]pyrroles
Abstract
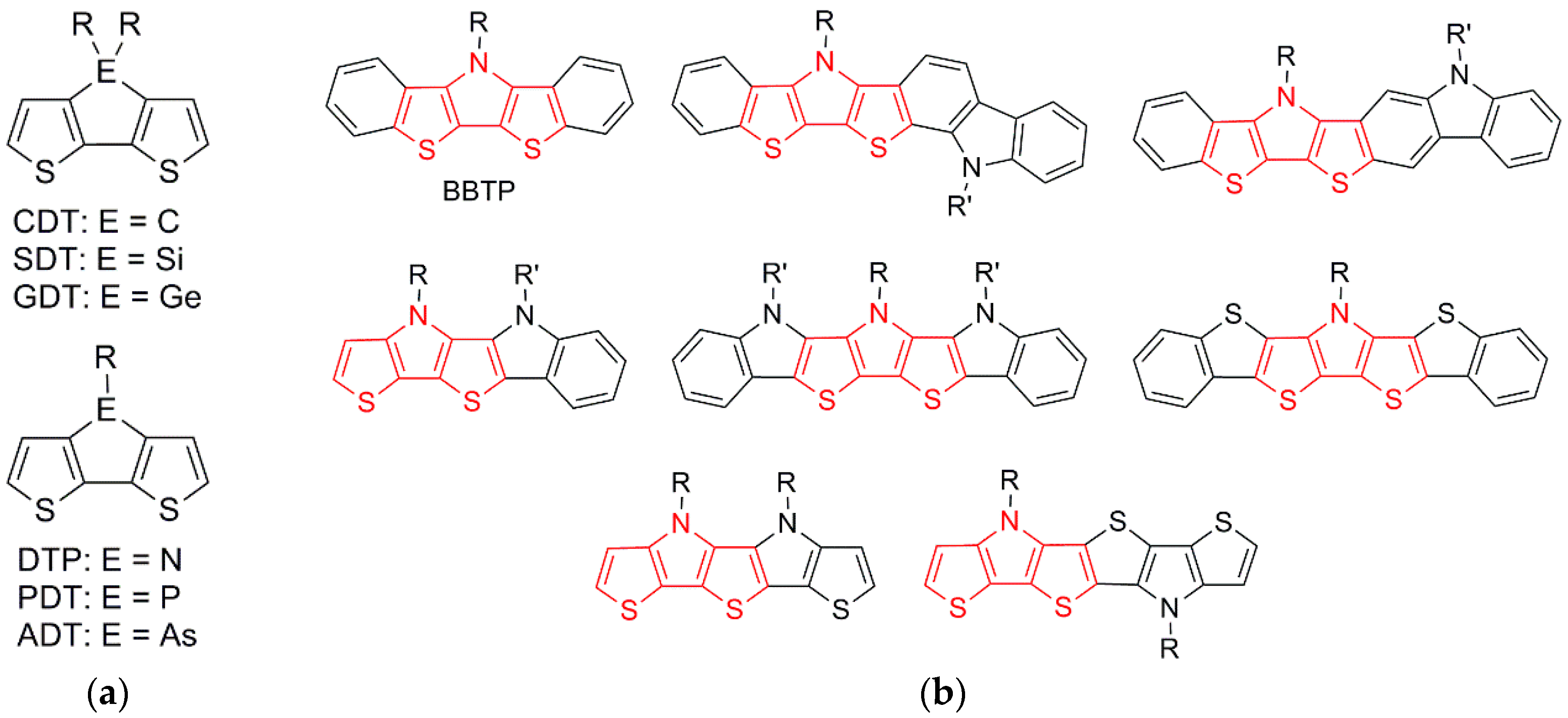
:1. Introduction
2. Results and Discussion
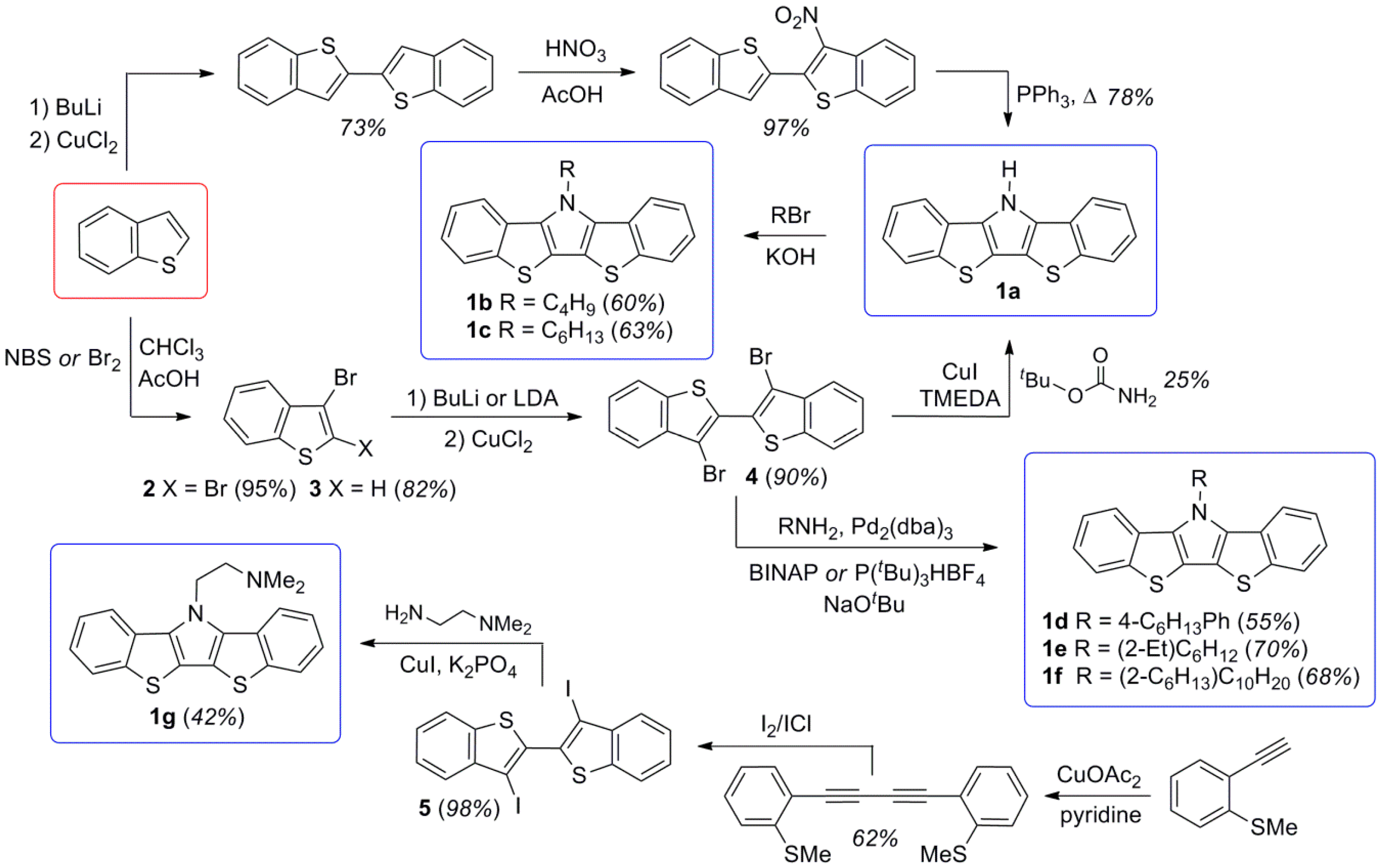
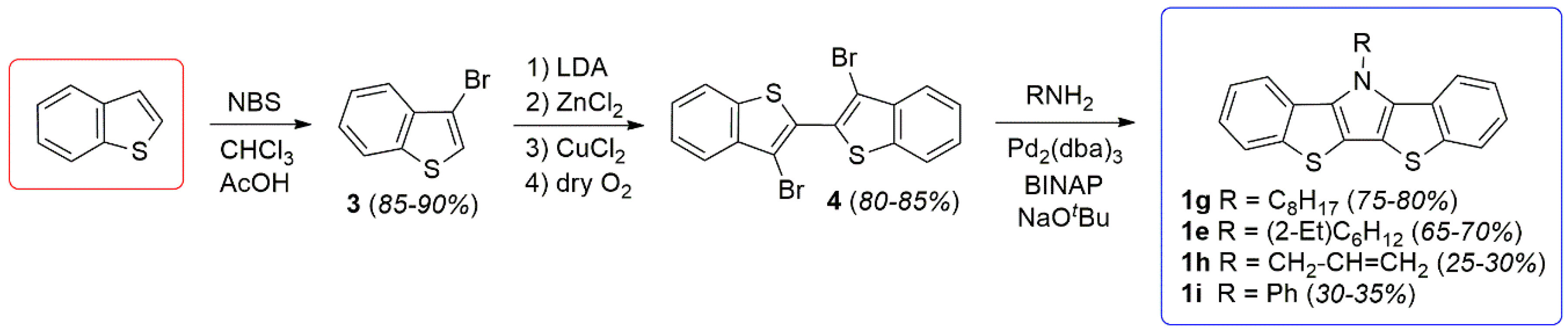
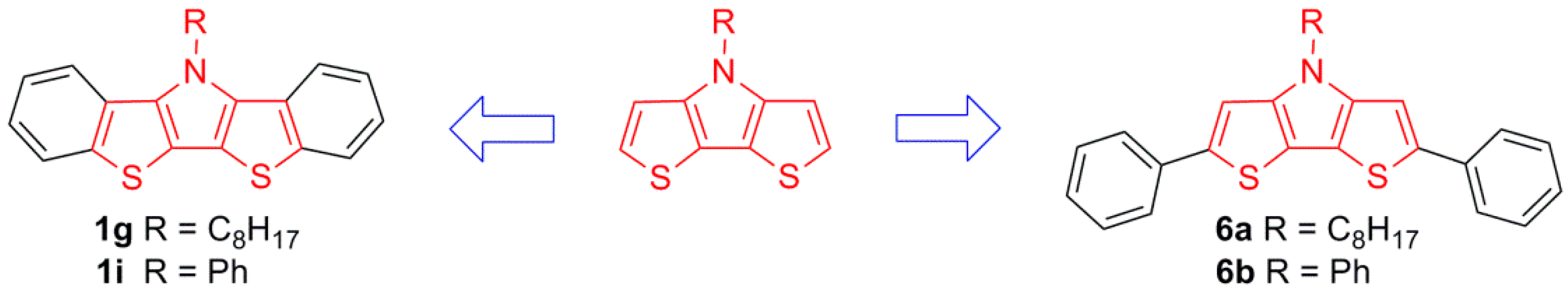
2.1. Synthesis
2.2. X-ray Crystallography and Structural Analysis of BBTPs
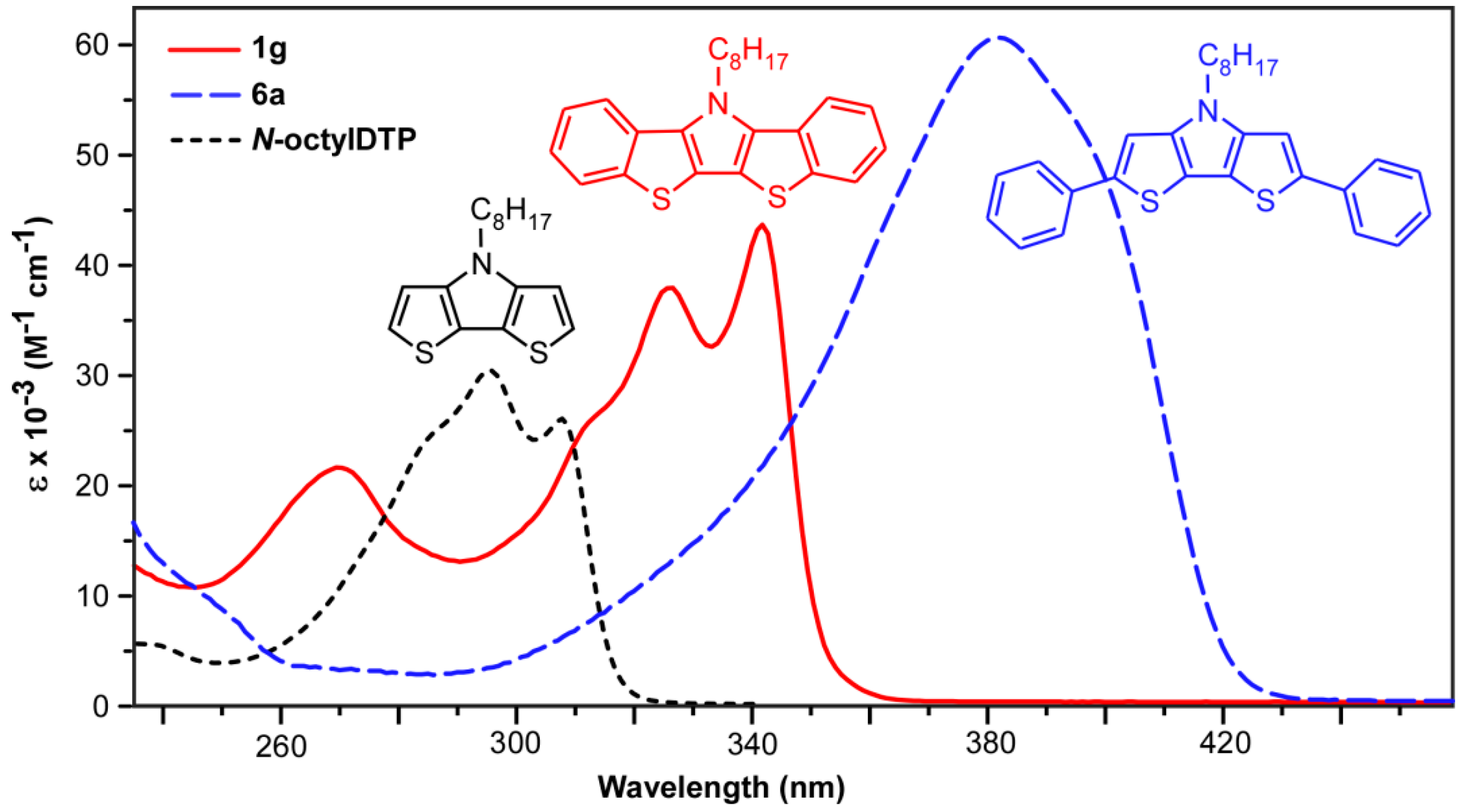
2.3. Absorption Spectroscopy
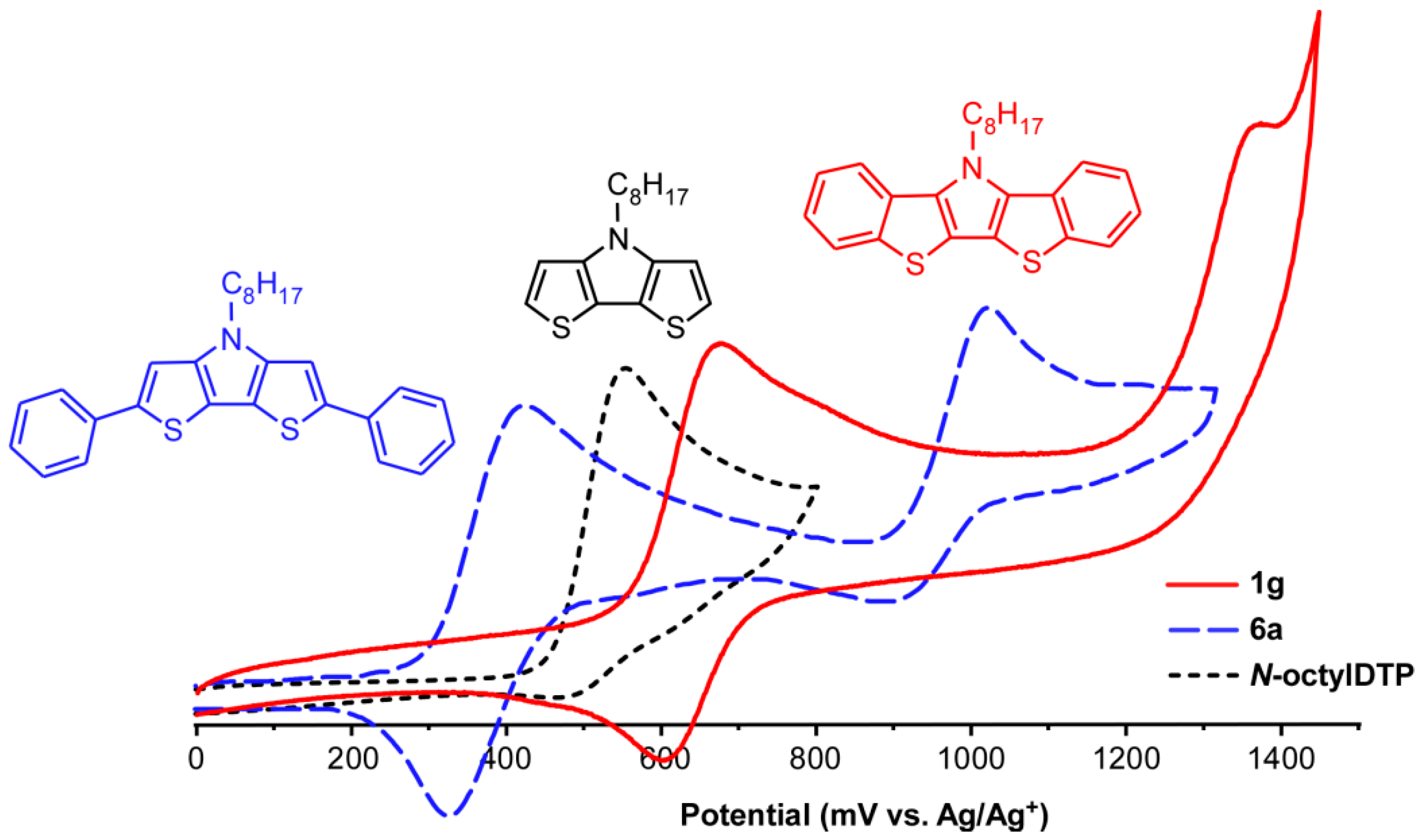
2.4. Electrochemistry
3. Materials and Methods
3.1. Synthesis of 3,3′-Dibromo-2,2′-bi(benzo[b]thiophene) (4)
3.2. General Synthesis of N-Functionalized Bis[1]benzothieno[3,2-b:2′,3′-d]pyrroles
3.2.1. N-Octylbis[1]benzothieno[3,2-b:2′,3′-d]pyrrole (1g)
3.2.2. N-(2-Ethylhexyl)bis[1]benzothieno[3,2-b:2′,3′-d]pyrrole (1e)
3.2.3. N-Allylbis[1]benzothieno[3,2-b:2′,3′-d]pyrrole (1h)
3.2.4. N-Phenylbis[1]benzothieno[3,2-b:2′,3′-d]pyrrole (1i)
3.3. X-ray Crystallography
3.4. Absorption Spectroscopy
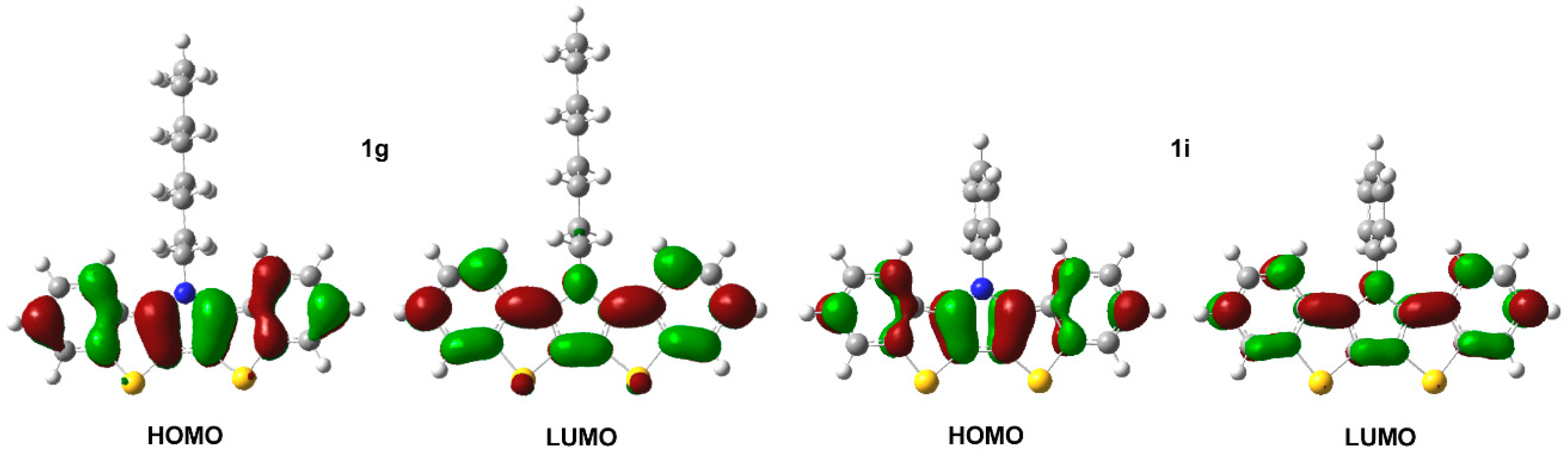
3.5. Computational Modeling
3.6. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Skotheim, T.A.; Reynolds, J.R. (Eds.) Handbook of Conducting Polymers, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2007; ISBN 978-1574446654. [Google Scholar]
- Rasmussen, S.C.; Ogawa, K.; Rothstein, S.D. Synthetic Approaches to Band Gap Control in Conjugated Polymeric Materials. In Handbook of Organic Electronics and Photonics; Nalwa, H.S., Ed.; American Scientific Publishers: Stevenson Ranch, CA, USA, 2008; Volume 1, pp. 1–50. ISBN 978-1-58883-095-5. [Google Scholar]
- Perepichka, I.F.; Perepichka, D.F. (Eds.) Handbook of Thiophene-Based Materials: Applications in Organic Electronics and Photonics; John Wiley & Sons: West Sussex, UK, 2009; ISBN 978-0470057322. [Google Scholar]
- Rasmussen, S.C. The Early History of Polyaniline: Discovery and Origins. Substantia 2017, 1, 99–109. [Google Scholar]
- Rasmussen, S.C. Revisiting the Early History of Synthetic Polymers: Critiques and New Insights. Ambix 2018, 65. in press. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, S.C. Early history of Conjugated Polymers: From their Origins to the Handbook of Conducting Polymers. In Handbook of Conducting Polymers, 4th ed.; Reynolds, J.R., Skotheim, T., Thompson, B., Eds.; CRC Press: Boca Raton, FL, USA, 2018; in press. [Google Scholar]
- Rasmussen, S.C. Electrically Conducting Plastics: Revising the History of Conjugated Organic Polymers. In 100+ Years of Plastics. Leo Baekeland and Beyond; Strom, E.T., Rasmussen, S.C., Eds.; ACS Symposium Series 1080; American Chemical Society: Washington, DC, USA, 2011; pp. 147–163. ISBN 9780841226777. [Google Scholar]
- Rasmussen, S.C. The Path to Conductive Polyacetylene. Bull. Hist. Chem. 2014, 39, 64–72. [Google Scholar]
- Rasmussen, S.C. Early History of Polypyrrole: The First Conducting Organic Polymer. Bull. Hist. Chem. 2015, 40, 45–55. [Google Scholar]
- Rasmussen, S.C. Early History of Conductive Organic Polymers. In Conductive Polymers: Electrical Interactions in Cell Biology and Medicine; Zhang, Z., Rouabhia, M., Moulton, S.E., Eds.; CRC Press: Boca Raton, FL, USA, 2017; pp. 1–21. ISBN 9781482259285. [Google Scholar]
- Baumgartner, T. π-Conjugated Heterocyclic fused Bithiophene Materials. J. Inorg. Organomet. Polym. Mater. 2005, 15, 389–409. [Google Scholar] [CrossRef]
- Rasmussen, S.C.; Evenson, S.J.; McCausland, C.B. Fluorescent Thiophene-based Materials and Their Outlook for Emissive Applications. Chem. Commun. 2015, 51, 4528–4543. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, S.C.; Evenson, S.J. Dithieno[3,2-b:2′,3′-d]pyrrole-based Materials: Synthesis and Applications to Organic Electronics. Prog. Polym. Sci. 2013, 38, 1773–1804. [Google Scholar] [CrossRef]
- Ogawa, K.; Rasmussen, S.C. A Simple and Efficient Route to N-Functionalized Dithieno[3,2-b:2′,3′-d]- pyrroles: Fused-Ring Building Blocks for New Conjugated Polymeric Systems. J. Org. Chem. 2003, 68, 2921–2928. [Google Scholar] [CrossRef] [PubMed]
- Evenson, S.J.; Seth, C.; Rasmussen, S.C. N-Acyldithieno[3,2-b:2′,3′-d]pyrroles: Second Generation Dithieno[3,2-b:2′,3′-d]pyrrole Building Blocks with Stabilized Energy Levels. Org. Lett. 2010, 12, 4054–4057. [Google Scholar] [CrossRef] [PubMed]
- Evenson, S.J.; Pappenfus, T.M.; Delgado, M.C.R.; Radke-Wohlers, K.; Navarrete, J.T.L.; Rasmussen, S.C. Molecular Tuning in Highly Fluorescent Dithieno[3,2-b:2′,3′-d]pyrrole-based Oligomers: Effects of N-Functionalization and Terminal Aryl Unit. Phys. Chem. Chem. Phys. 2012, 14, 6101–6111. [Google Scholar] [CrossRef] [PubMed]
- Amb, C.M.; Chen, S.; Graham, K.R.; Subbiah, J.; Small, C.E.; So, F.; Reynolds, J.R. Dithienogermole As a Fused Electron Donor in Bulk Heterojunction Solar Cells. J. Am. Chem. Soc. 2011, 133, 10062–10065. [Google Scholar] [CrossRef] [PubMed]
- Fei, Z.; Kim, J.S.; Smith, J.; Domingo, E.B.; Anthopoulos, T.D.; Stingelin, N.; Watkins, S.E.; Kim, J.-S.; Heeney, M. A low band gap co-polymer of dithienogermole and 2,1,3-benzothiadiazole by Suzuki polycondensation and its application in transistor and photovoltaic cells. J. Mater. Chem. 2011, 21, 16257–16263. [Google Scholar] [CrossRef] [Green Version]
- Gendron, D.; Morin, P.-O.; Berrouard, P.; Allard, N.; Aich, B.R.; Garon, C.N.; Tao, Y.; Leclerc, M. Synthesis and Photovoltaic Properties of Poly(dithieno[3,2-b:2′,3′-d]germole) Derivatives. Macromolecules 2011, 44, 7188–7193. [Google Scholar] [CrossRef]
- Green, J.P.; Han, Y.; Kilmurray, R.; McLachlan, M.A.; Anthopoulos, T.D.; Heeney, M. An Air-Stable Semiconducting Polymer Containing Dithieno[3,2-b:2′,3′-d]arsole. Angew. Chem. Int. Ed. 2016, 25, 7148–7151. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Imoto, H.; Tanaka, S.; Ishidoshiro, M.; Naka, K. Facile synthesis and properties of dithieno-[3,2-b:2′,3′-d]arsoles. Dalton Trans. 2016, 45, 11338–11345. [Google Scholar] [CrossRef] [PubMed]
- Green, J.P.; Cryer, S.J.; Marafie, J.; White, A.J.P.; Heeney, M. Synthesis of a Luminescent Arsolo-[2,3-d:5,4-d′]bis(thiazole) Building Block and Comparison to Its Phosphole Analogue. Organometallics 2017, 36, 2632–2636. [Google Scholar] [CrossRef]
- Qi, T.; Guo, Y.; Liu, Y.; Xi, H.; Zhang, H.; Gao, X.; Liu, Y.; Lu, K.; Du, C.; Yu, G.; Zhu, D. Synthesis and properties of the anti and syn isomers of dibenzothieno[b,d]pyrrole. Chem. Commun. 2008, 6227–6229. [Google Scholar] [CrossRef] [PubMed]
- Balaji, G.; Valiyaveettil, S. Synthesis and Properties of Symmetric and Unsymmetric Dibenzothieno- pyrroles. Org. Lett. 2009, 11, 3358–3361. [Google Scholar] [CrossRef] [PubMed]
- Balaji, G.; Della Pelle, A.M.; Popere, B.C.; Chandrasekaran, A.; Thayumanavan, S. Synthesis and properties of thienopyrrole based heteroacenes – indolodibenzothienopyrrole and dicarbazolodithienopyrrole. Org. Biomol. Chem. 2012, 10, 3455–3462. [Google Scholar] [CrossRef] [PubMed]
- Jung, I.H.; Kim, J.-H.; Nam, S.Y.; Lee, C.; Hwang, D.-H.; Yoon, S.C. Development of New Photovoltaic Conjugated Polymers Based on Di(1-benzothieno)[3,2-b:2′,3′-d]pyrrole: Benzene Ring Extension Strategy for Improving Open-Circuit Voltage. Macromolecules 2015, 48, 5213–5221. [Google Scholar] [CrossRef]
- Gupta, A.; Flynn, B.L. Linear and Angular Heteroacenes from Double-Electrophilic Cyclization (DEC) and DEC-Reductive Elimination of Diynes. Org. Lett. 2017, 19, 1939–1941. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Cho, D.; Yang, X.; Enkelmann, V.; Baumgarten, M.; Müllen, K. Heteroheptacenes with Fused Thiophene and Pyrrole Rings. Chem. Eur. J. 2010, 16, 5119–5128. [Google Scholar] [CrossRef] [PubMed]
- Balaji, G.; Phua, D.I.; Shim, W.L.; Valiyaveettil, S. Synthesis and Characterization of Unsymmetric Indolodithienopyrrole and Extended Diindolodithienopyrrole. Org. Lett. 2010, 12, 232–235. [Google Scholar] [CrossRef] [PubMed]
- Mitsudo, K.; Shimohara, S.; Mizoguchi, J.; Mandai, H.; Suga, S. Synthesis of Nitrogen-Bridged Terthiophenes by Tandem Buchwald-Hartwig Coupling and Their Properties. Org. Lett. 2012, 14, 2702–2705. [Google Scholar] [CrossRef] [PubMed]
- Wetzel, C.; Mishra, A.; Mena-Osteritz, E.; Liess, A.; Stolte, M.; Würthner, F.; Bäuerle, P. Synthesis and Structural Analysis of Thiophene-Pyrrole-Based S,N-Heteroacenes. Org. Lett. 2014, 16, 362–365. [Google Scholar] [CrossRef] [PubMed]
- Qin, P.; Kast, H.; Nazeeruddin, M.K.; Zakeeruddin, S.M.; Mishra, A.; Bäuerle, P.; Grätzel, M. Low band gap S,N-heteroacene-based oligothiophenes as hole-transporting and light absorbing materials for efficient perovskite-based solar cells. Energy Environ. Sci. 2014, 7, 2981–2985. [Google Scholar] [CrossRef]
- Rasmussen, S.C. The Nomenclature of Fused-ring Arenes and Heterocycles: A Guide to an Increasingly Important Dialect of Organic Chemistry. Chem. Texts 2016, 2, 16. [Google Scholar] [CrossRef]
- Jung, I.H.; Kim, J.-H.; Nam, S.Y.; Lee, C.; Hwang, D.-H.; Yoon, S.C. A di(1-benzothieno)-[3,2-b:2′,3′-d]pyrrole and isoindigo-based electron donating conjugated polymer for efficient organic photovoltaics. J. Mater. Chem. C 2016, 4, 663–667. [Google Scholar] [CrossRef]
- Dahlmann, U.; Neidlein, R. The Diyne Reaction of 3,3′-Bis(phenylethynyl)-2,2′-bithiophene Derivatives via Rhodium Complexes: A Novel Approach to Condensed Benzo[2,l-b:3,4-b’]dithiophenes. Helv. Chim. Acta 1997, 80, 111–120. [Google Scholar] [CrossRef]
- Gao, J.; Li, R.; Li, L.; Meng, Q.; Jiang, H.; Li, H.; Hu, W. High-Performance Field-Effect Transistor Based on Dibenzo[d,d′]thieno[3,2-b;4,5-b′]dithiophene, an Easily Synthesized Semiconductor with High Ionization Potential. Adv. Mater. 2007, 19, 3008–3011. [Google Scholar] [CrossRef]
- Dienes, Y.; Eggenstein, M.; Kárpáti, T.; Sutherland, T.C.; Nyulászi, L.; Baumgartner, T. Phosphorus-Based Heteropentacenes: Efficiently Tunable Materials for Organic n-Type Semiconductors. Chem.–Eur. J. 2008, 14, 9878–9889. [Google Scholar] [CrossRef] [PubMed]
- Alessandrini, L.; Braga, D.; Jaafari, A.; Miozzo, L.; Mora, S.; Silvestri, L.; Tavazzi, S.; Yassar, A. Optical Properties of Dibenzo[d,d′]thieno[3,2-b;4,5-b′]dithiophene Monocrystals: The Effect of Intermolecular Interactions. J. Phys. Chem. A 2011, 115, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Hao, W.; Ma, W.; Zang, Z.; Zhang, H.; Liu, X.; Zou, S.; Zhang, H.; Liu, W.; Gao, J. Easily-soluble heteroacene bis(benzothieno)silole derivatives for sensing of nitro explosives. New J. Chem. 2014, 38, 5754–5760. [Google Scholar] [CrossRef]
- Oechsle, P.; Paradies, J. Ambidextrous Catalytic Access to Dithieno[3,2-b:2′,3′-d]thiophene (DTT) Derivatives by Both Palladium-Catalyzed C−S and Oxidative Dehydro C−H Coupling. Org. Lett. 2014, 16, 4086–4089. [Google Scholar] [CrossRef] [PubMed]
- Kabir, S.M.H.; Miura, M.; Sasaki, S.; Harada, G.; Kuwatani, Y.; Yoshida, M.; Iyoda, M. New Syntheses of Tricyclic Thiophenes and Cyclic Tetrathiophenes Using Transition-Metal-catalyzed Cyclization. Heterocycles 2000, 52, 761–774. [Google Scholar]
- Katritzky, A.R.; Pozharskii, A.F. Handbook of Heterocyclic Chemistry, 2nd ed.; Pergamon Press: New York, NY, USA, 2000; p. 61. ISBN 0-08-042989-0. [Google Scholar]
- Katritzky, A.R.; Pozharskii, A.F. Handbook of Heterocyclic Chemistry, 2nd ed.; Pergamon Press: New York, NY, USA, 2000; p. 81. ISBN 0-08-042989-0. [Google Scholar]
- Barbarella, G.; Zambianchi, M.; Antolini, L.; Folli, U.; Goldoni, F.; Iarossi, D.; Schenetti, L.; Bongini, A. Conformational properties of 3,3′-, 3,4′- and 4,4′-dimethyl- and -bis(methylsulfanyl)-2,2′-bithiophenes. J. Chem. Soc. Penkin Trans. 2 1995, 10, 1869–1873. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Pozharskii, A.F. Handbook of Heterocyclic Chemistry, 2nd ed.; Pergamon Press: New York, NY, USA, 2000; p. 71. ISBN 0-08-042989-0. [Google Scholar]
- Cardona, C.M.; Li, W.; Kaifer, A.E.; Stockdale, D.; Bazan, G.C. Electrochemical Considerations for Determining Absolute Frontier Orbital Energy Levels of Conjugated Polymers for Solar Cell Applications. Adv. Mater. 2011, 23, 2367–2371. [Google Scholar] [CrossRef] [PubMed]
- Evenson, S.J.; Mumm, M.J.; Pokhodnya, K.I.; Rasmussen, S.C. Highly Fluorescent Dithieno[3,2-b:2′,3′-d]pyrrole-based Materials: Synthesis, Characterization and OLED Device Applications. Macromolecules 2011, 44, 835–841. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision A.02. Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.T.; Yang, W.T.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Larson, R.C.; Iwamoto, R.T.; Adams, R.N. Reference electrodes for voltammetry in acetonitrile. Anal. Chim. Acta 1961, 25, 371–374. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |
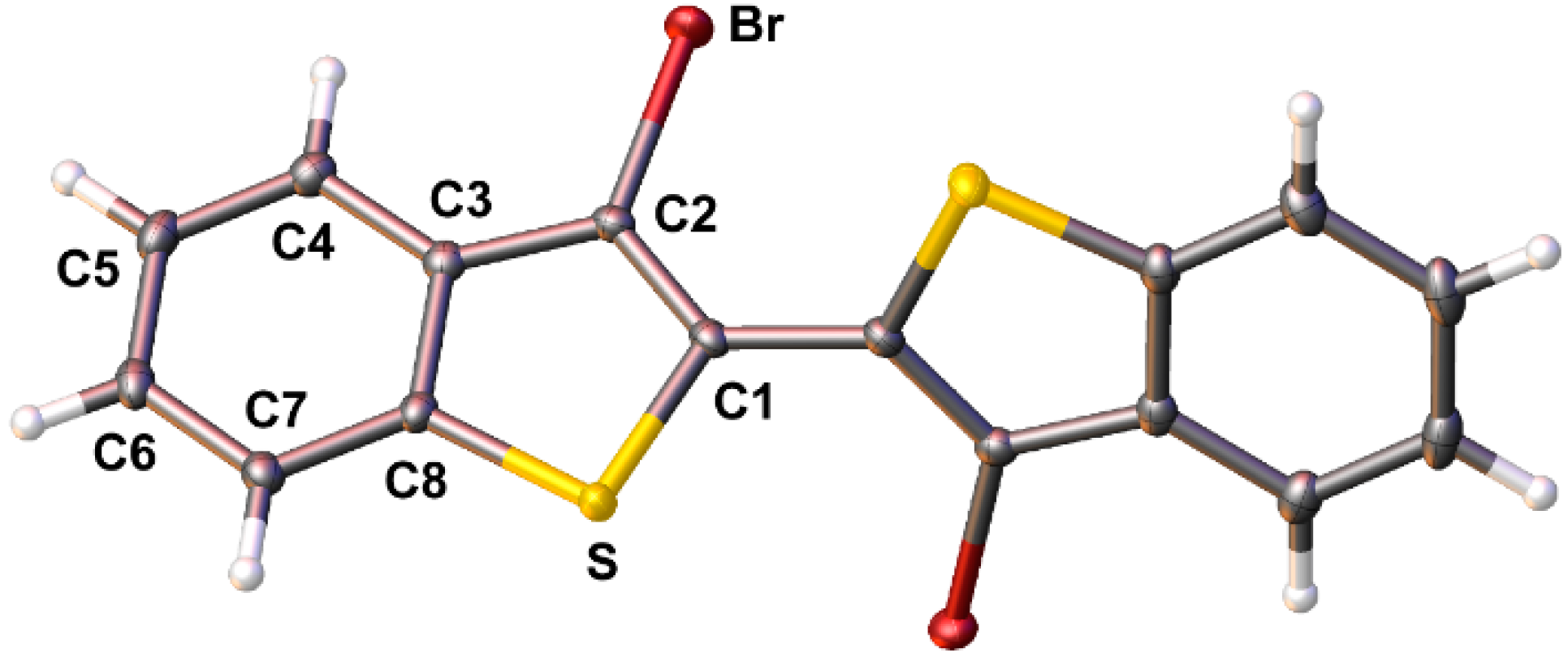


| Bond | 1a 1 | 1c 1 | 1g | 1e | 1i | 1d 2 | N-octylDTP 3 |
|---|---|---|---|---|---|---|---|
| S1-C1 | 1.732(2) | 1.7362(16) | 1.7338(14) | 1.731(6) | 1.7319(11) | 1.7325(13) | 1.719(3) |
| S1-C4 | 1.759(3) | 1.7582(17) | 1.7560(14) | 1.763(6) | 1.7546(12) | 1.7536(14) | 1.716(3) |
| C1-C2 | 1.389(3) | 1.3974(19) | 1.3960(18) | 1.404(8) | 1.3924(15) | 1.3894(17) | 1.384(4) |
| C2-N1 | 1.373(3) | 1.384(2) | 1.3842(17) | 1.378(8) | 1.3844(13) | 1.3802(16) | 1.379(5) |
| N1-C9 | 1.4706(16) | 1.4650(16) | 1.464(7) | 1.4347(14) | 1.4367(15) | 1.451(4) | |
| C2-C3 | 1.431(3) | 1.431(2) | 1.4382(19) | 1.443(8) | 1.4334(14) | 1.4307(18) | 1.416(5) |
| C3-C4 | 1.407(3) | 1.422(2) | 1.4201(19) | 1.412(8) | 1.4171(15) | 1.4159(18) | 1.349(6) |
| C1-C5 | 1.412(3) | 1.411(2) | 1.4080(19) | 1.410(8) | 1.4123(14) | 1.4163(18) | 1.420(4) |
| C3-C10 | 1.402(3) | 1.404(2) | 1.4079(18) | 1.406(8) | 1.4050(16) | 1.3984(19) | |
| C4-C13 | 1.392(3) | 1.394(2) | 1.393(2) | 1.387(9) | 1.3991(15) | 1.3951(19) | |
| C10-C11 | 1.372(4) | 1.382(3) | 1.384(2) | 1.387(9) | 1.3877(16) | 1.386(2) | |
| C11-C12 | 1.396(4) | 1.397(2) | 1.399(2) | 1.386(9) | 1.4011(18) | 1.389(2) | |
| C12-C13 | 1.384(4) | 1.378(2) | 1.386(2) | 1.381(9) | 1.3893(17) | 1.384(2) |
| Compound | λmax (nm) | ε (M−1 cm−1) | Eopt (eV) 2 |
|---|---|---|---|
| N-octylDTP | 309 | 26,100 | 3.88 |
| 296 | 30,400 | ||
| N-phenylDTP | 310 | 32,000 | 3.87 |
| 299 | 35,200 | ||
| 1g | 341 | 44,300 | 3.52 |
| 325 | 39,200 | ||
| 267 | 21,000 | ||
| 1e | 341 | 44,100 | 3.52 |
| 325 | 39,100 | ||
| 267 | 20,900 | ||
| 1h | 340 | 48,000 | 3.52 |
| 324 | 45,100 | ||
| 266 | 24,900 | ||
| 1i | 341 | 40,400 | 3.52 |
| 325 | 36,700 | ||
| 265 | 22,500 | ||
| 6a | 381 | 61,000 | 2.95 |
| 6b | 378 | 58,400 | 2.96 |
| Compound | E1/20/+1 (V) | E (mV) | Epa+1/+2 (V) | EHOMO (eV) 2 |
|---|---|---|---|---|
| N-octylDTP | 0.56 3 | −5.60 | ||
| N-phenylDTP | 0.65 3 | −5.69 | ||
| 1g | 0.64 | 75 | 1.37 | −5.68 |
| 1e | 0.65 | 80 | 1.37 | −5.69 |
| 1h | 0.65 | 75 | 1.37 | −5.69 |
| 1i | 0.70 | 90 | 1.37 | −5.70 |
| 6a | 0.38 | 80 | 1.05 | −5.42 |
| 6b | 0.47 | 80 | 1.09 | −5.51 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wolfe, R.M.W.; Culver, E.W.; Rasmussen, S.C. Synthesis and Characterization of Bis[1]benzothieno[3,2-b:2′,3′-d]pyrroles: Quantitative Effects of Benzannulation on Dithieno[3,2-b:2′,3′-d]pyrroles. Molecules 2018, 23, 2279. https://doi.org/10.3390/molecules23092279
Wolfe RMW, Culver EW, Rasmussen SC. Synthesis and Characterization of Bis[1]benzothieno[3,2-b:2′,3′-d]pyrroles: Quantitative Effects of Benzannulation on Dithieno[3,2-b:2′,3′-d]pyrroles. Molecules. 2018; 23(9):2279. https://doi.org/10.3390/molecules23092279
Chicago/Turabian StyleWolfe, Rylan M. W., Evan W. Culver, and Seth C. Rasmussen. 2018. "Synthesis and Characterization of Bis[1]benzothieno[3,2-b:2′,3′-d]pyrroles: Quantitative Effects of Benzannulation on Dithieno[3,2-b:2′,3′-d]pyrroles" Molecules 23, no. 9: 2279. https://doi.org/10.3390/molecules23092279






