Phenolic Composition Stability and Antioxidant Activity of Sour Cherry Liqueurs
Abstract
:1. Introduction
2. Results
2.1. Identification of Phenolic Compounds
2.1.1. Phenolic Acids
2.1.2. Flavanols
2.1.3. Flavonols
2.1.4. Anthocyanins
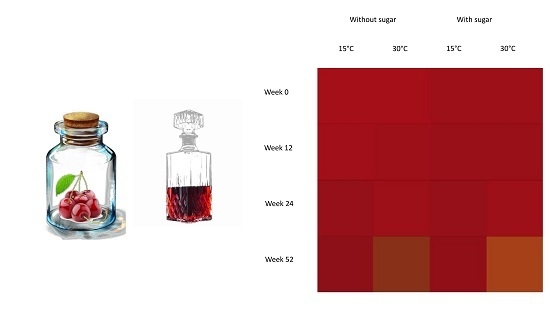
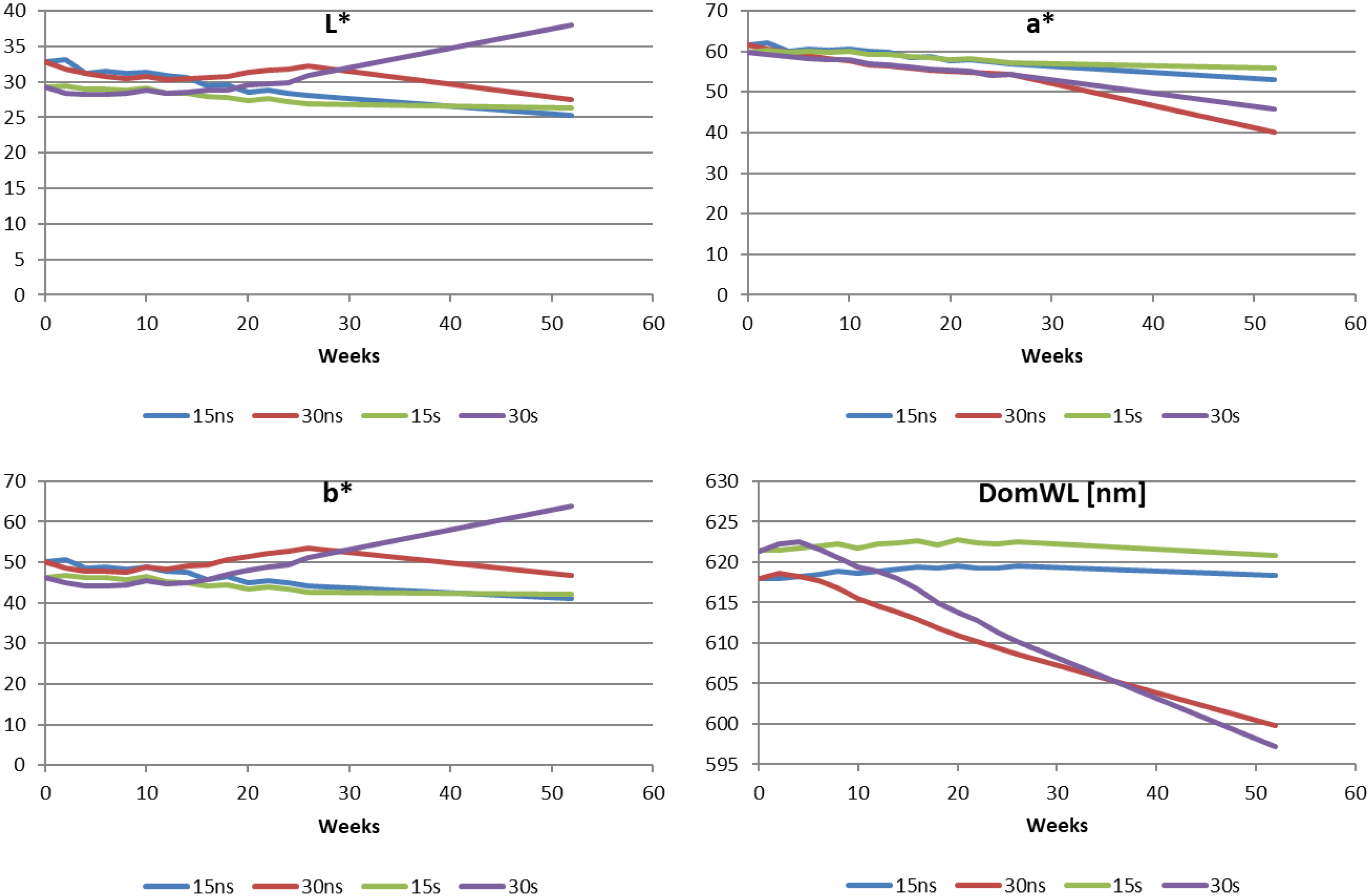
2.2. Color Changes
2.3. Aroma Compounds
2.4. Changes in Antioxidant Activity
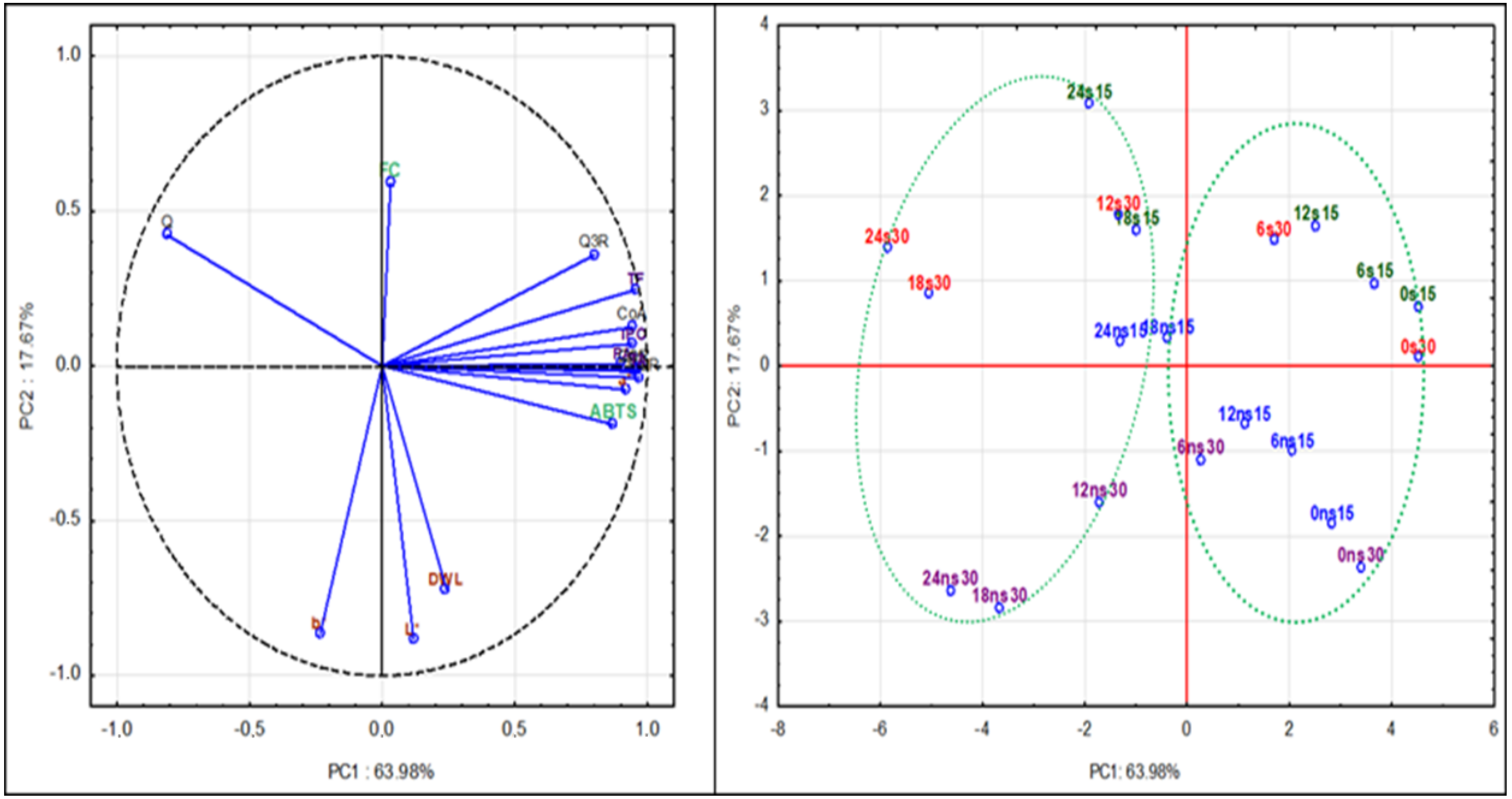
2.5. Principal Component Analysis
3. Materials and Methods
3.1. Preparation of Liqueurs
3.2. Identification of Phenolic Compounds by LC-MS Method
3.3. Quantitative Analysis of Phenolic Compounds by HPLC Method
3.4. Determination of Antioxidant Activity
3.5. Anthocyanins Polymers
3.6. GC/MS Analysis
3.7. Color
3.8. Organoleptic Evaluation
3.9. Kinetics
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alamprese, C.; Pompei, C. Influence of processing variables on some characteristics of Nocino liqueur. Food Chem. 2005, 92, 203–209. [Google Scholar] [CrossRef]
- Egea, T.; Adele Signorini, M.; Bruschi, P.; Rivera, D.; Obon, C.; Alcaraz, F.; Antonio Palazon, J. Spirits and liqueurs in European traditional medicine: Their history and ethnobotany in Tuscany and Bologna (Italy). J. Ethnopharmacol. 2015, 175, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Kucharska, A.Z.; Sokół-Łętowska, A.; Hudko, J.; Nawirska-Olszańska, A. Influence of the preparation procedure on the antioxidant activity and color of liqueurs from cornelian cherry (Cornus mas l.). Pol. J. Food Nutr. Sci. 2007, 57, 343–347. [Google Scholar]
- Sokół-Łętowska, A.; Kucharska, A.Z.; Nawirska-Olszańska, A. Wpływ dodatku cukru na jakość nalewek wiśniowych podczas przechowywania. Zesz. Nauk. UEP 2011, 205, 126–134. [Google Scholar]
- U.S. Department of Health and Human Services; U.S. Department of Agriculture (Eds.) 2010–2015 Dietary Guidelines for Americans, 7th ed.; U.S. Government Printing Office: Washington, WA, USA, 2010.
- U.S. Department of Health and Human Services; U.S. Department of Agriculture (Eds.) 2015–2020 Dietary Guidelines for Americans, 8th ed.; U.S. Government Printing Office: Washington, WA, USA, 2015. Available online: https://health.gov/dietaryguidelines/2015/guidelines/ (accessed on 10 July 2018).
- De Lorimier, A.A. Alcohol, wine, and health. Am. J. Surg. 2000, 180, 357–361. [Google Scholar] [CrossRef]
- Alrgei, H.O.S.; Dabić, D.Č.; Natić, M.M.; Rakonjac, V.S.; Milojković-Opsenica, D.; Tešić, Ž.L.; Fotirić Akšić, M.M. Chemical profile of major taste- and health-related compounds of Oblačinska sour cherry. J. Sci. Food Agric. 2015, 96, 1241–1251. [Google Scholar] [CrossRef] [PubMed]
- Burkhardt, S.; Tan, D.X.; Manchester, L.C.; Hardeland, R.; Reiter, R.J. Detection and quantification of the antioxidant melatonin in Montmorency and Balaton tart cherries (Prunus cerasus). J. Agric. Food Chem. 2001, 49, 4898–4902. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Jiang, Q.; Lin, J.; Li, X.; Sun, C.; Chen, K. Physicochemical characterisation of four cherry species (Prunus spp.) grown in China. Food Chem. 2015, 173, 855–863. [Google Scholar] [CrossRef] [PubMed]
- Bonerz, D.; Würth, K.; Dietrich, H.; Will, F. Analytical characterization and the impact of ageing on anthocyanin composition and degradation in juices from five sour cherry cultivars. Eur. Food Res. Technol. 2007, 224, 355–364. [Google Scholar] [CrossRef]
- Sokół-Łętowska, A. Związki fenolowe w nalewkach z wybranych owoców; Wydawnictwo Uniwersytetu Przyrodniczego we Wrocławiu: Wrocław, Poland, 2013; p. 112. [Google Scholar]
- Nikićević, N.; Veličković, M.; Jadranin, M.; Vučković, I.; Novaković, M.; Vujisić, L.; Stanković, M.; Urošević, I.; Tešević, V. The effects of the cherry variety on the chemical and sensorial characteristics of cherry brandy. J. Serb. Chem. Soc. 2011, 9, 1219–1228. [Google Scholar] [CrossRef]
- Rødtjer, A.; Skibsted, L.; Andersen, M. Identification and quantification of phenolics in aromatic bitter and cherry liqueur by HPLC with electrochemical detection. Eur. Food Res. Technol. 2006, 223, 663–668. [Google Scholar] [CrossRef]
- Sokół-Łętowska, A.; Kucharska, A.Z.; Wińska, K.; Szumny, A.; Nawirska-Olszańska, A.; Mizgier, P.; Wyspiańska, D. Composition and antioxidant activity of red fruit liqueurs. Food Chem. 2014, 157, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Nowicka, P.; Wojdylo, A. Stability of phenolic compounds, antioxidant activity and colour through natural sweeteners addition during storage of sour cherry puree. Food Chem. 2016, 196, 925–934. [Google Scholar] [CrossRef] [PubMed]
- Toydemir, G.; Capanoglu, E.; Gomez Roldan, M.V.; de Vos, R.C.H.; Boyacioglu, D.; Hall, R.D.; Beekwilder, J. Industrial processing effects on phenolic compounds in sour cherry (Prunus cerasus l.) fruit. Food Res. Int. 2013, 53, 218–225. [Google Scholar] [CrossRef]
- Wojdyło, A.; Nowicka, P.; Laskowski, P.; Oszmiański, J. Evaluation of sour cherry (Prunus cerasus l.) fruits for their polyphenol content, antioxidant properties, and nutritional components. J. Agric. Food Chem. 2014, 62, 12332–12345. [Google Scholar] [CrossRef] [PubMed]
- Kallithraka, S.; Salacha, M.I.; Tzourou, I. Changes in phenolic composition and antioxidant activity of white wine during bottle storage: Accelerated browning test versus bottle storage. Food Chem. 2009, 113, 500–505. [Google Scholar] [CrossRef]
- Brauch, J.E.; Kroner, M.; Schweiggert, R.M.; Carle, R. Studies into the stability of 3-O-glycosylated and 3,5-O-diglycosylated anthocyanins in differently purified liquid and dried maqui (Aristotelia chilensis (mol.) Stuntz) preparations during storage and thermal treatment. J. Agric. Food Chem. 2015, 63, 8705–8714. [Google Scholar] [CrossRef] [PubMed]
- De Rosso, V.V.; Mercadante, A.Z. Evaluation of colour and stability of anthocyanins from tropical fruits in an isotonic soft drink system. Innov. Food Sci. Emerg. Technol. 2007, 8, 347–352. [Google Scholar] [CrossRef]
- Macheix, J.J.; Fleuriet, A.; Billot, J. Fruit Phenolics; CRC Press, Inc.: Boca Raton, FL, USA, 1990; p. 378. [Google Scholar]
- Hellström, J.; Mattila, P.; Karjalainen, R. Stability of anthocyanins in berry juices stored at different temperatures. J. Food Compos. Anal. 2013, 31, 12–19. [Google Scholar] [CrossRef]
- Patras, A.; Brunton, N.P.; O’Donnell, C.; Tiwari, B.K. Effect of thermal processing on anthocyanin stability in foods; mechanisms and kinetics of degradation. Trends Food Sci. Technol. 2010, 21, 3–11. [Google Scholar] [CrossRef]
- Pérez-Magariño, S.; González-Sanjosé, M.L. Application of absorbance values used in wineries for estimating CIELAB parameters in red wines. Food Chem. 2003, 81, 301–306. [Google Scholar] [CrossRef]
- Nikkhah, E.; Khayamy, M.; Heidari, R.; Jamee, R. Effect of sugar treatment on stability of anthocyanin pigments in berries. J. Biol. Sci. 2007, 7, 1412–1417. [Google Scholar]
- Bolarinwa, I.F.; Orfila, C.; Morgan, M.R.A. Amygdalin content of seeds, kernels and food products commercially-available in the UK. Food Chem. 2014, 152, 133–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolken, W.A.M.; Tramper, J.; van der Werf, M.J. Amino acid-catalysed retroaldol condensation: The production of natural benzaldehyde and other flavour compounds. Flavour Frag. J. 2004, 19, 115–120. [Google Scholar] [CrossRef]
- Shen, Y.F. Study on the formation mechanism of four main kinds of ethyl esters in the fermentation of liquour. Liquor-Making Sci. Technol. 2003, 5, 005. [Google Scholar]
- Niu, Y.W.; Zhang, X.M.; Xiao, Z.B.; Song, S.Q.; Eric, K.; Jia, C.S.; Yu, H.Y.; Zhu, J.C. Characterization of odor-active compounds of various cherry wines by gas chromatography-mass spectrometry, gas chromatography-olfactometry and their correlation with sensory attributes. J. Chromatogr. B.-Anal. Technol. Biomed. Life Sci. 2011, 879, 2287–2293. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.Y.; Jiang, W.G.; Zhao, Y.P. Comparison of aromatic and phenolic compounds in cherry wines with different cherry cultivars by HS-SPME-GC-MS and HPLC. Int. J. Food Sci. Technol. 2012, 47, 100–106. [Google Scholar] [CrossRef]
- Loch, C.; Reusch, H.; Ruge, I.; Godelmann, R.; Pflaum, T.; Kuballa, T.; Schumacher, S.; Lachenmeier, D.W. Benzaldehyde in cherry flavour as a precursor of benzene formation in beverages. Food Chem. 2016, 206, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Heinonen, I.M.; Lehtonen, P.J.; Hopia, A.I. Antioxidant activity of berry and fruit wines and liquors. J. Agric. Food Chem. 1998, 46, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Piljac-Žegarac, J.; Valek, L.; Martinez, S.; Belščak, A. Fluctuations in the phenolic content and antioxidant capacity of dark fruit juices in refrigerated storage. Food Chem. 2009, 113, 394–400. [Google Scholar] [CrossRef]
- Montoro, P.; Tuberoso, C.I.G.; Perrone, A.; Piacente, S.; Cabras, P.; Pizza, C. Characterisation by liquid chromatography-electrospray tandem mass spectrometry of anthocyanins in extracts of Myrtus communis l. berries used for the preparation of myrtle liqueur. J. Chromatogr. A 2006, 1112, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Klimczak, I.; Małecka, M.; Szlachta, M.; Gliszczyńska-Świgło, A. Effect of storage on the content of polyphenols, vitamin C and the antioxidant activity of orange juices. J. Food Compos. Anal. 2007, 20, 313–322. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Wrolstad, R.E.; Acree, T.E.; Decker, E.A.; Penner, M.H.; Reid, D.S.; Schwartz, S.J.; Shoemaker, C.F.; Smith, D.; Sporns, P. Anthocyanins. In Handbook of Food Analytical Chemistry; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005; pp. 5–69. [Google Scholar]
- Tešević, V.; Nikićević, N.; Milosavljević, S.; Bajić, D.; Vajs, V.; Vučković, I.; Vujisić, L.; Dordević, I.; Stanković, M.; Veličković, M. Characterization of volatile compounds of “Drenja”, an alcoholic beverage obtained from the fruits of Cornelian cherry. J. Serb. Chem. Soc. 2009, 74, 117–128. [Google Scholar] [CrossRef]
Sample Availability: Samples of the liqueurs are available from the authors. |
| Compounds | Week | Temperature 15 °C | Temperature 30 °C | ||
|---|---|---|---|---|---|
| Without Sugar Added | With Sugar Added | Without Sugar Added | With Sugar Added | ||
| Neochlorogenic acid | 0 | 47.61 ± 0.39 1 | 50.85 ± 0.42 | 47.81 ± 0.64 | 50.43 ± 0.36 |
| 6 | 47.66 ± 0.40 | 50.87 ± 0.11 | 46.16 ± 0.49 | 50.16 ± 0.47 | |
| 12 | 45.88 ± 0.43 | 49.27 ± 0.13 | 43.83 ± 0.59 | 46.47 ± 0.61 | |
| 18 | 46.47 ± 0.58 | 41.75 ± 0.00 | 44.33 ± 0.13 | 42.64 ± 0.17 | |
| 24 | 45.73 ± 0.16 | 40.81 ± 0.24 | 43.44 ± 0.25 | 42.55 ± 0.39 | |
| Coumaroylquinic acid | 0 | 100.35 ± 0.09 | 108.24 ± 1.13 | 101.93 ± 0.65 | 107.52 ± 0.53 |
| 6 | 100.98 ± 0.23 | 108.03 ± 0.02 | 98.93 ± 0.25 | 106.72 ± 0.72 | |
| 12 | 97.82 ± 0.83 | 105.16 ± 0.74 | 94.29 ± 1.08 | 99.48 ± 0.66 | |
| 18 | 94.62 ± 0.11 | 96.81 ± 1.15 | 89.74 ± 0.52 | 83.8 ± 1.19 | |
| 24 | 92.9 ± 1.22 | 92.98 ± 0.34 | 89.53 ± 0.00 | 85.25 ± 1.12 | |
| Chlorogenic acid | 0 | 40.43 ± 0.27 | 44.35 ± 0.47 | 41.63 ± 0.57 | 43.41 ± 0.26 |
| 6 | 40.76 ± 0.42 | 43.32 ± 0.28 | 39.95 ± 0.44 | 43.43 ± 0.01 | |
| 12 | 39.79 ± 0.47 | 42.98 ± 0.26 | 37.9 ± 0.31 | 40.77 ± 0.22 | |
| 18 | 38.63 ± 0.51 | 40.59 ± 0.03 | 44.46 ± 0.30 | 36.85 ± 0.16 | |
| 24 | 37.55 ± 0.19 | 36.9 ± 0.26 | 44.06 ± 0.04 | 37.27 ± 0.44 | |
| Sum of Phenolic acids | 0 | 188.40 ± 1.15 | 203.43 ± 0.52 | 191.36 ± 1.76 | 201.36 ± 0.00 |
| 6 | 189.41 ± 2.25 | 202.22 ± 0.31 | 185.03 ± 1.99 | 200.31 ± 0.11 | |
| 12 | 183.49 ± 2.57 | 197.41 ± 1.40 | 176.02 ± 2.14 | 186.73 ± 0.77 | |
| 18 | 179.72 ± 1.17 | 179.15 ± 0.35 | 178.53 ± 1.59 | 163.29 ± 1.52 | |
| 24 | 176.18 ± 0.42 | 170.69 ± 1.86 | 177.03 ± 1.35 | 165.06 ± 1.63 | |
| Compounds | Week | Temperature 15 °C | Temperature 30 °C | ||
|---|---|---|---|---|---|
| Without Sugar Added | With Sugar Added | Without Sugar Added | With Sugar Added | ||
| Procyanidin B1 | 0 | 7.73 ± 0.03 1 | 6.68 ± 0.01 | 5.64 ± 0.01 | 6.17 ± 0.05 |
| 6 | 7.28 ± 0.03 | 5.64 ± 0.02 | 5.88 ± 0.06 | 7.21 ± 0.06 | |
| 12 | 4.35 ± 0.04 | 5.47 ± 0.01 | 4.99 ± 0.00 | 4.89 ± 0.05 | |
| 18 | tr | tr | tr | nd | |
| 24 | tr | tr | 0.00 ± 0.00 | nd | |
| Procyanidin B2 | 0 | 80.46 ± 0.32 | 90.88 ± 1.11 | 82.54 ± 1.13 | 89.84 ± 0.50 |
| 6 | 75.72 ± 0.02 | 84.67 ± 1.17 | 68.89 ± 0.48 | 73.66 ± 0.51 | |
| 12 | 70.68 ± 0.08 | 78.14 ± 0.35 | 55.89 ± 0.51 | 58.93 ± 0.71 | |
| 18 | 55.34 ± 0.76 | 37.85 ± 0.32 | 31.49 ± 0.06 | 0.00 ± 0.00 | |
| 24 | 53.22 ± 0.71 | 39.64 ± 0.51 | 0.00 ± 0.00 | 0.00 ± 0.00 | |
| (−)-Epicatechin + dimer | 0 | 33.03 ± 0.01 | 67.33 ± 0.80 | 50.83 ± 0.30 | 54.81 ± 0.75 |
| 6 | 46.17 ± 0.56 | 50.59 ± 0.25 | 44.03 ± 0.57 | 48.73 ± 0.34 | |
| 12 | 37.21 ± 0.28 | 54.38 ± 0.57 | 38.11 ± 0.34 | 46.97 ± 0.51 | |
| 18 | 44.31 ± 0.41 | 28.04 ± 0.34 | 0.00 ± 0.00 | 0.00 ± 0.00 | |
| 24 | 48.17 ± 0.24 | 28.69 ± 0.24 | 0.00 ± 0.00 | 0.00 ± 0.00 | |
| Procyanidin C1 + tetramer | 0 | 28.70 ± 0.09 | 33.21 ± 0.32 | 32.45 ± 0.30 | 31.14 ± 0.07 |
| 6 | 25.72 ± 0.28 | 35.01 ± 0.24 | 32.69 ± 0.02 | 38.65 ± 0.40 | |
| 12 | 30.44 ± 0.39 | 34.42 ± 0.36 | 24.21 ± 0.33 | 30.66 ± 0.22 | |
| 18 | 13.67 ± 0.07 | 14.78 ± 0.05 | 11.07 ± 0.04 | 12.65 ± 0.08 | |
| 24 | 15.78 ± 0.05 | 15.39 ± 0.11 | 8.08 ± 0.05 | 10.79 ± 0.08 | |
| Sum of flavan 3-ols | 0 | 149.92 ± 1.42 | 198.11 ± 1.74 | 171.46 ± 1.84 | 181.96 ± 2.11 |
| 6 | 154.89 ± 0.88 | 175.90 ± 1.72 | 151.50 ± 0.17 | 168.24 ± 2.26 | |
| 12 | 142.68 ± 2.00 | 172.42 ± 0.73 | 123.20 ± 0.05 | 141.45 ± 0.74 | |
| 18 | 113.85 ± 0.61 | 80.84 ± 1.14 | 42.86 ± 0.24 | 12.65 ± 0.10 | |
| 24 | 117.67 ± 0.95 | 83.88 ± 0.93 | 8.08 ± 0.06 | 10.79 ± 0.10 | |
| Compounds | Week | Temperature 15 °C | Temperature 30 °C | ||
|---|---|---|---|---|---|
| Without Sugar Added | With Sugar Added | Without Sugar Added | With Sugar Added | ||
| Kaempferol-trihexoside 1 | 0 | 22.29 ± 0.14 1 | 28.35 ± 0.18 | 25.63 ± 0.31 | 28.16 ± 0.12 |
| 6 | 20.41 ± 0.28 | 24.74 ± 0.07 | 13.83 ± 0.13 | 16.18 ± 0.19 | |
| 12 | 18.29 ± 0.01 | 21.79 ± 0.15 | 8.47 ± 0.10 | 10.28 ± 0.09 | |
| 18 | 22.07 ± 0.14 | 18.94 ± 0.03 | 7.32 ± 0.06 | 5.34 ± 0.06 | |
| 24 | 18.86 ± 0.16 | 16.87 ± 0.17 | 3.93 ± 0.05 | 3.54 ± 0.04 | |
| Kaempferol-trihexoside 2 | 0 | 7.63 ± 0.01 | 11.45 ± 0.14 | 10.42 ± 0.10 | 11.29 ± 0.05 |
| 6 | 6.55 ± 0.08 | 9.59 ± 0.12 | 4.39 ± 0.04 | 5.38 ± 0.06 | |
| 12 | 7.91 ± 0.07 | 7.83 ± 0.04 | 1.12 ± 0.00 | 2.05 ± 0.03 | |
| 18 | 6.27 ± 0.05 | 6.90 ± 0.03 | 1.64 ± 0.00 | 5.34 ± 0.01 | |
| 24 | 0.00 ± 0.00 | 5.91 ± 0.06 | 0.00 ± 0.00 | 3.54 ± 0.03 | |
| Kaempferol-dihexoside | 0 | 16.98 ± 0.01 | 18.38 ± 0.01 | 17.24 ± 0.05 | 18.20 ± 0.23 |
| 6 | 17.23 ± 0.01 | 18.53 ± 0.01 | 16.84 ± 0.08 | 18.40 ± 0.01 | |
| 12 | 16.64 ± 0.07 | 17.97 ± 0.06 | 16.11 ± 0.13 | 17.35 ± 0.09 | |
| 18 | 15.80 ± 0.02 | 15.82 ± 0.07 | 15.44 ± 0.05 | 15.12 ± 0.06 | |
| 24 | 15.51 ± 0.15 | 15.72 ± 0.19 | 15.34 ± 0.11 | 15.25 ± 0.09 | |
| Quercetin-rutinoside-rhamnoside | 0 | 3.79 ± 0.01 | 3.54 ± 0.05 | 3.34 ± 0.01 | 3.51 ± 0.04 |
| 6 | 3.35 ± 0.01 | 3.58 ± 0.03 | 3.27 ± 0.01 | 3.56 ± 0.02 | |
| 12 | 3.21 ± 0.01 | 3.44 ± 0.04 | 3.14 ± 0.02 | 3.43 ± 0.00 | |
| 18 | 3.32 ± 0.00 | 3.28 ± 0.02 | 3.32 ± 0.01 | 2.57 ± 0.03 | |
| 24 | 3.28 ± 0.03 | 3.29 ± 0.04 | 2.83 ± 0.03 | 2.53 ± 0.02 | |
| Quercetin-rutinoside | 0 | 48.28 ± 0.20 | 52.15 ± 41 | 48.16 ± 0.27 | 51.71 ± 0.34 |
| 6 | 48.36 ± 0.40 | 52.21 ± 0.52 | 47.58 ± 0.65 | 52.78 ± 0.32 | |
| 12 | 47.01 ± 0.08 | 51.06 ± 0.60 | 46.28 ± 0.63 | 49.23 ± 0.02 | |
| 18 | 45.64 ± 0.54 | 46.61 ± 0.65 | 43.64 ± 0.30 | 43.78 ± 0.03 | |
| 24 | 44.29 ± 0.28 | 46.30 ± 0.10 | 43.35 ± 0.59 | 46.69 ± 0.22 | |
| Quercetin-glucoside | 0 | 5.90 ± 0.07 | 6.48 ± 0.07 | 5.37 ± 0.07 | 6.55 ± 0.07 |
| 6 | 5.52 ± 0.03 | 6.57 ± 0.03 | 5.65 ± 0.07 | 6.71 ± 0.00 | |
| 12 | 6.04 ± 0.01 | 6.32 ± 0.01 | 6.17 ± 0.01 | 5.91 ± 0.06 | |
| 18 | 5.83 ± 0.05 | 5.91 ± 0.06 | 5.79 ± 0.04 | 5.64 ± 0.07 | |
| 24 | 5.68 ± 0.08 | 6.01 ± 0.08 | 6.01 ± 0.05 | 6.26 ± 0.02 | |
| Kaempferol-rutinoside | 0 | 12.42 ± 0.02 | 14.19 ± 0.18 | 13.43 ± 0.00 | 14.25 ± 0.06 |
| 6 | 13.32 ± 0.04 | 14.46 ± 0.11 | 13.26 ± 0.13 | 14.52 ± 0.07 | |
| 12 | 12.90 ± 0.06 | 14.17 ± 0.18 | 12.72 ± 0.08 | 13.60 ± 0.06 | |
| 18 | 12.39 ± 0.10 | 12.72 ± 0.04 | 11.81 ± 0.12 | 12.02 ± 0.11 | |
| 24 | 13.92 ± 0.10 | 12.48 ± 0.02 | 11.63 ± 0.12 | 12.68 ± 0.13 | |
| Isorhamnetine-rutinoside | 0 | 20.92 ± 0.24 | 23.11 ± 0.20 | 21.58 ± 0.21 | 22.75 ± 0.03 |
| 6 | 21.41 ± 0.14 | 23.25 ± 0.16 | 21.37 ± 0.14 | 23.33 ± 0.05 | |
| 12 | 20.77 ± 0.06 | 22.68 ± 0.15 | 20.45 ± 0.20 | 21.93 ± 0.23 | |
| 18 | 20.38 ± 0.09 | 20.76 ± 0.15 | 19.46 ± 0.07 | 19.75 ± 0.05 | |
| 24 | 20.11 ± 0.04 | 20.46 ± 0.19 | 19.30 ± 0.10 | 12.68 ± 0.16 | |
| Quercetin | 0 | 0.88 ± 0.00 | 0.90 ± 0.00 | 0.97 ± 0.00 | 0.94 ± 0.00 |
| 6 | 1.62 ± 0.00 | 1.53 ± 0.00 | 2.01 ± 0.02 | 2.43 ± 0.02 | |
| 12 | 1.15 ± 0.01 | 1.47 ± 0.00 | 1.76 ± 0.01 | 3.38 ± 0.01 | |
| 18 | 2.17 ± 0.02 | 2.06 ± 0.03 | 2.04 ± 0.01 | 2.76 ± 0.03 | |
| 24 | 2.30 ± 0.03 | 2.97 ± 0.03 | 2.58 ± 0.00 | 4.34 ± 0.05 | |
| Sum of flavonols | 0 | 139.73 ± 0.79 | 158.56 ± 1.48 | 146.16 ± 0.54 | 157.38 ± 0.87 |
| 6 | 138.85 ± 0.04 | 154.45 ± 1.62 | 128.21 ± 0.33 | 143.30 ± 1.30 | |
| 12 | 132.56 ± 0.73 | 146.72 ± 0.62 | 116.23 ± 0.77 | 127.18 ± 1.10 | |
| 18 | 135.50 ± 1.15 | 133.02 ± 0.19 | 110.46 ± 0.41 | 112.33 ± 1.38 | |
| 24 | 130.21 ± 1.10 | 130.01 ± 0.96 | 104.97 ± 1.05 | 107.52 ± 1.46 | |
| Compounds | Week | Temperature 15 °C | Temperature 30 °C | ||
|---|---|---|---|---|---|
| Without Sugar Added | With Sugar Added | Without Sugar Added | With Sugar Added | ||
| Cyanidin 3-O-sophoroside | 0 | 19.11 ± 0.13 1 | 20.21 ± 0.10 | 18.98 ± 0.01 | 20.15 ± 0.24 |
| 6 | 15.57 ± 0.07 | 17.17 ± 0.18 | 7.95 ± 0.04 | 9.37 ± 0.10 | |
| 12 | 12.32 ± 0.01 | 14.57 ± 0.15 | 3.81 ± 0.02 | 4.74 ± 0.01 | |
| 18 | 8.12 ± 0.00 | 12.50 ± 0.16 | 1.57 ± 0.02 | 2.81 ± 0.02 | |
| 24 | 6.91 ± 0.09 | 11.80 ± 0.06 | 0.88 ± 0.01 | 1.59 ± 0.01 | |
| Cyanidin 3-O-glucosyl rutinoside | 0 | 135.23 ± 0.84 | 143.26 ± 1.07 | 134.83 ± 1.79 | 142.62 ± 1.03 |
| 6 | 113.56 ± 0.29 | 125.12 ± 0.85 | 64.42 ± 0.91 | 75.92 ± 0.24 | |
| 12 | 92.85 ± 0.30 | 105.45 ± 1.31 | 35.04 ± 0.12 | 43.86 ± 0.61 | |
| 18 | 65.27 ± 0.61 | 55.17 ± 0.71 | 17.06 ± 0.16 | 15.50 ± 0.14 | |
| 24 | 56.14 ± 0.71 | 48.93 ± 0.23 | 11.01 ± 0.08 | 9.82 ± 0.01 | |
| Cyanidin 3-O-glucoside | 0 | 5.18 ± 0.01 | 6.06 ± 0.08 | 5.54 ± 0.01 | 6.13 ± 0.01 |
| 6 | 4.30 ± 0.05 | 4.77 ± 0.03 | 1.73 ± 0.01 | 2.30 ± 0.01 | |
| 12 | 3.17 ± 0.02 | 4.55 ± 0.01 | 0.68 ± 0.00 | 0.89 ± 0.00 | |
| 18 | 2.75 ± 0.02 | 2.44 ± 0.02 | 0.21 ± 0.00 | 0.37 ± 0.00 | |
| 24 | 1.76 ± 0.02 | 2.01 ± 0.02 | 0.11 ± 0.00 | 0.15 ± 0.00 | |
| Cyanidin 3-O-sambubioside-5-rhamnoside | 0 | 2.21 ± 0.02 | 2.70 ± 0.00 | 2.54 ± 0.01 | 2.85 ± 0.04 |
| 6 | 2.33 ± 0.02 | 2.31 ± 0.03 | 0.87 ± 0.00 | 1.33 ± 0.01 | |
| 12 | 1.60 ± 0.02 | 2.54 ± 0.03 | 0.38 ± 0.00 | 0.59 ± 0.01 | |
| 18 | 1.73 ± 0.02 | 1.46 ± 0.01 | tr | 0.31 ± 0.00 | |
| 24 | 1.18 ± 0.01 | 1.21 ± 0.01 | tr | tr | |
| Cyanidin 3-O-rutinoside | 0 | 55.55 ± 0.05 | 58.62 ± 0.60 | 54.97 ± 0.13 | 58.58 ± 0.31 |
| 6 | 44.24 ± 0.33 | 48.64 ± 0.43 | 21.75 ± 0.01 | 26.15 ± 0.30 | |
| 12 | 33.87 ± 0.29 | 40.34 ± 0.47 | 10.3 ± 0.11 | 13.52 ± 0.17 | |
| 18 | 25.38 ± 0.13 | 26.29 ± 0.18 | 4.95 ± 0.00 | 5.70 ± 0.03 | |
| 24 | 20.49 ± 0.13 | 22.66 ± 0.12 | 2.95 ± 0.02 | 3.19 ± 0.02 | |
| Sum of anthocyanins | 0 | 217.28 ± 0.74 | 230.84 ± 2.32 | 216.85 ± 2.64 | 230.33 ± 1.50 |
| 6 | 180.00 ± 1.99 | 198.01 ± 0.20 | 96.72 ± 0.23 | 115.07 ± 0.41 | |
| 12 | 143.81 ± 0.63 | 167.46 ± 1.66 | 50.20 ± 0.09 | 63.61 ± 0.68 | |
| 18 | 103.26 ± 0.38 | 101.42 ± 1.35 | 23.93 ± 0.07 | 24.68 ± 0.18 | |
| 24 | 86.48 ± 0.54 | 90.19 ± 0.78 | 15.05 ± 0.14 | 14.88 ± 0.07 | |
| Week | Temperature 15 °C | Temperature 30 °C | |||
|---|---|---|---|---|---|
| Without Sugar Added | With Sugar Added | Without Sugar Added | With Sugar Added | ||
| Polymeric anthocyanin percentage | 0 | 9.5 | 8.9 | 9.3 | 9.2 |
| 8 | 12.6 | 10.7 | 20.5 | 18.5 | |
| 16 | 22.7 | 20.7 | 57.5 | 42 | |
| 24 | 25.8 | 21.9 | 75.8 | 65.3 | |
| k (1/week) | 0.042 | 0.048 | 0.105 | 0.118 | |
| t1/2 (week) | 16.4 | 14.5 | 6.6 | 5.9 | |
| R2 | 0.994 | 0.969 | 0.996 | 0.993 | |
| Compounds | tR (min) | Week | Temperature 15 °C | Temperature 30 °C | ||
|---|---|---|---|---|---|---|
| Without Sugar Added | With Sugar Added | Without Sugar Added | With Sugar Added | |||
| Benzaldehyde | 6.85 | 6 | 9491.0 ± 54.76 1 | 10,800.0 ± 106.30 | 10,150.0 ± 44.79 | 8600.0 ± 24.32 |
| 24 | 10,900.0 ± 54.26 | 11,500.0 ± 19.52 | 12,600.0 ± 51.32 | 12,600.0 ± 25.66 | ||
| 1-Octanol | 8.60 | 6 | 5.0 ± 0.04 | 10.0 ± 0.01 | 10.0 ± 0.01 | 30.0 ± 0.17 |
| 24 | 30.0 ± 0.00 | 40.0 ± 0.29 | 20.0 ± 0.03 | 60.0 ± 0.68 | ||
| Hexyl acetate | 8.67 | 6 | 4.0 ± 0.01 | 40.0 ± 0.34 | 20.0 ± 0.04 | 20.0 ± 0.17 |
| 24 | 20.0 ± 0.04 | 68.0 ± 0.29 | 35.0 ± 0.15 | 54.0 ± 0.01 | ||
| Benzyl alcohol | 9.64 | 6 | 45.0 ± 0.38 | 60.0 ± 0.25 | 30.0 ± 0.13 | 48.0 ± 0.02 |
| 24 | 78.0 ± 0.43 | 47.0 ± 0.13 | 35.0 ± 0.27 | 47.0 ± 0.15 | ||
| Limonene | 10.05 | 6 | 1.0 ± 0.00 | 10.0 ± 0.06 | 1.0 ± 0.00 | 1.0 ± 0.00 |
| 24 | 1.0 ± 0.00 | 1.0 ± 0.01 | 1.0 ± 0.00 | 1.0 ± 0.01 | ||
| 2-Octenal | 10.56 | 6 | 1.0 ± 0.01 | 1.0 ± 0.01 | 1.0 ± 0.00 | 1.0 ± 0.01 |
| 24 | 1.0 ± 0.01 | 1.0 ± 0.00 | 1.0 ± 0.01 | 1.0 ± 0.01 | ||
| 1-Nonanol | 11.55 | 6 | 2.0 ± 0.00 | 2.0 ± 0.02 | 3.0 ± 0.00 | 3.0 ± 0.02 |
| 24 | 2.0 ± 0.02 | 1.0 ± 0.01 | 3.0 ± 0.00 | 2.0 ± 0.02 | ||
| Unknown | 11.97 | 6 | 0.0 ± 0.00 | 1.0 ± 0.00 | 0.2 ± 0.00 | 1.0 ± 0.01 |
| 24 | 1.0 ± 0.01 | 1.0 ± 0.00 | 1.0 ± 0.00 | 1.0 ± 0.00 | ||
| 2-Nonen-1-ol + linalool | 12.68 | 6 | 26.0 ± 0.12 | 36.0 ± 0.29 | 26.0 ± 0.12 | 48.0 ± 0.08 |
| 24 | 26.0 ± 0.11 | 78.0 ± 0.04 | 48.0 ± 0.46 | 68.0 ± 0.38 | ||
| Ethyl benzoate | 15.25 | 6 | 55.0 ± 0.44 | 95.0 ± 0.05 | 73.0 ± 0.22 | 87.0 ± 0.28 |
| 24 | 45.0 ± 0.20 | 68.0 ± 0.41 | 28.0 ± 0.18 | 78.0 ± 0.64 | ||
| Diethyl succinate | 15.66 | 6 | 34.0 ± 0.38 | 69.0 ± 0.30 | 48.0 ± 0.03 | 89.0 ± 0.36 |
| 24 | 68.0 ± 0.61 | 45.0 ± 0.25 | 65.0 ± 0.63 | 67.0 ± 0.27 | ||
| Octanoic acid ethyl ester | 17.02 | 6 | 7.0 ± 0.01 | 8.0 ± 0.07 | 9.0 ± 0.02 | 87.0 ± 0.79 |
| 24 | 48.0 ± 0.30 | 68.0 ± 0.74 | 59.0 ± 0.18 | 71.0 ± 0.44 | ||
| Ethyl acetal of benzaldehyde | 18.13 | 6 | 23.0 ± 0.20 | 160.0 ± 1.18 | 120.0 ± 1.34 | 140.0 ± 0.19 |
| 24 | 980.0 ± 5.99 | 680.0 ± 3.54 | 780.0 ± 3.09 | 470.0 ± 2.34 | ||
| Diethyl malate | 19.41 | 6 | 17.0 ± 0.08 | 580.0 ± 1.38 | 80.0 ± 0.33 | 180.0 ± 1.91 |
| 24 | 150.0 ± 0.63 | 950.0 ± 3.87 | 870.0 ± 5.32 | 680.0 ± 2.69 | ||
| Phenylacetalde-hyde diethyl acetal | 22.24 | 6 | 6.0 ± 0.03 | 9.0 ± 0.08 | 9.0 ± 0.05 | 3.0 ± 0.03 |
| 24 | 24.0 ± 0.04 | 48.0 ± 0.30 | 35.0 ± 0.07 | 78.0 ± 0.19 | ||
| Methyl decanoate | 22.55 | 6 | 20.0 ± 0.17 | 53.0 ± 0.40 | 35.0 ± 0.18 | 47.0 ± 0.04 |
| 24 | 24.0 ± 0.03 | 48.0 ± 0.22 | 26.0 ± 0.10 | 87.0 ± 0.34 | ||
| ar-Ionone | 23.480 | 6 | 2.0 ± 0.01 | 78.0 ± 0.68 | 6.0 ± 0.03 | 59.0 ± 0.24 |
| 24 | 20.0 ± 0.13 | 45.0 ± 0.26 | 65.0 ± 0.24 | 70.0 ± 0.73 | ||
| Ethyl decanoate | 24.593 | 6 | 4.0 ± 0.02 | 48.0 ± 0.29 | 3.0 ± 0.02 | 45.0 ± 0.50 |
| 24 | 520.0 ± 4.71 | 68.0 ± 0.55 | 41.0 ± 0.20 | 78.0 ± 0.60 | ||
| α-Ionone | 24.954 | 6 | 5.0 ± 0.02 | 7.0 ± 0.07 | 9.0 ± 0.09 | 5.0 ± 0.02 |
| 24 | 48.0 ± 0.51 | 48.0 ± 0.47 | 65.0 ± 0.05 | 36.0 ± 0.33 | ||
| β-Ionone | 25.749 | 6 | 9.0 ± 0.07 | 7.0 ± 0.00 | 7.0 ± 0.00 | 5.0 ± 0.04 |
| 24 | 40.0 ± 0.27 | 50.0 ± 0.50 | 650.0 ± 6.03 | 98.0 ± 0.01 | ||
| Total | 6 | 9760.0 ± 59.6 | 12070.0 ± 12.3 | 10640.0 ± 78.3 | 9500.0 ± 55.9 | |
| 24 | 13030.0 ± 147.4 | 13860.0 ± 142.7 | 15430.0 ± 117.0 | 14650.0 ± 54.7 | ||
| Test | Week | Temperature 15 °C | Temperature 30 °C | ||
|---|---|---|---|---|---|
| Without Sugar Added | With Sugar Added | Without Sugar Added | With Sugar Added | ||
| FC (mg GAE/mL) | 0 | 1.16 ± 0.04 1 | 1.16 ± 0.02 | 1.13 ± 0.01 | 1.15 ± 0.07 |
| 6 | 1.15 ± 0.01 | 1.16 ± 0.06 | 1.12 ± 004 | 1.15 ± 0.01 | |
| 12 | 1.19 ± 0.04 | 1.18 ± 0.05 | 1.06 ± 0.06 | 1.18 ± 0.04 | |
| 18 | 1.21 ± 0.04 | 1.10 ± 0.03 | 0.98 ± 0.02 | 1.21 ± 0.03 | |
| 24 | 1.10 ± 0.03 | 1.21 ± 0.05 | 1.14 ± 0.01 | 1.25 ± 0.05 | |
| ABTS (µM TE/mL) | 0 | 11.56 ± 0.29 | 11.77 ± 0.14 | 12.28 ± 0.64 | 12.62 ± 0.47 |
| 6 | 11.56 ± 0.70 | 11.86 ± 0.23 | 11.46 ± 0.71 | 10.83 ± 0.61 | |
| 12 | 11.67 ± 0.28 | 11.30 ± 0.22 | 10.64 ± 0.38 | 8.66 ± 0.43 | |
| 18 | 11.51 ± 0.18 | 10.74 ± 0.39 | 9.47 ± 0.05 | 8.78 ± 0.07 | |
| 24 | 10.83 ± 0.29 | 10.68 ± 0.48 | 9.90 ± 0.32 | 7.85 ± 0.19 | |
| Compound | Linear Range (µg/mL) | Equation of Calibration | Correlation Coefficient R² | LOD (µg/mL) | LOQ (µg/mL) |
|---|---|---|---|---|---|
| Cyanidin 3-O-glucoside | 10–75 | y = 0.8538x | 0.9994 | 0.073 | 0.241 |
| Quercetin 3-O-rutinoside | 20–300 | y = 1.9793x | 0.9961 | 0.107 | 0.354 |
| (+) Catechin | 20–200 | y = 4.1786x | 0.9996 | 0.438 | 1.446 |
| Chlorogenic acid | 20–300 | y = 0.9195x | 0.9998 | 0.062 | 0.199 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sokół-Łętowska, A.; Kucharska, A.Z.; Szumny, A.; Wińska, K.; Nawirska-Olszańska, A. Phenolic Composition Stability and Antioxidant Activity of Sour Cherry Liqueurs. Molecules 2018, 23, 2156. https://doi.org/10.3390/molecules23092156
Sokół-Łętowska A, Kucharska AZ, Szumny A, Wińska K, Nawirska-Olszańska A. Phenolic Composition Stability and Antioxidant Activity of Sour Cherry Liqueurs. Molecules. 2018; 23(9):2156. https://doi.org/10.3390/molecules23092156
Chicago/Turabian StyleSokół-Łętowska, Anna, Alicja Z. Kucharska, Antoni Szumny, Katarzyna Wińska, and Agnieszka Nawirska-Olszańska. 2018. "Phenolic Composition Stability and Antioxidant Activity of Sour Cherry Liqueurs" Molecules 23, no. 9: 2156. https://doi.org/10.3390/molecules23092156