Imidazolium Chloride: An Efficient Catalyst for Transamidation of Primary Amines
Abstract
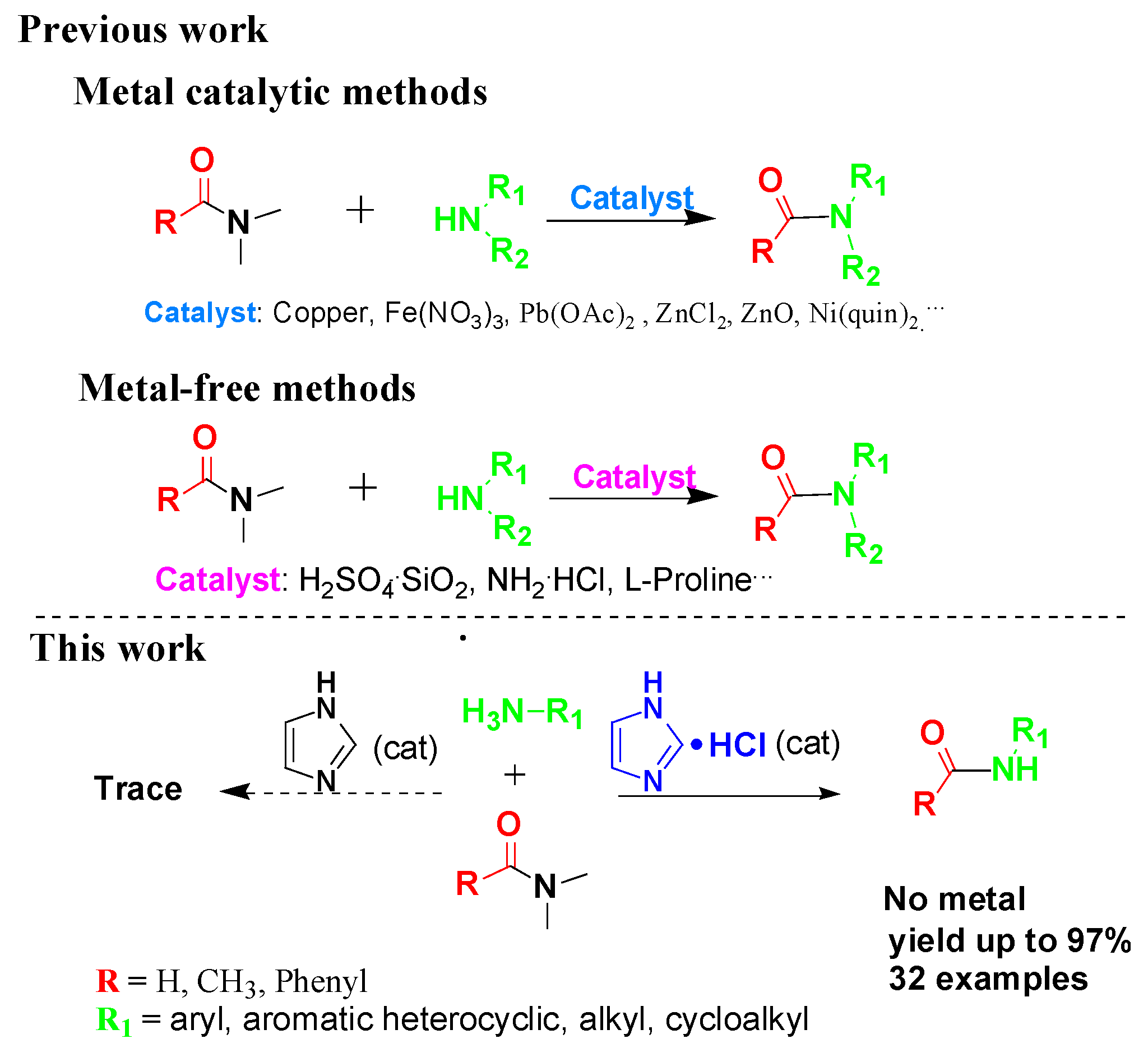
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. General Procedures for the Synthesis of N-Acetamides 1a–1w
3.3. General Procedures for the Synthesis of N-Formamides 2a–2e
3.4. General Procedures for the Synthesis of N-Benzoylation 3a–3d
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cupido, T.; Tulla-Puche, J.J.; Albericio, F. The synthesis of naturally occurring peptides and their analogs. Curr. Opin. Drug Discov. Dev. 2007, 10, 768–783. [Google Scholar]
- Lundberg, H.; Tinnis, F.; Selander, N.; Adolfsson, H. ChemInform Abstract: Catalytic Amide Formation from Non-Activated Carboxylic Acids and Amines. Chem. Soc. Rev. 2014, 43, 2714–2742. [Google Scholar] [CrossRef] [PubMed]
- Pattabiraman, V.R.; Bode, J.W. Rethinking amide bond synthesis. Nature 2011, 480, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Valeur, E.; Bradley, M. Amide bond formation: Beyond the myth of coupling reagents. Chem. Soc. Rev. 2009, 40, 606–631. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Mercado, N.; Millerlarsson, A.; Barnes, P.J.; Ito, K. Increased corticosteroid sensitivity by a long acting β2 agonist formoterol via β2 adrenoceptor independent protein phosphatase 2A activation. Pulm. Pharmacol. Ther. 2012, 25, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Iwanaga, Y.; Kimura, T.; Miyashita, N.; Morikawa, K.; Nagata, O.; Itoh, Z. Characterization of acetylcholinesterase-inhibition by itopride. Jpn. J. Pharmacol. 1994, 66, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Abe, H.; Kikuchi, S.; Hayakawa, K.; Iida, T.; Nagahashi, N.; Maeda, K. Discovery of a highly potent and selective mek inhibitor: Gsk1120212 (jtp-74057 dmso solvate). ACS Med. Chem. Lett. 2011, 2, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Perez-Aso, M.; Montesinos, M.C.; Mediero, A.; Wilder, T.; Schafer, P.H.; Cronstein, B. Apremilast, a novel phosphodiesterase 4 (pde4) inhibitor, regulates inflammation through multiple camp downstream effectors. Arthritis Res. Ther. 2015, 17, 249. [Google Scholar] [CrossRef] [PubMed]
- Hinz, B.; Cheremina, O.; Brune, K. Acetaminophen (paracetamol) is a selective cyclooxygenase-2 inhibitor in man. FASEB J. 2008, 22, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Zamponi, G.W.; Sui, X.; Codding, P.W.; French, R.J. Dual actions of procainamide on batrachotoxin-activated sodium channels: Open channel block and prevention of inactivation. Biophys. J. 1993, 65, 2324–2334. [Google Scholar] [CrossRef]
- Gernigon, N.; Alzoubi, R.M.; Hall, D.G. Direct amidation of carboxylic acids catalyzed by ortho-iodo arylboronic acids: Catalyst optimization, scope, and preliminary mechanistic study supporting a peculiar halogen acceleration effect. J. Org. Chem. 2012, 77, 8386–8400. [Google Scholar] [CrossRef] [PubMed]
- Ueki, H.; Ellis, T.K.; Martin, C.H.; Boettiger, T.U.; Bolene, S.B.; Soloshonok, V.A. Improved synthesis of proline-derived Ni (II) complexes of glycine: Versatile chiral equivalents of nucleophilic glycine for general asymmetric synthesis of alpha-amino acids. J. Org. Chem. 2003, 68, 7104–7107. [Google Scholar] [CrossRef] [PubMed]
- Ueki, H.; Ellis, T.K.; Martin, C.H.; Soloshonok, V.A. Efficient large-scale synthesis of picolinic acid-derived Ni (II) complexes of glycine. Eur. J. Org. Chem. 2010, 2003, 1954–1957. [Google Scholar] [CrossRef]
- Lanigan, R.M.; Sheppard, T.D. Recent developments in amide synthesis: Direct amidation of carboxylic acids and transamidation reactions. Eur. J. Org. Chem. 2013, 33, 7453–7465. [Google Scholar] [CrossRef]
- Kissounko, D.A.; Hoerter, J.M.; Guzei, I.A.; Cui, Q.; Gellman, S.H.; Stahl, S.S. Ti(iv)-mediated reactions between primary amines and secondary carboxamides: Amidine formation versus transamidation. J. Am. Chem. Soc. 2007, 129, 1776–1783. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, N.A.; Zhu, J.; Gellman, S.H.; Stahl, S.S. Catalytic transamidation reactions compatible with tertiary amide metathesis under ambient conditions. J. Am. Chem. Soc. 2009, 131, 10003–10008. [Google Scholar] [CrossRef] [PubMed]
- Hoerter, J.M.; Otte, K.M.; Gellman, S.H.; Cui, Q.; Stahl, S.S. Discovery and mechanistic study of AlIII-catalyzed transamidation of tertiary amides. J. Am. Chem. Soc. 2008, 130, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Hoerter, J.M.; Otte, K.M.; Gellman, S.H.; Stahl, S.S. Mechanism of AlIII-catalyzed transamidation of unactivated secondary carboxamides. J. Am. Chem. Soc. 2006, 128, 5177–5183. [Google Scholar] [CrossRef] [PubMed]
- Kissounko, D.A.; Guzei, I.A.; And, S.H.G.; Stahl, S.S. Titanium(iv)-mediated conversion of carboxamides to amidines and implications for catalytic transamidation. Organometallics. 2005, 24, 5208–5210. [Google Scholar] [CrossRef]
- Watson, A.J.; Maxwell, A.C.; Williams, J.M. Ruthenium-catalyzed oxidation of alcohols into amides. Org. Lett. 2009, 11, 2667–2670. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, B.N.; Chhatwal, A.R.; Lomax, H.V.; Walton, J.W.; Williams, J.M.J. Transamidation of primary amides with amines catalyzed by zirconocene dichloride. Chem. Commun. 2012, 48, 11626–11628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dineen, T.A.; Zajac, M.A.; Myers, A.G. Efficient transamidation of primary carboxamides by in situ activation with N,N-dialkylformamide dimethyl acetals. J. Am. Chem. Soc. 2007, 38, 16406–16409. [Google Scholar] [CrossRef]
- Zhang, M.; Imm, S.; Bähn, S.; Neubert, L.; Neumann, H.; Beller, M. Efficient copper (II)-catalyzed transamidation of non-activated primary carboxamides and ureas with amines. Angew. Chem. Int. Ed. 2012, 124, 3905–3975. [Google Scholar] [CrossRef] [PubMed]
- Tamura, M.; Tonomura, T.; Shimizu, K.; Satsuma, A. Transamidation of amides with amines under solvent-free conditions using a CeO2 catalyst. Green Chem. 2012, 14, 717–724. [Google Scholar] [CrossRef]
- Becerra-Figueroa, L.; Ojeda-Porras, A.; Gamba-Sanchez, D. Transamidation of carboxamides catalyzed by Fe (III) and water. J. Org. Chem. 2015, 45, 4544–4552. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Singh, D.P.; Tiwari, K.; Mishra, M.; Singh, A.K.; Singh, V.P. Synthesis, structural investigations and corrosion inhibition studies on Mn (II), Co (II), Ni (II), Cu (II) and Zn (II) complexes with 2-amino-benzoic acid (phenyl-pyridin-2-yl-methylene)-hydrazide. RSC Adv. 2015, 5, 45217–45230. [Google Scholar] [CrossRef]
- Nageswara, R.S.; Chandra, M.D.; Adimurthy, S. Chitosan: An Efficient Recyclable Catalyst for Transamidation of Carboxamides with Amines under Neat Conditions. Green Chem. 2014, 16, 4122–4126. [Google Scholar] [CrossRef]
- Pathare, S.; Jain, A.; Akamanchi, K. Sulfated tungstate: A highly efficient catalyst for transamidation of carboxamides with amines. RSC Adv. 2013, 3, 7697–7703. [Google Scholar] [CrossRef]
- Liu, H.; Liu, J.; Zhang, Y.; Shao, C.; Yu, J. copper-catalyzed amide bond formation from formamides and carboxylic acids. Chin. Chem. Lett. 2015, 46, 11–14. [Google Scholar] [CrossRef]
- Becerrafigueroa, L.; Ojedaporras, A.; Gambasánchez, D. Transamidation of Carboxamides Catalyzed by Fe (III) and Water. J. Org. Chem. 2014, 79, 4544–4552. [Google Scholar] [CrossRef] [PubMed]
- Gu, D.-W.; Guo, X.-X. Synthesis of N-arylcarboxamides by the efficient transamidation of dmf and derivatives with anilines. Tetrahedron 2015, 71, 9117–9122. [Google Scholar] [CrossRef]
- Shekhar, A.C.; Kumar, A.R.; Sathaiah, G.; Paul, V.L.; Sridhar, M.; Rao, P.S. Facile N-formylation of amines using lewis acids as novel catalysts. Tetrahedron Lett. 2009, 50, 7099–7101. [Google Scholar] [CrossRef]
- Hosseini-Sarvari, M.; Sharghi, H. ZnO as a new catalyst for N-formylation of amines under solvent-free conditions. J. Org. Chem. 2006, 37, 6652–6654. [Google Scholar] [CrossRef] [PubMed]
- Sonawane, R.B.; Rasal, N.K.; Jagtap, S.V. Nickel-(II)-Catalyzed N-Formylation and N-Acylation of Amines. Org. Lett. 2017, 19, 2078–2081. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.L.; Atkinson, B.N.; Williams, J.M. Transamidation of primary amides with amines using hydroxylamine hydrochloride as an inorganic catalyst. Angew. Chem. Int. Ed. 2012, 51, 1383–1386. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.N.; Mohan, D.C.; Adimurthy, S. l-Proline: An Efficient Catalyst for Transamidation of Carboxamides with Amines. Org. Lett. 2013, 15, 1496–1499. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, S.; Rao, D.N.; Reddy, A.S. Sulphuric acid immobilized on silica gel (H2SO4-SiO2) as an eco-friendly catalyst for transamidation. RSC Adv. 2015, 5, 10567–10574. [Google Scholar] [CrossRef]
- Suchý, M.; Elmehriki, A.A.; Hudson, R.H. A remarkably simple protocol for the N-formylation of amino acid esters and primary amines. Org. Lett. 2011, 13, 3952–3955. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |


 | |||||
|---|---|---|---|---|---|
| Entry | Base (equiv.) | Solvent (mL) | Temp (°C) | Time (h) | Yield b (%) |
| 1 | CuCl2 | DMA | 150 | 12 | Trace |
| 2 | Zr(SO4)2 | DMA | 150 | 12 | Trace |
| 3 | CH3COOH(2) | DMA | 150 | 6 | 8 |
| 4 | HCl(2) | DMA | 150 | 6 | 46 |
| 5 | H2SO4(2) | DMA | 150 | 6 | 52 |
| 6 | p-toluenesulfonic acid | DMA | 150 | 6 | 50 |
| 7 | Pyridine(3) | DMA | 150 | 6 | Trace |
| 8 | KOtBu(2) | DMA | 150 | 6 | 12 |
| 9 | NaOH(2) | DMA | 150 | 6 | 18 |
| 10 | 2,6-lutidine(3) | DMA | 150 | 6 | Trace |
| 11 | TEA(3) | DMA | 150 | 6 | Trace |
| 12 | Imidazolium chloride(3) | DMA | rt | 6 | Trace |
| 13 | Imidazolium chloride(3) | DMA | 150 | 2 | 96 |
| 14 | Imidazolium chloride(0.5) | DMA | 150 | 2 | 95 |
| 15 | Imidazolium chloride(0.3) | DMA | 150 | 2 | 96 |
| 16 | Imidazolium chloride(0.1) | DMA | 150 | 6 | 65 |
| 17 | Imidazolium chloride(3) | DMA | 30 | 2 | Trace |
| 18 | Imidazolium chloride(3) | DMA | 60 | 2 | 59 |
| 19 | Imidazolium chloride(3) | DMA | 90 | 2 | 68 |
| 20 | Imidazolium chloride(3) | DMA | 135 | 2 | 85 |
| 21 | Imidazole(3) | DMA | 150 | 12 | Trace |
| 22 | - | DMA | 150 | 12 | NOC |
 | ||||
|---|---|---|---|---|
| Entry | Substrate | Time | Product | Yield b (%) |
| 1 |  | 2 h |  | 92 |
| 2 |  | 2 h |  | 97 |
| 3 |  | 3 h |  | 90 |
| 4 |  | 2 h |  | 75 |
| 5 |  | 2 h |  | 82 |
| 6 |  | 4 h |  | 84 |
| 7 |  | 6 h |  | 83 |
| 8 |  | 6 h |  | 80 |
| 9 |  | 5 h |  | 89 |
| 10 |  | 6 h |  | 90 |
| 11 |  | 4 h |  | 89 |
| 12 |  | 4 h |  | 63 |
| 13 |  | 5 h |  | 57 |
| 14 |  | 4 h |  | 68 |
| 15 |  | 3 h |  | 93 |
| 16 |  | 3 h |  | 94 |
| 17 |  | 5 h |  | 60 |
| 18 |  | 5 h |  | 72 |
| 19 |  | 5 h |  | 75 |
| 20 |  | 3 h |  | 95 |
| 21 |  | 3 h |  | 94 |
| 22 |  | 3 h |  | 95 |
| 23 |  | 3 h |  | 89 |
 | ||||
|---|---|---|---|---|
| Entry | Substrate | Time | Product | Yield b (%) |
| 1 |  | 4 h |  | 89 |
| 2 |  | 4 h |  | 85 |
| 3 |  | 4 h |  | 89 |
| 4 |  | 4 h |  | 71 |
| 5 |  | 3 h |  | 92 |
 | ||||
|---|---|---|---|---|
| Entry | Substrate | Time | Product | Yield b (%) |
| 1 |  | 4 h |  | 89 |
| 2 |  | 3 h |  | 94 |
| 3 |  | 3 h |  | 97 |
| 4 |  | 3 h |  | 93 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, Q.; Gan, Z.; Wang, X.; Li, D.; Luo, W.; Wang, H.; Dai, Z.; Yuan, J. Imidazolium Chloride: An Efficient Catalyst for Transamidation of Primary Amines. Molecules 2018, 23, 2234. https://doi.org/10.3390/molecules23092234
Tian Q, Gan Z, Wang X, Li D, Luo W, Wang H, Dai Z, Yuan J. Imidazolium Chloride: An Efficient Catalyst for Transamidation of Primary Amines. Molecules. 2018; 23(9):2234. https://doi.org/10.3390/molecules23092234
Chicago/Turabian StyleTian, Qingqiang, Zongjie Gan, Xuetong Wang, Dan Li, Wen Luo, Huajun Wang, Zeshu Dai, and Jianyong Yuan. 2018. "Imidazolium Chloride: An Efficient Catalyst for Transamidation of Primary Amines" Molecules 23, no. 9: 2234. https://doi.org/10.3390/molecules23092234





