Hematological, Biochemical, Histopathological and 1H-NMR Metabolomics Application in Acute Toxicity Evaluation of Clinacanthus nutans Water Leaf Extract
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physical and Behavioral Changes Related to Acute Oral Toxicity
2.2. Hematology and Biochemical Analysis
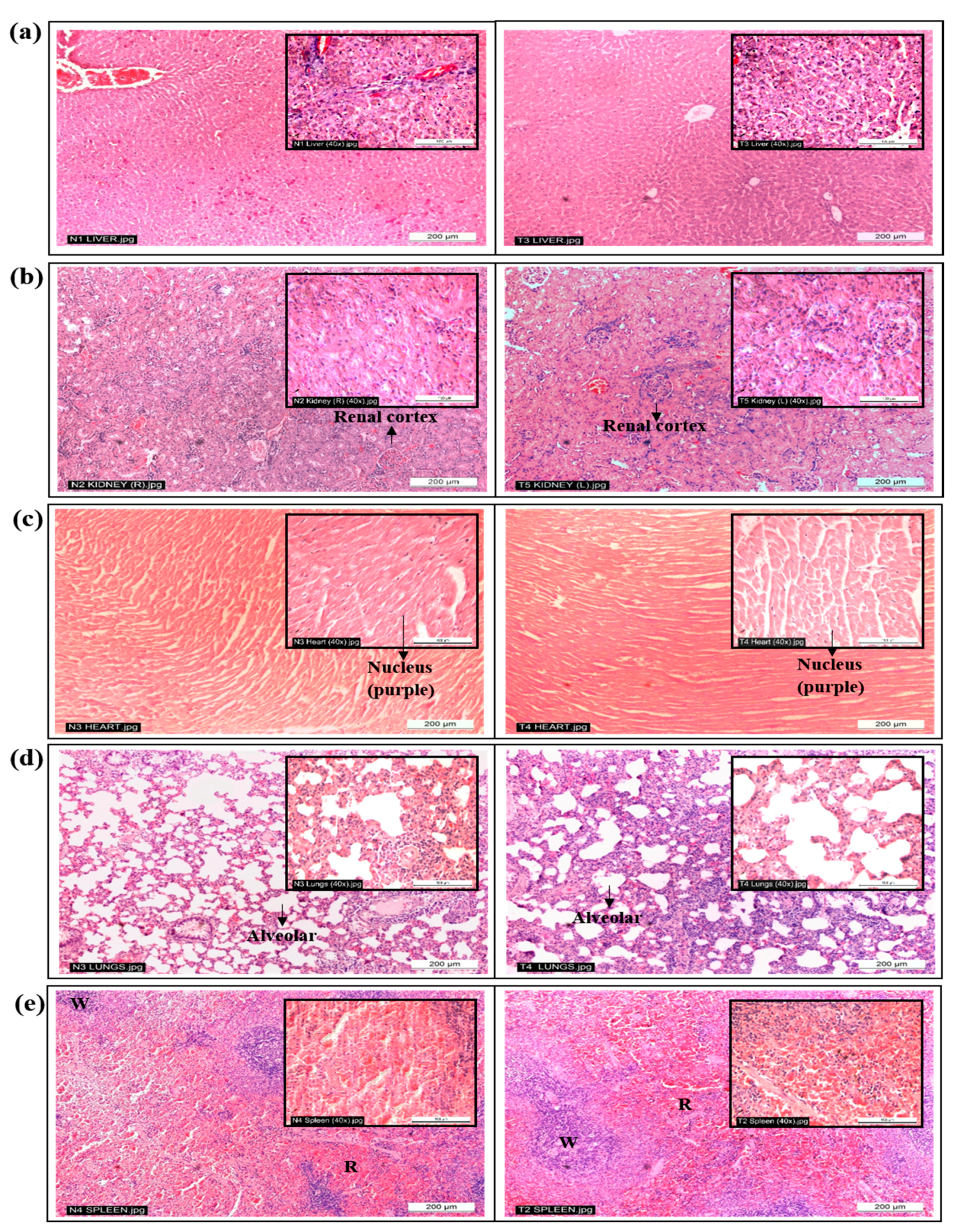
2.3. Histopathological Analysis
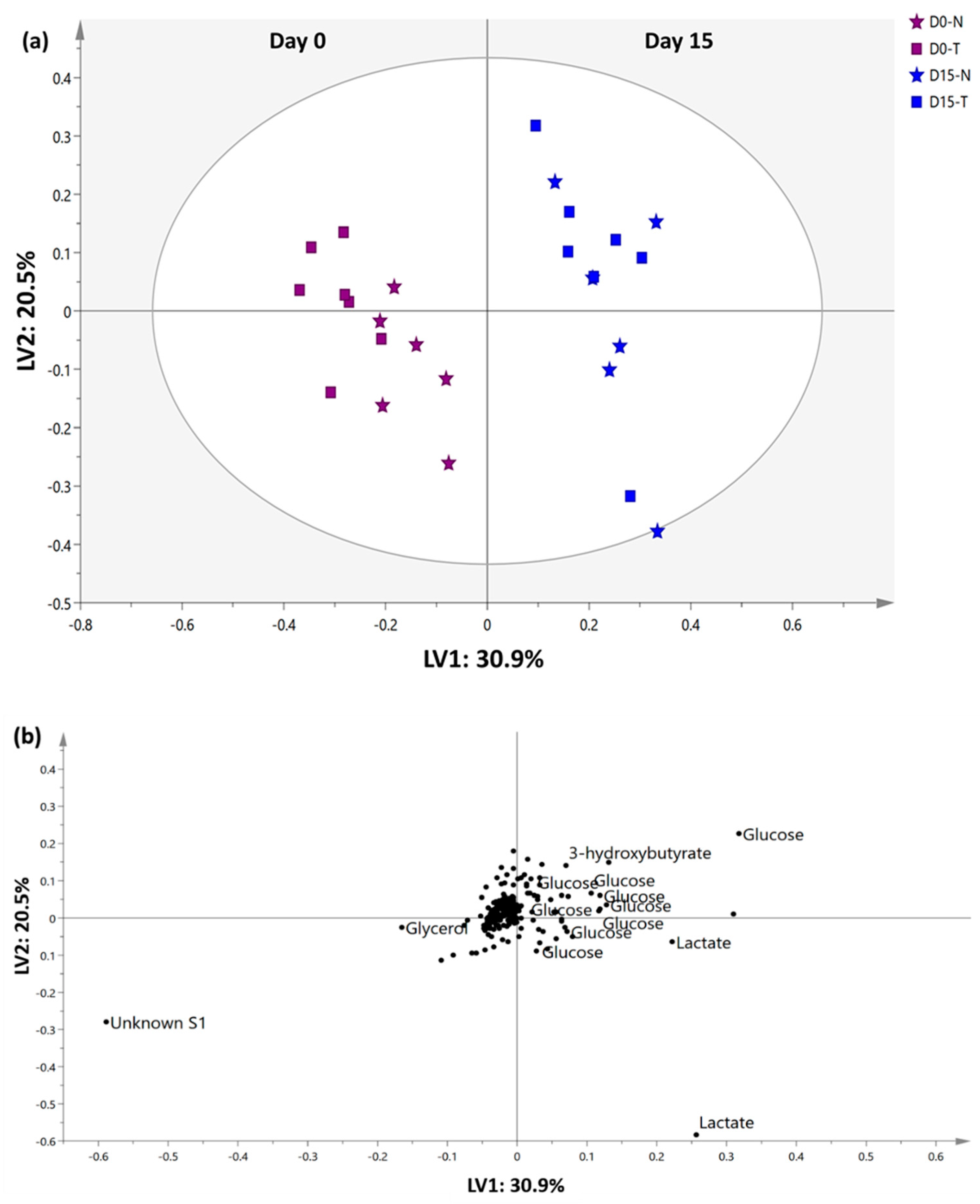
2.4. 1H-NMR Serum Metabolomics Evaluation
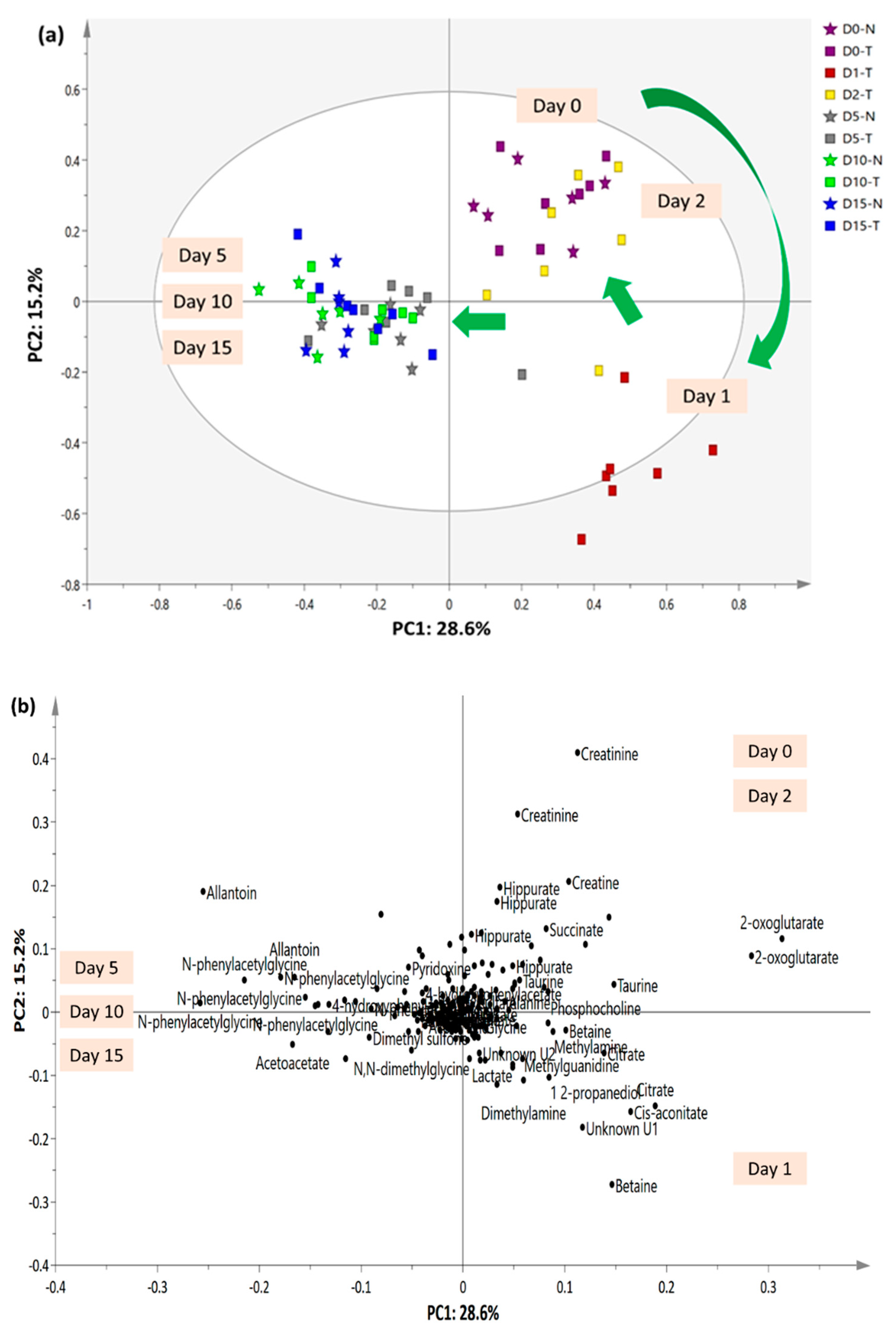
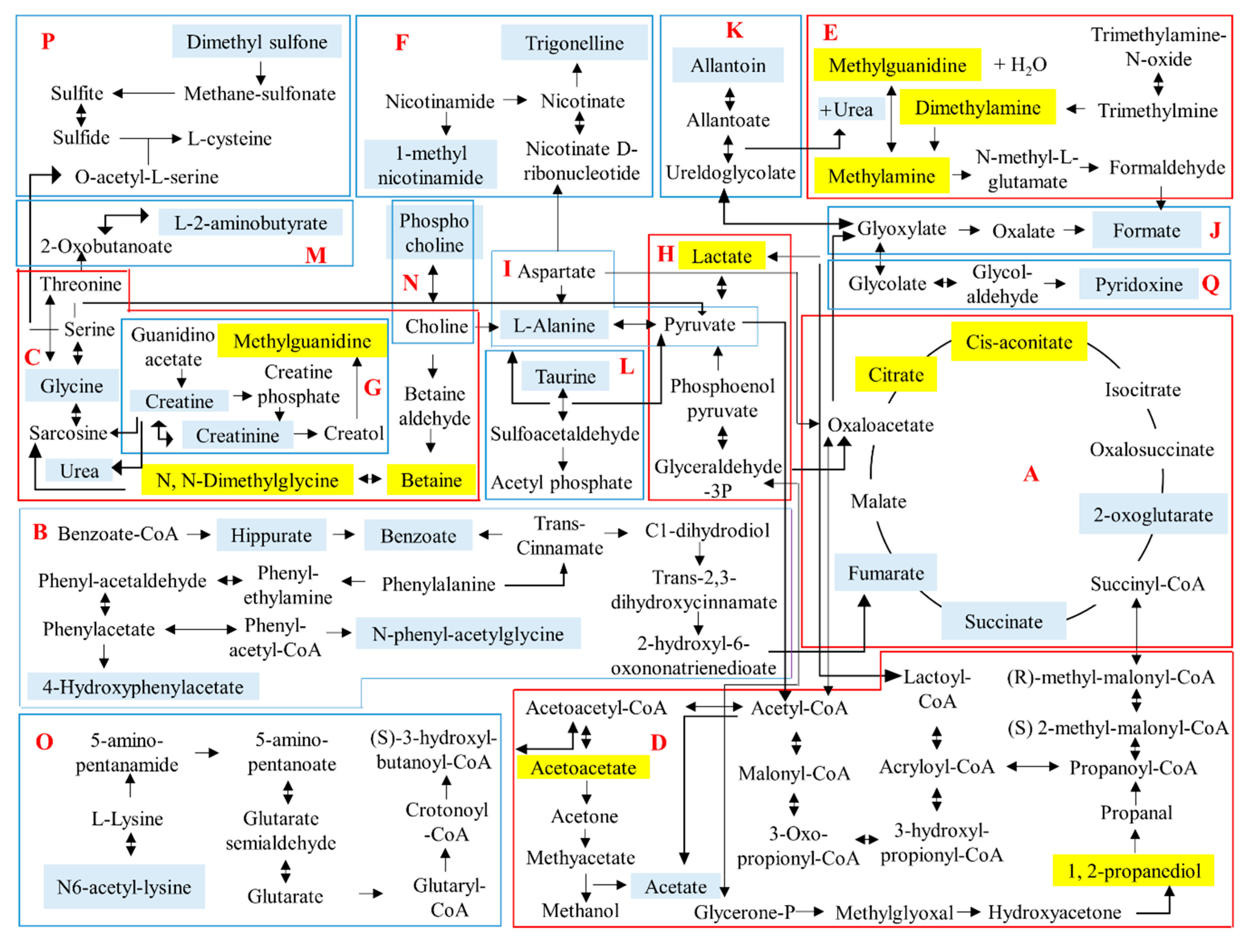
2.5. 1H-NMR Urinary Metabolomics Analysis with Suggested Metabolic Pathways
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Plant Extract Preparation
3.3. Experimental Animals
3.4. Acute Oral Toxicity Study
3.5. Body and Organ Weight Analysis
3.6. Food and Water Consumption
3.7. Hematology and Biochemical Analysis
3.8. Histopathological Analysis
3.9. Serum and Urine Sample Preparation and 1H-NMR Spectra Acquisition and Processing
3.10. Metabolic Pathway Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Putwatana, P.; Sanmanowong, P.; Oonprasertpong, L.; Junda, T.; Pitiporn, S.; Narkwong, L. Relief of radiation-induced oral mucositis in head and neck cancer. Cancer Nurs. 2009, 32, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.S.; Ahmad, M.S.; Mamat, A.S.; Ahmad, M.Z.; Salam, F. Antioxidant and wound healing activity of polyherbal fractions of Clinacanthus nutans and Elephantopus scaber. Evid-Based Compl. Alt. 2016, 2016, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.Q.; Chen, H.; Chen, L.; Tang, D.D.; Miao, H.; Zhao, Y.Y. Metabolomic application in toxicity evaluation and toxicological biomarker identification of natural product. Chem. Biol. Interact. 2016, 252, 114–130. [Google Scholar] [CrossRef] [PubMed]
- Arome, D.; Chinedu, E. The importance of toxicity testing. J. Pharm. Biosci. 2014, 4, 146–148. [Google Scholar]
- Satatyavivad, J.; Bunyaprephatsara, N.; Kitisiripornkul, S.; Tanasomwang, W. Analgesic and anti-inflammatory activities of extract of Clinacanthus nutans (Burm.F.) Lindau. Thai J. Pharm. Sci. 1996, 3, 7–17. [Google Scholar]
- Chavalittumrong, P.; Attawish, A.; Rugsamon, P.; Chuntapet, P. Toxicological study of Clinacanthus nutans (Burm.f.) Lindau. Bull. Dept. Med. Sci. 1995, 37, 323–338. [Google Scholar]
- Tiew, W.P.; Wen, P.X.; Chin, J.H.; Akowuah, G.A. Effect of methanol extract of Clinacanthus nutans on serum biochemical parameters in rats. J. Appl. Pharm. 2014, 6, 77–86. [Google Scholar]
- P’ng, X.W.; Akowuah, G.A.; Chin, J.H. Acute oral toxicity study of Clinacanthus nutans in mice. Int. J. Pharm. Sci. Res. 2012, 3, 4202–4205. [Google Scholar]
- Farsi, E.; Esmailli, K.; Shafaei, A.; Moradi Khaniabadi, P.; Al Hindi, B.; Khadeer Ahamed, M.B.; Sandai, D.; Abdul Sattar, M.; Ismail, Z.; Abdul Majid, A.M.S.; et al. Mutagenicity and preclinical safety assessment of the aqueous extract of Clinacanthus nutans leaves. Drug Chem. Toxicol. 2016, 545, 1–13. [Google Scholar]
- Shi, J.; Cao, B.; Wang, X.-W.; Aa, J.-Y.; Duan, J.-A.; Zhu, X.-X.; Wang, G.-J.; Liu, C.-X. Metabolomics and its application to the evaluation of the efficacy and toxicity of traditional chinese herb medicines. J. Chromatogr. B 2016, 1026, 204–216. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.N.; Hu, H.J.; Shi, Y.F.; Yang, X.Z.; Cao, L.G.; Wu, J.; Asweto, C.O.; Feng, L.; Duan, J.C.; Sun, Z.W. 1H NMR-based metabolomics study on repeat dose toxicity of fine particulate matter in rats after intratracheal instillation. Sci. Total Environ. 2017, 589, 212–221. [Google Scholar] [CrossRef] [PubMed]
- EClinpath Veterinary Clinical Pathology. Available online: http://www.eclinpath.com/ (accessed on 13 October 2017).
- Petterino, C.; Argentino-Storino, A. Clinical chemistry and haematology historical data in control Sprague-Dawley rats from pre-clinical toxicity studies. Exp. Toxicol. Pathol. 2006, 57, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Hussein, R.R.S.; Soliman, R.H.; Abdelhaleem Ali, A.M.; Tawfeik, M.H.; Abdelrahim, M.E.A. Effect of antiepileptic drugs on liver enzymes. Beni-Suef University J. Basic Appl. Sci. 2013, 2, 14–19. [Google Scholar] [CrossRef]
- Dollah, M.A.; Parhizkar, S.; Abdul Latiff, L.; Hassan, M.H. Bin Toxicity effect of Nigella sativa on the liver function of rats. Adv. Pharm. Bull. 2013, 3, 97–102. [Google Scholar] [PubMed]
- Brzóska, M.M.; Moniuszko-Jakoniuk, J.; Piłat-Marcinkiewicz, B.; Sawicki, B. Liver and kidney function and histology in rats exposed to cadmium and ethanol. Alcohol Alcohol. 2003, 38, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Uchôa Junqueira, L.C.; Carneiro, J. Basic Histology, Text and Atlas, 10th ed.; Carneiro, J., Ed.; Lange Medical Books McGraw-Hill: New York, NY, USA, 2003. [Google Scholar]
- Meyer, F.; Lizana, J.N.; Dziedricki, L.F.; Bleggi-Torres, L.F. Histologic alterations of rat kidneys perfused with a Euro-Collins diltiazem solution. Acta Cir. Bras. 2010, 25, 496–500. [Google Scholar] [CrossRef] [PubMed]
- Greaves, P. Histopathology of Preclinical Toxicity Studies: Interpretation and Relevance in Drug Safety Studies, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Gado, A.M.; Aldahmash, B.A. Protective effect of l-carnitine against amiodarone-induced lung toxicity in rats. Internet J. Toxicol. 2013, 10, 1–9. [Google Scholar]
- Maamar, M.S.; Al-Griw, M.A.; Al-Ghazeer, R.O.; Al-Azreg, S.A.; Salama, N.M.; Bennour, E.M. Oxidative stress mediated cytotoxicity of trichloroethane in a model of murine splenic injury. Am. J. Biosci. 2016, 4, 1–8. [Google Scholar] [CrossRef]
- Wishart, D.S.; Tzur, D.; Knox, C.; Eisner, R.; Guo, A.C.; Young, N.; Cheng, D.; Jewell, K.; Arndt, D.; Sawhney, S.; et al. HMDB: The human metabolome database. Nucl. Acids Res. 2007, 35, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Maulidiani; Abas, F.; Khatib, A.; Perumal, V.; Suppaiah, V.; Ismail, A.; Hamid, M.; Shaari, K.; Lajis, N.H. Metabolic alteration in obese diabetes rats upon treatment with Centella asiatica extract. J. Ethnopharmacol. 2016, 180, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, L.; Johansson, E.; Kettaneh-Wold, N.; Trygg, J.; Wikstrom, C.; Wold, S. Multi- and megavariate data analysis part I: Basic principles and applications; Umetrics, Inc.: Umea, Sweden, 2006. [Google Scholar]
- Chatham, J.C. Lactate-The forgotten fuel! J. Physiol. 2002, 542, 333. [Google Scholar] [CrossRef] [PubMed]
- Couto De Andrade Júnior, M.; Andrade, D.; Mc, J. Metabolism during fasting and starvation: Understanding the basics to glimpse new boundaries. J. Nutr. Diet. 2018, 1, 14–16. [Google Scholar]
- Illingworth, J. Specialised Metabolism of Differentiated Tissues. Available online: http://www.bmb.leeds.ac.uk/teaching/icu3/lecture/22/index.htm (accessed on 3 August 2018).
- Gutierrez, E.N.; Lamberti, V. Preparation of cis and trans Aconitic Acids and their Salts. U.S. Patent 4,123,458, 31 October 1978. [Google Scholar]
- Laffel, L. Ketone bodies: A review of physiology, pathophysiology and application of monitoring to diabetes. Diabetes Metab. Res. Rev. 1999, 15, 412–426. [Google Scholar] [CrossRef]
- Yoo, W.; Kim, D.; Yoon, H.; Ryu, S. Enzyme IIA Ntr regulates Salmonella invasion via 1,2-propanediol and propionate catabolism. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kelava, T.; Cavar, I.; Culo, F. Biological actions of drug solvent. Period. Biol. 2011, 113, 311–320. [Google Scholar]
- Denning, D.; Webster, A. Detrimental effect of propylene glycol on natural killer cell and neutrophil function. J. Pharm. Pharmacol. 1987, 39, 236–238. [Google Scholar] [CrossRef] [PubMed]
- Oshiro, T.T.; Teixeira, C.F.P.; Oga, S. Propylene glycol enhances anti-inflammatory effects of phenylbutazone. Gen. Pharmacol. 1990, 21, 131–134. [Google Scholar] [CrossRef]
- Kanehisa, M.; Araki, M.; Goto, S.; Hattori, M.; Hirakawa, M.; Itoh, M.; Katayama, T.; Kawashima, S.; Okuda, S.; Tokimatsu, T.; et al. KEGG for linking genomes to life and the environment. Nucleic Acids Res. 2008, 36, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Marzocco, S.; Di Paola, R.; Serraino, I.; Sorrentino, R.; Meli, R.; Mattaceraso, G.; Cuzzocrea, S.; Pinto, A.; Autore, G. Effect of methylguanidine in carrageenan-induced acute inflammation in the rats. Eur. J. Pharmacol. 2004, 484, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Marzocco, S.; Di Paola, R.; Ribecco, M.T.; Sorrentino, R.; Domenico, B.; Genesio, M.; Pinto, A.; Autore, G.; Cuzzocrea, S. Effect of methylguanidine in a model of septic shock induced by LPS. Free Radic. Res. 2004, 38, 1143–1153. [Google Scholar] [CrossRef] [PubMed]
- Mak Dimethylamine. The MAK Collection for Occupational Health and Safety; Wiley-VCH: Weinheim, Germany, 2012; Volume 7, pp. 76–89. [Google Scholar]
- Dzhanashvili, G.D. Hygienic substantiation of the maximum permissible content of dimethylamine in water bodies. Gig. I Sanit. 1967, 6, 16. [Google Scholar]
- Mitchell, S.C.; Zhang, A.Q. Methylamine in human urine. Clin. Chim. Acta 2001, 312, 107–114. [Google Scholar] [CrossRef]
- Lever, M.; Slow, S. The clinical significance of betaine, an osmolyte with a key role in methyl group metabolism. Clin. Biochem. 2010, 43, 732–744. [Google Scholar] [CrossRef] [PubMed]
- Amiraslani, B.; Sabouni, F.; Abbasi, S.; Nazem, H.; Sabet, M. Recognition of betaine as an inhibitor of lipopolysaccharide-induced nitric oxide production in activated microglial cells. Iran. Biomed. J. 2012, 16, 84–89. [Google Scholar] [PubMed]
- Kendall, R.V.; Lawson, J.W. Treatment of Arthritis and Inflammation Using N,N-dimethylglycine. U.S. Patent 5,026,728, 25 June 1991. [Google Scholar]
- Organisation of Economic Cooperation and Development (OECD). 423 Guideline for Testing of Chemicals. Available online: https://www.oecd-ilibrary.org/environment/test-no-423-acute-oral-toxicity-acute-toxic-class-method_9789264071001-en (accessed on 20 March 2016).
- Lorke, D. A new approach to practical acute toxicity testing. Arch. Toxicol. 1983, 54, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Liju, V.B.; Jeena, K.; Kuttan, R. Acute and subchronic toxicity as well as mutagenic evaluation of essential oil from turmeric (Curcuma longa L.). Food Chem. Toxicol. 2013, 53, 52–61. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: The samples of the extracts are available from the authors. |


| Parameter | Unit | Treatment | |
|---|---|---|---|
| Untreated (Distilled Water) | Treated (C. nutans Water Extract 5000 mg/kg) | ||
| Red blood cells | 1012/L | 7.132 ± 0.367 | 6.811 ± 0.237 |
| Hemaglobin | g/L | 136.6 ± 5.307 | 131.143 ± 3.362 |
| Packed cells volume | L/L | 0.418 ± 0.013 | 0.381 ± 0.01 *, p = 0.0489 |
| Mean corpuscular volume | fL | 59.0 ± 1.581 | 56.142 ± 0.738 |
| Mean corpuscular hemaglobin concentration | g/L | 326.6 ± 4.925 | 344.0 ± 2.093 **, p = 0.0046 |
| White blood cells | 109/L | 9.650 ± 1.049 | 10.024 ± 1.345 |
| B Neutrophils | 109/L | 0.134 ± 0.021 | 0.146 ± 0.030 |
| S Neutrophils | 109/L | 1.912 ± 0.225 | 2.059 ± 0.387 |
| Lymphocytes | 109/L | 6.910 ± 0.861 | 7.047 ± 0.942 |
| Monocytes | 109/L | 0.536 ± 0.052 | 0.614 ± 0.116 |
| Eosin | 109/L | 0.166 ± 0.067 | 0.163 ± 0.041 |
| Basophil | 109/L | 0.000 | 0.000 |
| Thrombocytes | 109/L | 927.4 ± 29.763 | 837.429 ± 59.503 |
| Plasma protein | g/L | 70.0 ± 2.025 | 72.857 ± 1.639 |
| Icterus index | U | 2.0 ± 0.00 | 2.0 ± 0.00 |
| Parameter | Unit | Treatment | |
|---|---|---|---|
| Untreated | Treated | ||
| Liver Function Test | |||
| ALT | U/L | 100.6 ± 36.57 | 94.17 ± 21.47 |
| ALP | U/L | 126.8 ± 11.46 | 116.9 ± 6.808 |
| AST | U/L | 340.3 ± 107.5 | 299.9 ± 42.69 |
| Total Bilirubin | μmol/L | 1.100 ± 0.1033 | 1.043 ± 0.1716 |
| Kidney Function Test | |||
| Creatinine | μmol/L | 73.50 ± 4.478 | 70.86 ± 1.056 |
| Urea | mmol/L | 8.733 ± 0.4937 | 7.614 ± 0.9647 |
| Metabolites | δH ppm | VIP | Day 0 | Day 1 | Day 2 | Day 1 vs. Day 0 | Day 2 vs. Day 0 | Day 2 vs. Day 1 | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fold Change | p | Fold Change | p | Fold Change | p | ||||||
| Betaine | 3.26 | 3.55 | 0.0188 ± 0.0038 | 0.0527 ± 0.0218 | 0.0243 ± 0.0076 | 2.803 | 0.002 | 1.290 | 0.115 | 0.460 | 0.007 |
| Dimethylamine | 2.70 | 2.72 | 0.0124 ± 0.0052 | 0.0253 ± 0.0124 | 0.0248 ± 0.0059 | 2.047 | 0.025 | 2.004 | 0.001 | 0.979 | 0.919 |
| Cis aconitate | 3.10 | 2.52 | 0.0071 ± 0.0017 | 0.0207 ± 0.0018 | 0.0138 ± 0.0060 | 2.899 | 0.000 | 1.932 | 0.016 | 0.667 | 0.013 |
| Citrate | 2.54 | 2.46 | 0.0206 ± 0.0096 | 0.0399 ± 0.0174 | 0.0306 ± 0.0009 | 1.933 | 0.025 | 1.484 | 0.072 | 0.768 | 0.238 |
| 1,2-propanediol | 1.14 | 1.60 | 0.0026 ± 0.0004 | 0.0080 ± 0.0012 | 0.0029 ± 0.0010 | 3.106 | 0.000 | 1.143 | 0.389 | 0.368 | 0.000 |
| Methylguanidine | 2.82 | 1.27 | 0.0022 ± 0.0003 | 0.0059 ± 0.0012 | 0.0029 ± 0.0008 | 2.726 | 0.000 | 1.346 | 0.032 | 0.494 | 0.000 |
| Methylamine | 2.58 | 1.16 | 0.0020 ± 0.0004 | 0.0060 ± 0.0040 | 0.0046 ± 0.0028 | 2.942 | 0.023 | 2.263 | 0.031 | 0.769 | 0.467 |
| Lactate | 1.30 | 0.94 | 0.0036 ± 0.0007 | 0.0061 ± 0.0016 | 0.0038 ± 0.0011 | 1.679 | 0.003 | 1.044 | 0.752 | 0.622 | 0.009 |
| N,N-dimethylglycine | 2.90 | 0.72 | 0.0032 ± 0.0005 | 0.0051 ± 0.0012 | 0.0036 ± 0.0019 | 1.604 | 0.002 | 1.124 | 0.610 | 0.701 | 0.098 |
| Acetoacetate | 2.30 | 0.65 | 0.0040 ± 0.0015 | 0.0060 ± 0.0007 | 0.0046 ± 0.0027 | 1.499 | 0.008 | 1.164 | 0.584 | 0.777 | 0.230 |
| Unknown U1 | 3.30 | 2.48 | 0.0024 ± 0.0003 | 0.0158 ± 0.0029 | 0.0056 ± 0.0016 | 6.580 | 0.000 | 2.307 | 0.000 | 0.351 | 0.000 |
| Unknown U2 | 2.74 | 1.03 | 0.0028 ± 0.0010 | 0.0046 ± 0.0008 | 0.0022 ± 0.0004 | 1.680 | 0.002 | 0.801 | 0.199 | 0.477 | 0.000 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khoo, L.W.; Foong Kow, A.S.; Maulidiani, M.; Lee, M.T.; Tan, C.P.; Shaari, K.; Tham, C.L.; Abas, F. Hematological, Biochemical, Histopathological and 1H-NMR Metabolomics Application in Acute Toxicity Evaluation of Clinacanthus nutans Water Leaf Extract. Molecules 2018, 23, 2172. https://doi.org/10.3390/molecules23092172
Khoo LW, Foong Kow AS, Maulidiani M, Lee MT, Tan CP, Shaari K, Tham CL, Abas F. Hematological, Biochemical, Histopathological and 1H-NMR Metabolomics Application in Acute Toxicity Evaluation of Clinacanthus nutans Water Leaf Extract. Molecules. 2018; 23(9):2172. https://doi.org/10.3390/molecules23092172
Chicago/Turabian StyleKhoo, Leng Wei, Audrey Siew Foong Kow, M. Maulidiani, Ming Tatt Lee, Chin Ping Tan, Khozirah Shaari, Chau Ling Tham, and Faridah Abas. 2018. "Hematological, Biochemical, Histopathological and 1H-NMR Metabolomics Application in Acute Toxicity Evaluation of Clinacanthus nutans Water Leaf Extract" Molecules 23, no. 9: 2172. https://doi.org/10.3390/molecules23092172
APA StyleKhoo, L. W., Foong Kow, A. S., Maulidiani, M., Lee, M. T., Tan, C. P., Shaari, K., Tham, C. L., & Abas, F. (2018). Hematological, Biochemical, Histopathological and 1H-NMR Metabolomics Application in Acute Toxicity Evaluation of Clinacanthus nutans Water Leaf Extract. Molecules, 23(9), 2172. https://doi.org/10.3390/molecules23092172







