Diverse Derivatives of Selenoureas: A Synthetic and Single Crystal Structural Study
Abstract
:1. Introduction
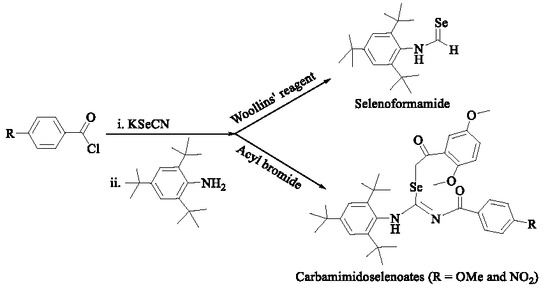
2. Results and Discussion
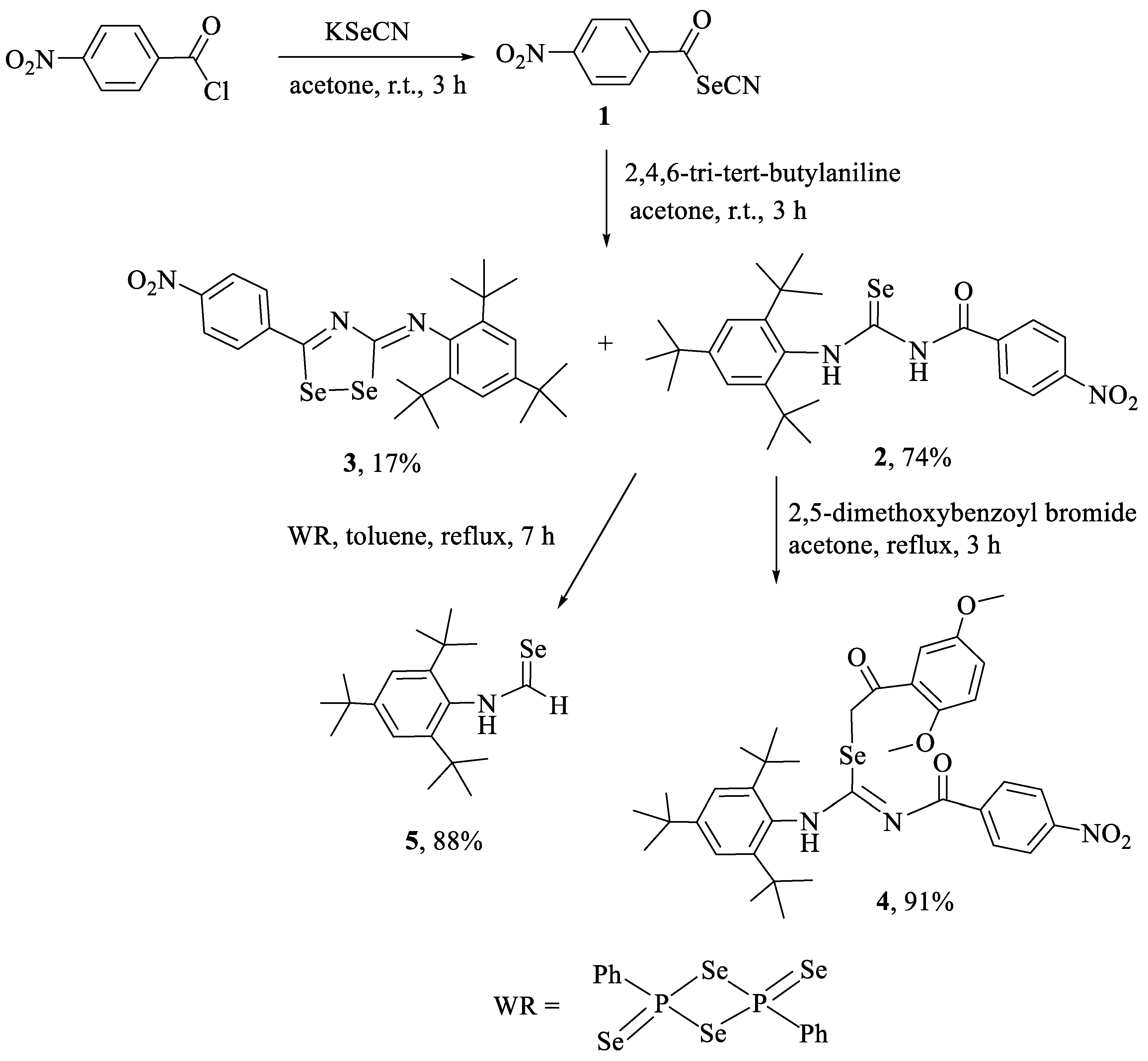
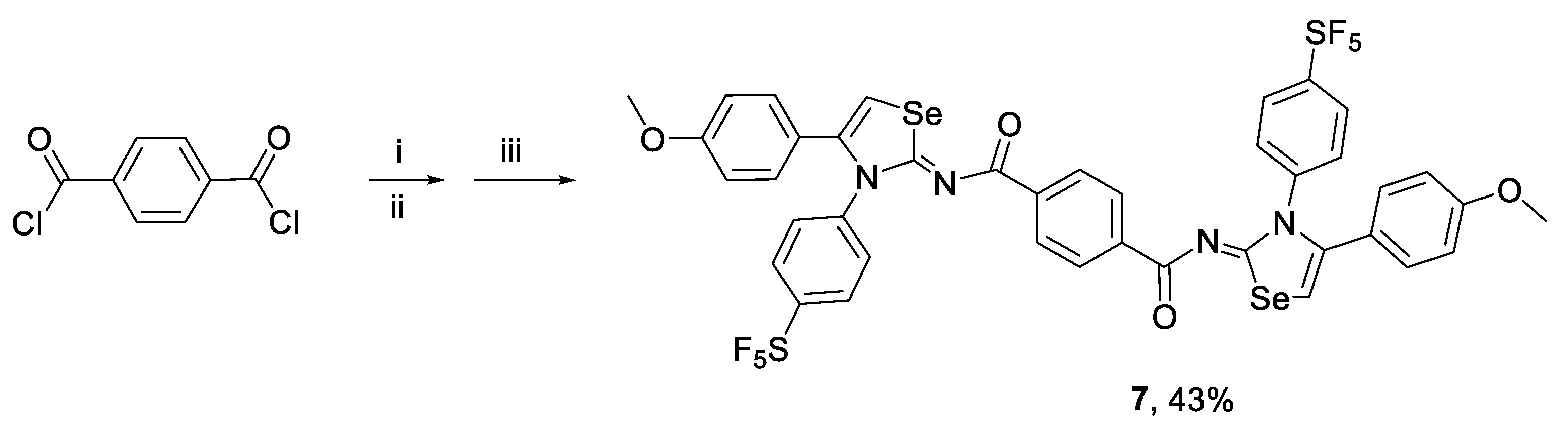
2.1. Synthesis and Characterization
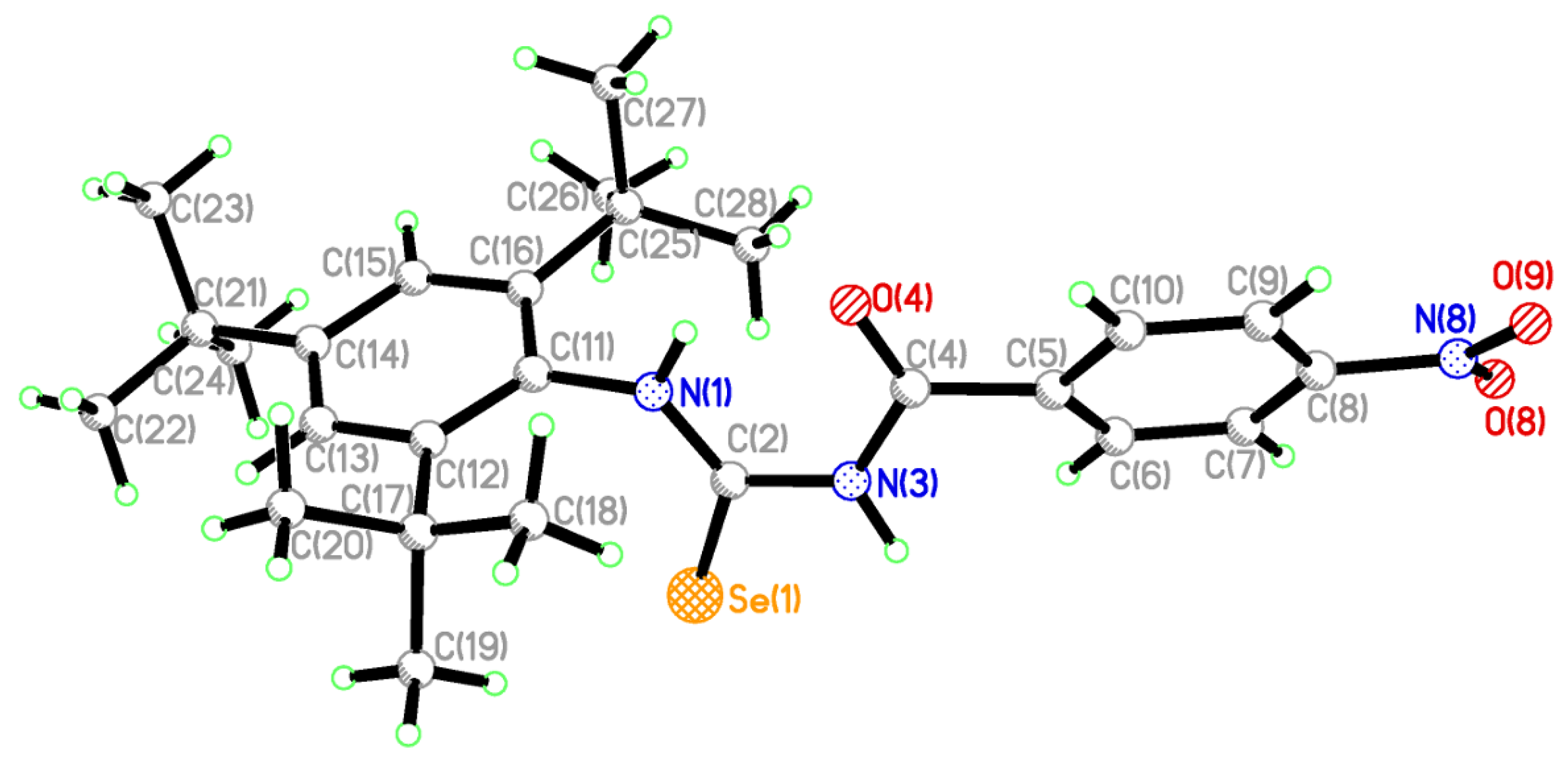
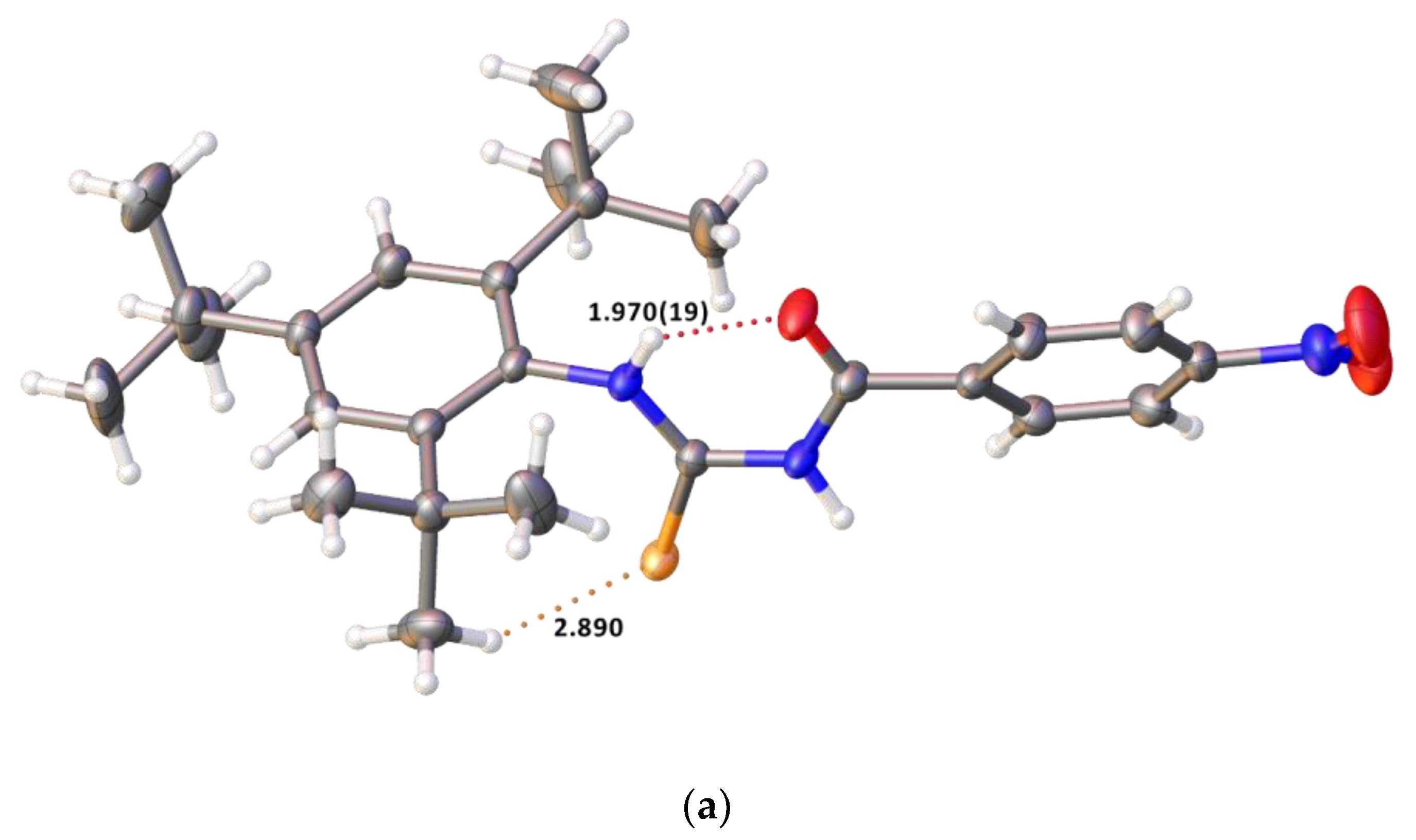
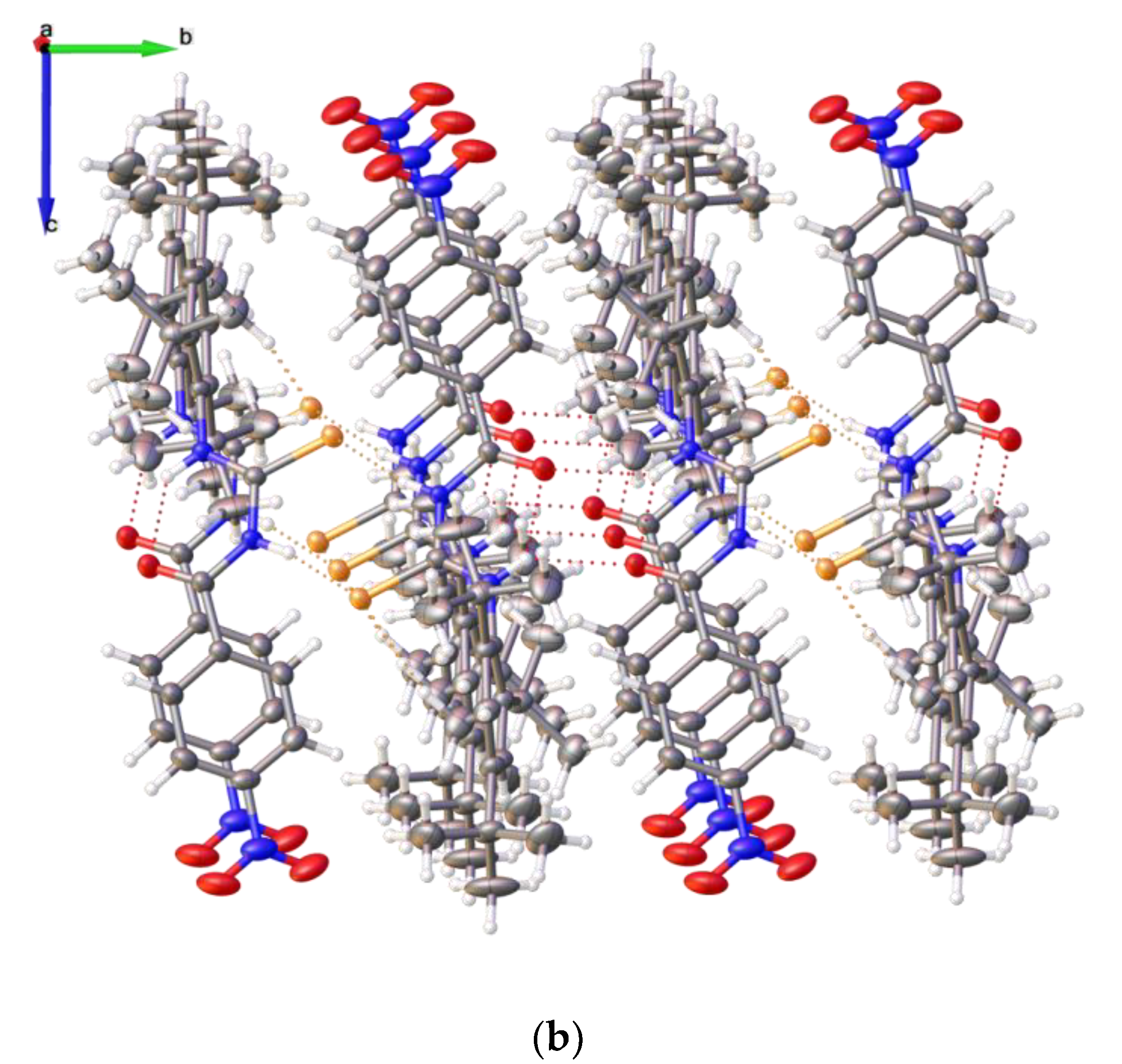
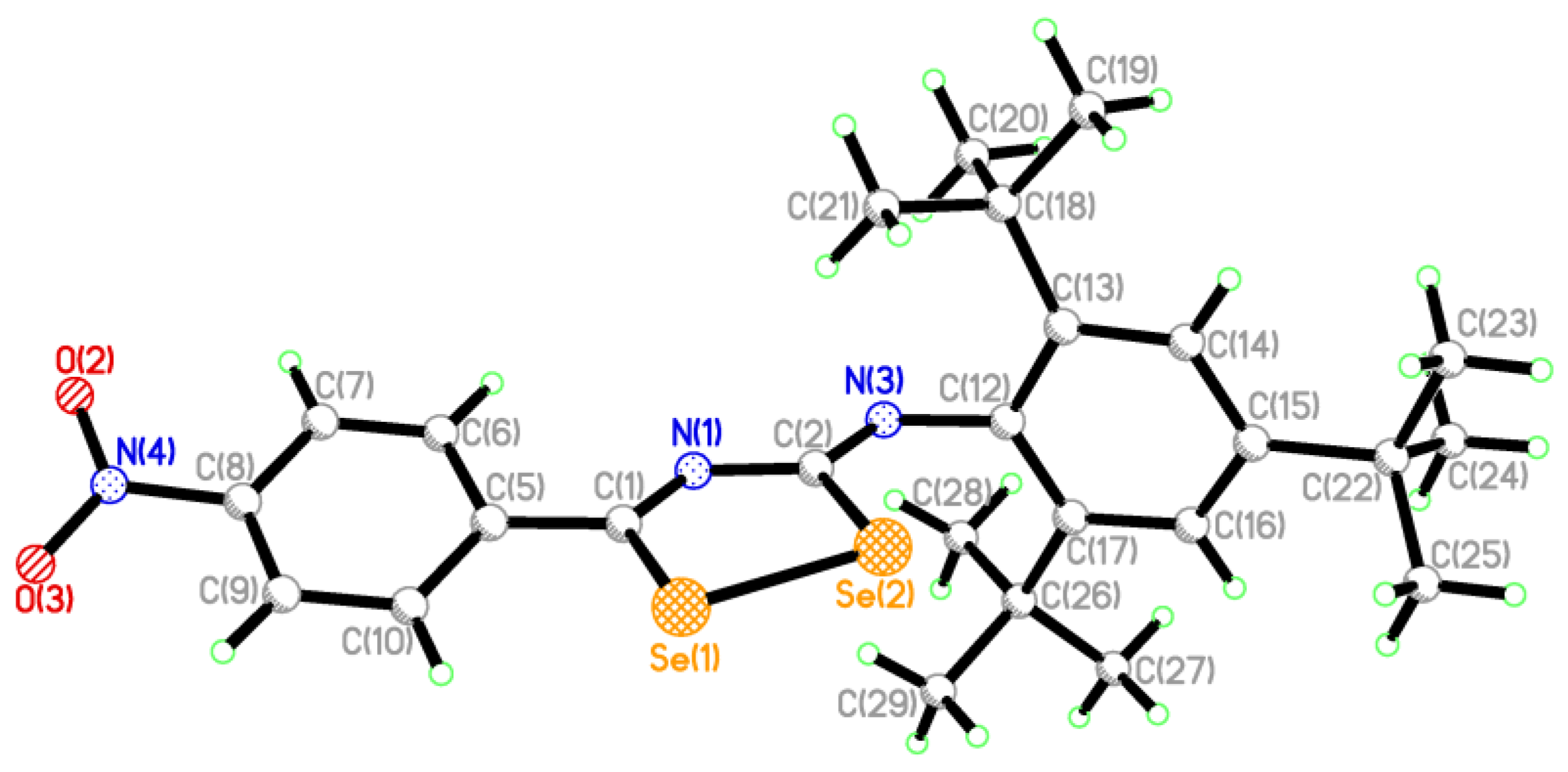
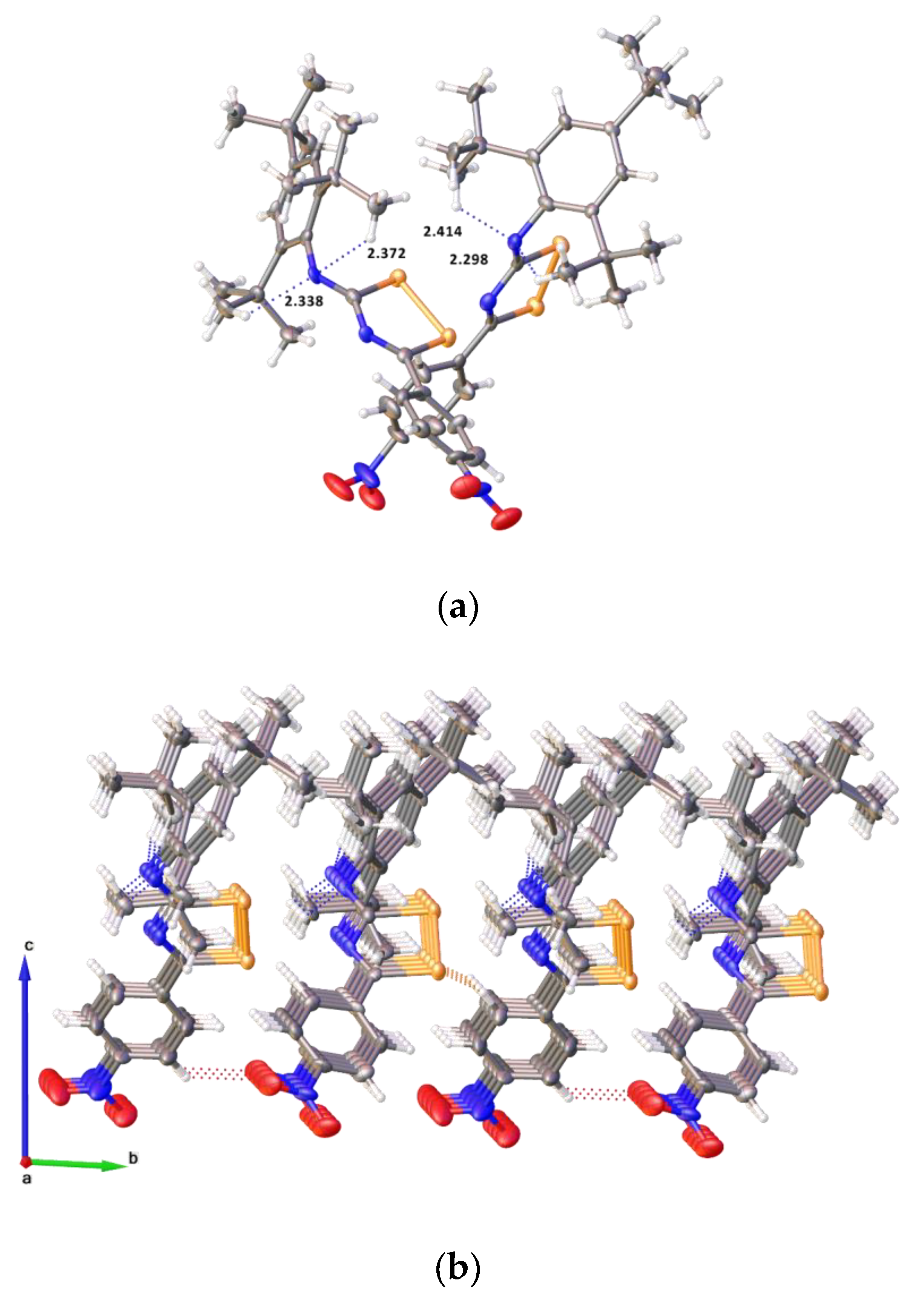
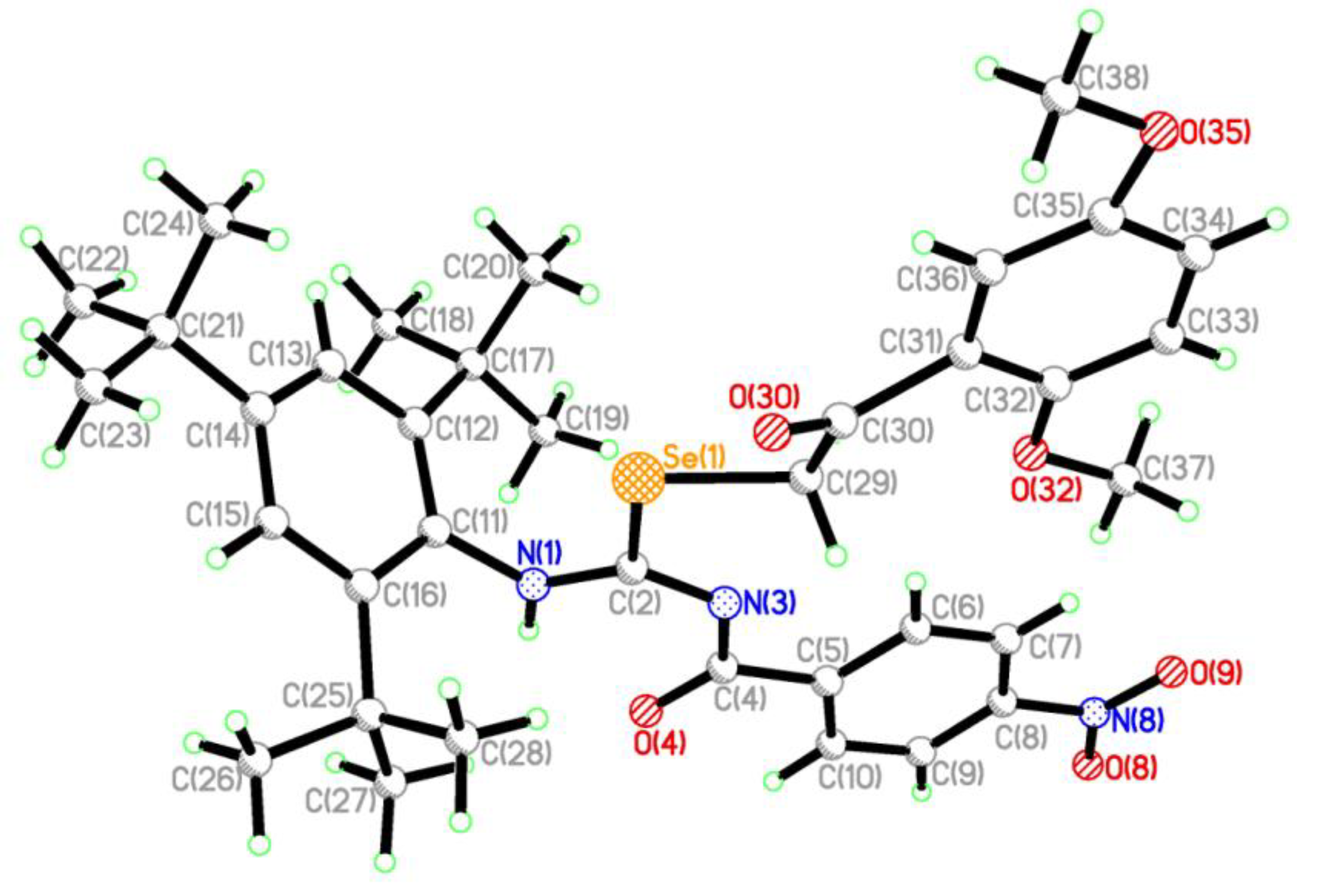
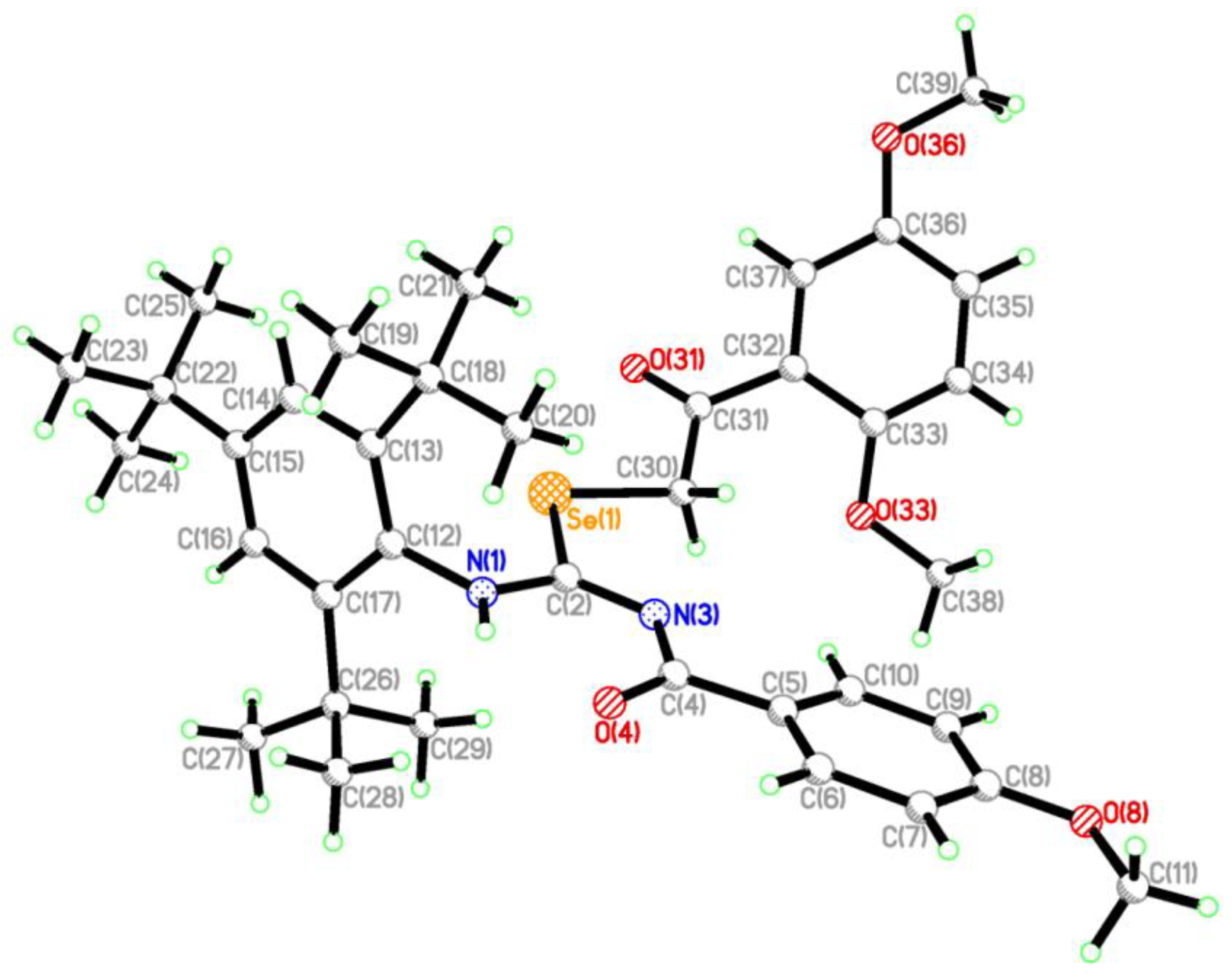
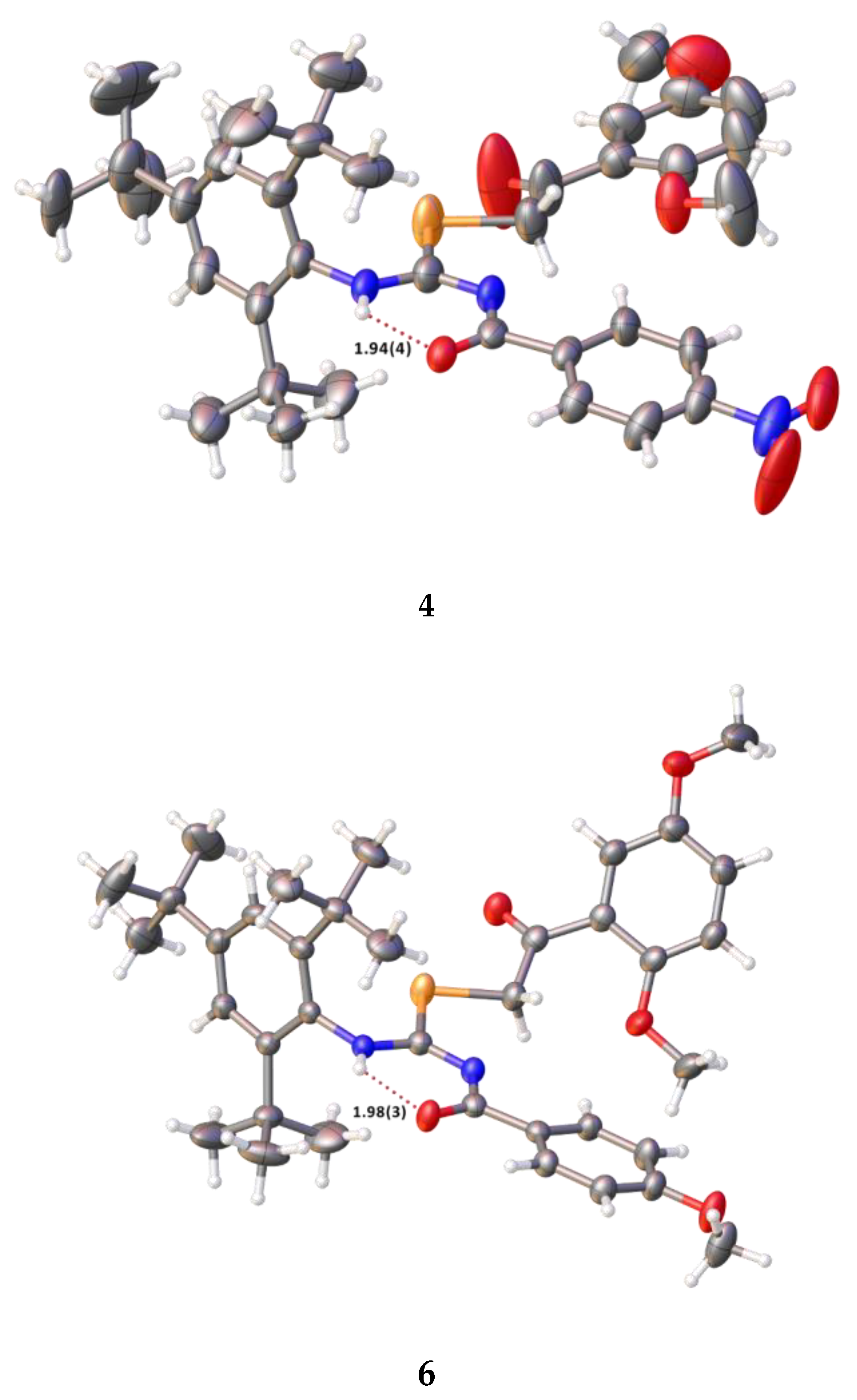
2.2. Single Crystal Structure Analysis
3. Materials and Methods
3.1. General
3.2. Synthesis
3.2.1. Synthesis of Compounds 2 and 3
3.2.2. Synthesis of 2-(2,5-dimethoxyphenyl)-2-oxoethyl-N′-(4-nitrobenzoyl)-N-(2,4,6-tri-tert-butylphenyl)-carbamimidoselenoate (4)
3.2.3. Synthesis of 2-(2,5-dimethoxyphenyl)-2-oxoethyl-N′-(4-methoxybenzoyl)-N-(2,4,6-tri-tert-butylphenyl)-carbamimidoselenoate (6)
3.2.4. Synthesis of 1,4-bis[N-(4-(4-methoxyphenyl)-3-(4-(pentafluoro-l6-sulfanyl)phenyl)-1,3-selenazol-2(3H)-ylidene)]terephthalamide (7)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Garud, D.R.; Koketsu, M.; Ishihara, H. Isoselenocyanates: A power tool for the synthesis of selenium-containing heterocycles. Molecules 2007, 12, 504–535. [Google Scholar] [CrossRef] [PubMed]
- Heimgartner, H.; Zhou, Y.; Atanassov, P.K.; Sommen, G.L. Isoselenocyantates as building blocks for selenium-containing heterocycles. Phosphorus Sulfur Silicon Relat. Elem. 2008, 183, 840–855. [Google Scholar] [CrossRef]
- Ninomiya, M.; Garud, D.R.; Koketsu, M. Selenium-containing heterocycles using selenoamides, selenoureas, selenazadienes, and isoselenocyanates. Heterocycles 2010, 81, 2027–2055. [Google Scholar]
- Barton, D.H.R.; Parekh, S.I.; Tajbakhsh, M.; Theodorakis, E.A.; Tse, C.L. A convenient and high yielding procedure for the preparation of isoselenocyanates. Synthesis and reactivity of O-alkylselenocarbamates. Tetrahedron 1994, 50, 639–654. [Google Scholar] [CrossRef]
- Atanassov, P.K.; Zhou, Y.; Linden, A. Heimgartner, H. Synthesis of bis(2,4-diarylimidazol-5-yl) diselennides from N-benzylbenzidoyl isoselenocyanates. Helv. Chim. Acta 2002, 85, 1102–1117. [Google Scholar] [CrossRef]
- Zakrzewski, J.; Krawczyk, M. Synthesis and pesticidal properties of thio and seleno analogs of some urea herbicides. Phosphorus Sulfur Silicon Relat. Elem. 2009, 184, 1880–1903. [Google Scholar] [CrossRef]
- Koketsu, M.; Takahashi, A.; Ishihara, H. A facile preparation of selenohydantoins using isoselenocyanate. J. Heterocycl. Chem. 2007, 44, 79–81. [Google Scholar] [CrossRef]
- Köhler, R.; Beyer, L.; Moll, M.; Hantschmann, A.; Richter, R.; Sieler, J.; Szargan, R.; Weber, L.; Hoyer, E. 2-Benzoylamino-5-dimethylamino-1,6-6aλ4-triselena-3,4-diazapentalene. Tetrahedron 1990, 46, 7735–7738. [Google Scholar]
- Zhou, Y.; Heimgartner, H. Selenium-containing heterocycles from isoselenocyanates: synthesis of 1,2,3-selenadiazole derivatives. Helv. Chim. Acta 2000, 83, 539–553. [Google Scholar] [CrossRef]
- L’abbé, G.; Dekerk, J.P.; Martens, C.; Toppet, S. Chemistry of N-sulfonyl-substituted thiiranimines. J. Org. Chem. 1980, 45, 4366–4371. [Google Scholar] [CrossRef]
- Koketsu, M.; Suzuki, N.; Ishihara, H. Preparation of isoselenocyanate and synthesis of carbodiimide by oxidation of selenourea. J. Org. Chem. 1999, 64, 6473–6475. [Google Scholar] [CrossRef]
- Fujiwara, S.I.; Kambe, N.; Sonoda, N. Organoselenium Chemistry: A Practical Approach; Back, T.G., Ed.; Oxford University Press: London, UK, 1999; pp. 223–240. [Google Scholar]
- Koketsu, M.; Ishihara, H. Thioureas and selenoureas and their applications. Curr. Org. Synth. 2006, 3, 439–455. [Google Scholar] [CrossRef]
- Koketsu, M.; Yamamura, Y.; Ishihara, H. Synthesis of selenosemicarbazides and 1,2,4-triazoles. Heterocycles 2006, 68, 1191–1200. [Google Scholar] [CrossRef]
- Koketsu, M.; Yamamura, Y.; Ando, H.; Ishihara, H. Synthesis of 1,3-selenazetidines and 4H-1,3,5-oxadiazines using acyl isoselenocyanates. Heterocycles 2006, 68, 1267–1273. [Google Scholar] [CrossRef]
- Koketsu, M.; Otsuka, T.; Ishihara, H. Steroids. Synthesis of 1,3-selenazetidine derivatives from imines and thiocarbamoyl isoselenocycanate. Heterocycles 2006, 68, 2107–2112. [Google Scholar] [CrossRef]
- Koketsu, M.; Sakai, T.; Kiyokuni, T.; Garud, D.R.; Ando, H.; Ishihara, H. One-pot synthesis of 2-imino-1,3-selenazolidines by reaction of isoselenocyanates with propargylamine. Heterocycles 2006, 68, 1607–1615. [Google Scholar] [CrossRef]
- Koketsu, M.; Yamamura, Y.; Ishihara, H. Synthesis of 2-selenoxoperhydro-1,3-selenazin-4-ones and 2-selenoxo-1,3-selenazolidin-4-ones via diselenocarbamate intermediates. Synthesis 2006, 2738–2742. [Google Scholar] [CrossRef]
- Koketsu, M.; Otsuka, T.; Swenson, D.; Ishihara, H. The synthesis of 1-thia-6-oxa-6aλ4-seleno-3-azapentalene and a 3H-1,2,4-dithiazole. Org. Biomol. Chem. 2007, 5, 613–616. [Google Scholar] [CrossRef] [PubMed]
- Koketsu, M.; Yamamura, Y.; Aoki, H.; Ishihara, H. The preparation of acylselenourea and selenocarbamate using isoselenocyanate. Phosphorus Sulfur Silicon Relat. Elem. 2006, 181, 2699–2708. [Google Scholar] [CrossRef]
- Segi, M.; Kojima, A.; Nakajima, T.; Suga, S. A facile synthesis of seleno- and telluroformamides. Synlett 1991, 105–106. [Google Scholar] [CrossRef]
- Li, G.M.; Zingaro, R.A. Chalcogenoamides: Convenient preparations and reactions with metal compounds. Phosphorus Sulfur Silicon Relat. Elem. 1998, 136–138, 525–530. [Google Scholar] [CrossRef]
- Shibara, F.; Sugiura, R.; Murai, T. Direct thionation and selenation of amides using elemental sulfur and selenium and hydrochlorosilanes in the presence of amines. Org. Lett. 2009, 11, 3064–3067. [Google Scholar] [CrossRef] [PubMed]
- Linden, A.; Zhou, Y.; Hermgartner, H. Intra- and intermolecular Se···X (X = Se, O) interactions in selenium-containing heterocycles: 3-benzoylimino-5-(morpholin-4-yl)-1,2,4-diselenazole. Acta Crystallogr. 2014, C70, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Richter, R.; Sieler, J.; Hansen, L.K.; Kohler, R.; Beyer, L.; Hoyer, E. Structure of 2-benzoylamino-5-diethylamino-1,66aλ4-triselena-3,4-diazapentalene. Acta Chem. Scand. 1991, 45, 1–5. [Google Scholar] [CrossRef]
- Li, G.M.; Zingaro, R.A.; Segi, M.; Reibenspies, J.H.; Nakajima, T. Synthesis and structure of telluroamides and selenoamides. The first crystallographic study of telluroamides. Organometallics 1997, 16, 756–762. [Google Scholar] [CrossRef]
- Hummel, H.U.; Fischer, T.; Gruss, D.; Franke, A.; Dietzsch, W. Synthesis and molecular structures of some novel anionic diselenides. J. Chem. Soc. Dalton Trans. 1992, 2781–2785. [Google Scholar] [CrossRef]
- Landman, M.; van der Westhuizen, B.; Bezuidenhout, D.I.; Liles, D.C. N-(2,4,6-Trimethylphenyl)formamide. Acta Crystallogr. 2011, E67, o120. [Google Scholar] [CrossRef] [PubMed]
- Hua, G.; Li, Y.; Slawin, A.M.Z.; Woollins, J.D. Synthesis of primary arylselenoamids by reaction of aryl nitriles with Woollins’ reagent. Org. Lett. 2006, 8, 5251–5254. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hua, G.; Slawin, A.M.Z.; Woollins, J.D. The X-ray crystal structures of primary aryl substituted selenoamides. Molecules 2009, 14, 884–892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuller, A.L.; Scott-Hayward, L.A.S.; Li, Y.; Bühl, M.; Slawin, A.M.Z.; Woollins, J.D. Automated Chemical Crystallography. J. Am. Chem. Soc. 2010, 132, 5799–5802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rigaku. CrystalClear-SM Expert (Version 3.1b27); Rigaku Americas: The Woodlands, TX, USA; Rigaku Corporation: Tokyo, Japan, 2013. [Google Scholar]
- Rigaku. CrystalClear-SM Expert (Version 2.1); Rigaku Americas: The Woodlands, TX, USA; Rigaku Corporation: Tokyo, Japan, 2014. [Google Scholar]
- Altomare, A.; Cascarano, G.; Giacovazzo, C.; Guagliardi, A. SIR92—A program for automatic solution of crystal structures by direct methods. J. Appl. Cryst. 1994, 27, 435. [Google Scholar] [CrossRef]
- Burla, M.C.; Caliandro, R.; Camalli, M.; Carrozzini, B.; Cascarano, G.L.; De Caro, L.; Giacovazzo, C.; Polidori, G.; Spagna, R. SIR2004: An improved tool for crystal structure determination and refinement. J. Appl. Crystallogr. 2005, 38, 381–388. [Google Scholar] [CrossRef]
- Burla, M.C.; Caliandro, R.; Camalli, M.; Carrozzini, B.; Cascarano, G.L.; Giacovazzo, C.; Mallamo, M.; Mazzone, A.; Polidori, G.; Spagna, R. SIR2011: A new package for crystal structure determination and refinement. J. Appl. Crystallogr. 2012, 45, 357–361. [Google Scholar] [CrossRef]
- Beurskens, P.T.; Beurskens, G.; de Gelder, R.; Garcia-Granda, S.; Gould, R.O.; Israel, R.; Smits, J.M.M. The DIRDIF-99 Program System; Crystallography Laboratory, University of Nijmegen: Nijmegen, The Netherlands, 1999. [Google Scholar]
- Spek, A.L. Platon squeeze: A tool for the calculation of the disordered solvent contribution to the calculated structure factors. Acta Crystallogr. Sect. C 2015, 71, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Rigaku. CrystalStructure (Version 4.3.0); Rigaku Americas: The Woodlands, TX, USA; Rigaku Corporation: Tokyo, Japan, 2018. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
Sample Availability: Samples of all compounds are not available from the authors. |
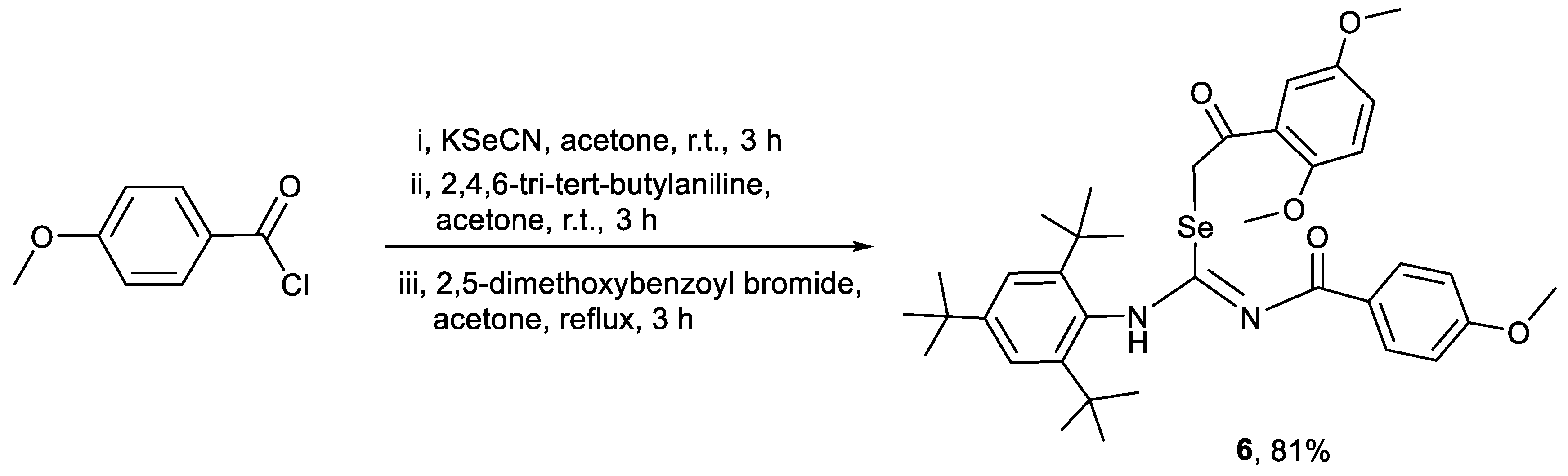
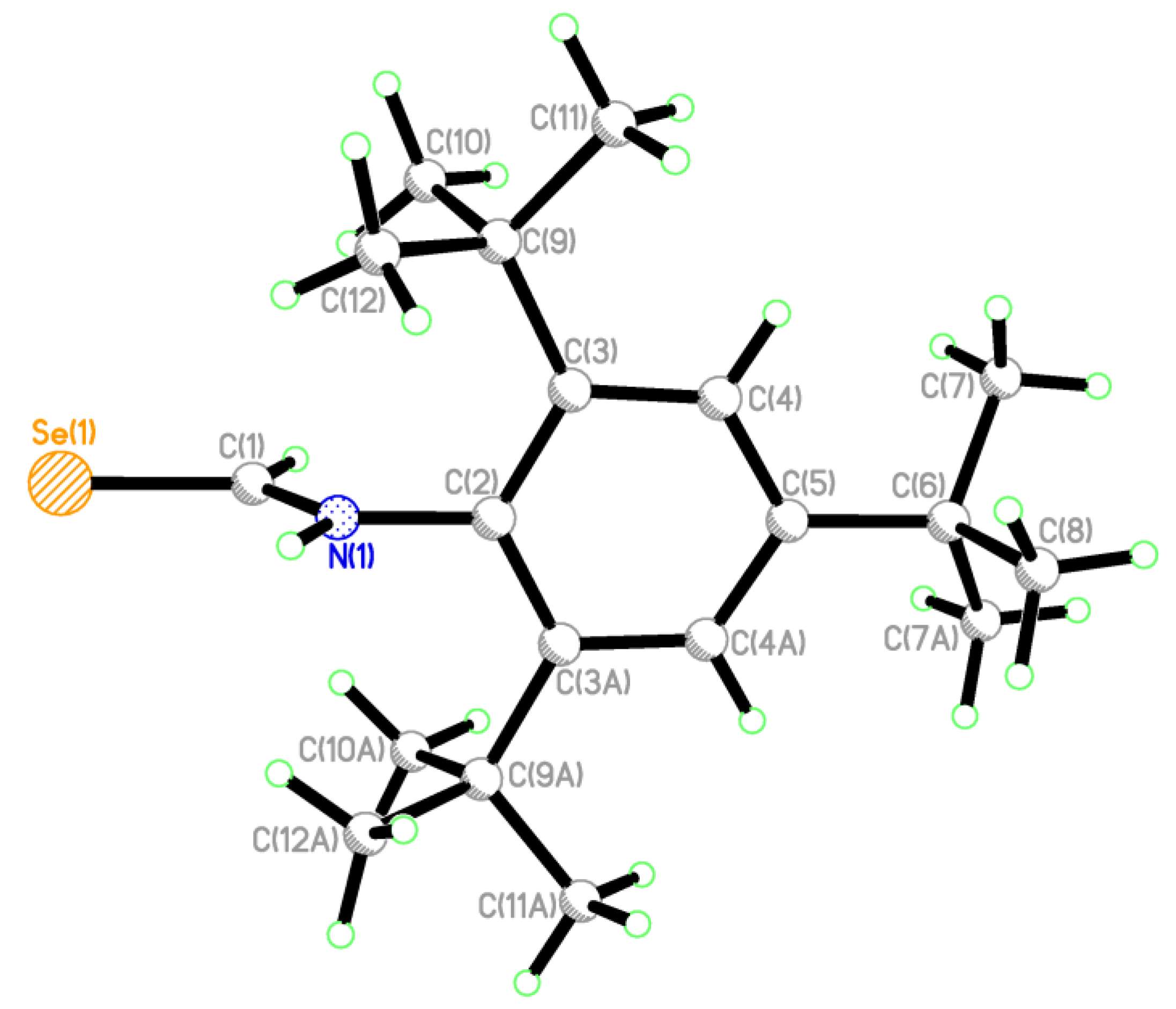
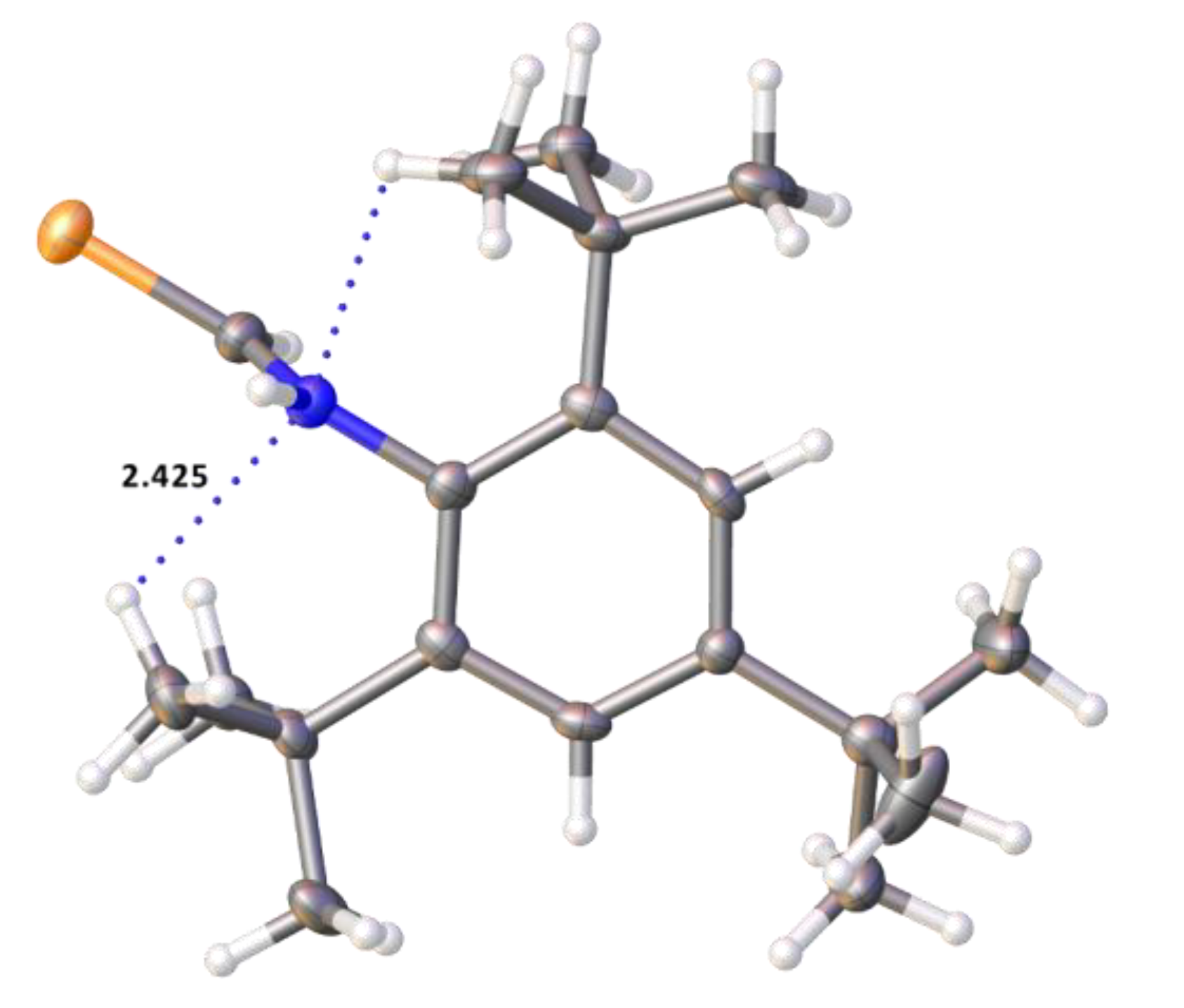
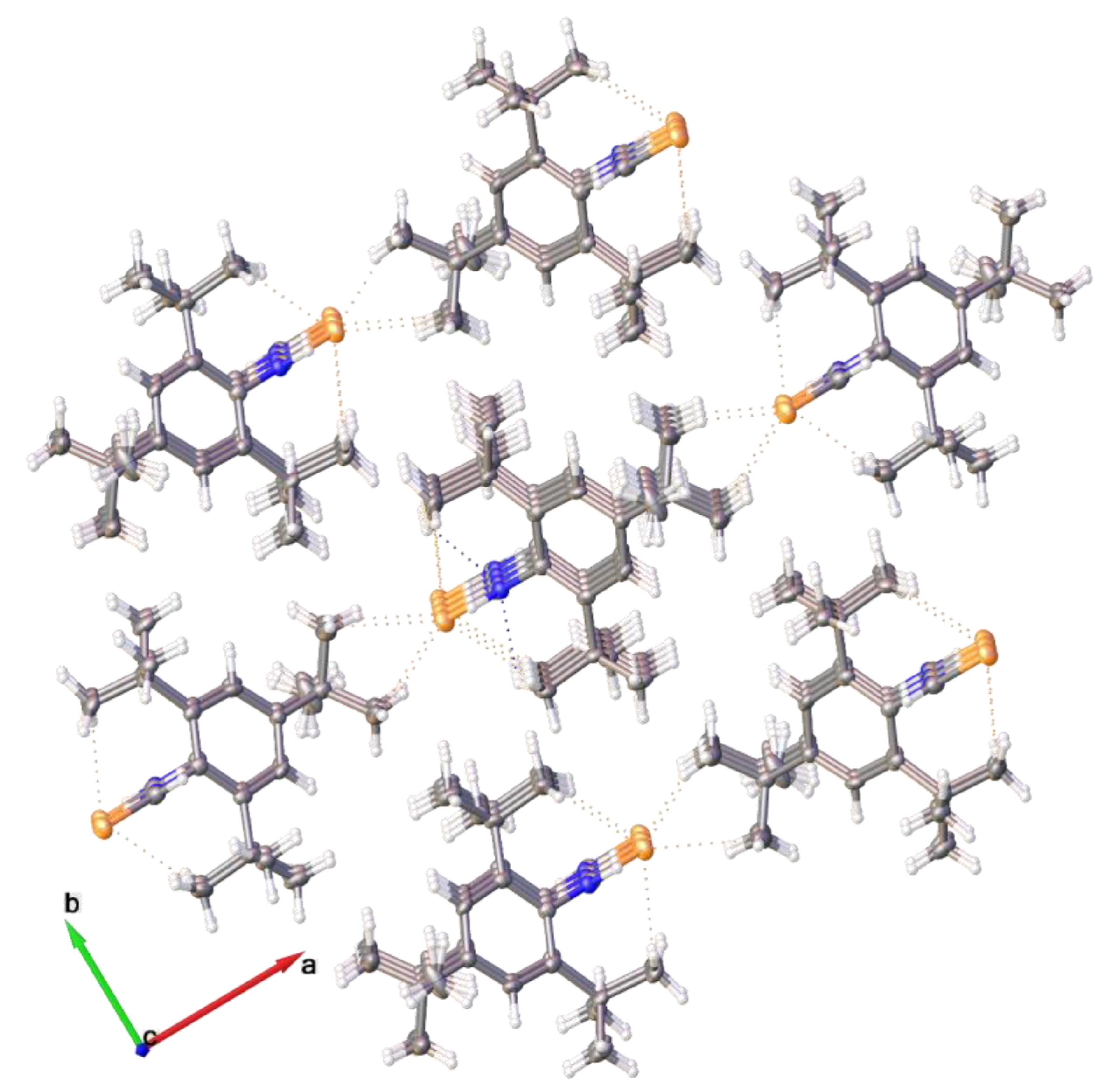
| Compound | 2·CHCl3 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|
| Formula | C27H36Cl3N3O3Se | C26H33N3O2Se2 | C36H45N3O6Se | C19H31NSe | C37H48N2O5Se |
| M | 635.92 | 577.49 | 694.73 | 352.42 | 679.76 |
| T/K | 173 | 125 | 173 | 125 | 173 |
| Crystal system | triclinic | triclinic | triclinic | orthorhombic | monoclinic |
| Space group | P | P | P | Pnma | P21/n |
| a/Å | 9.6580 (12) | 9.4547 (7) | 15.5888 (3) | 20.2224 (14) | 18.003 (3) |
| b/Å | 10.9397 (12) | 11.4917 (8) | 16.3917 (4) | 15.2835 (11) | 9.5828 (16) |
| c/Å | 15.5479 (17) | 27.2316 (19) | 24.7330 (10) | 5.8839 (4) | 21.764 (4) |
| α/° | 91.094 (4) | 93.099 (6) | 71.062 (6) | 90 | 90 |
| β/° | 91.4028 (15) | 95.470 (6) | 74.004 (7) | 90 | 106.755 (4) |
| γ/° | 113.824 (3) | 90.116 (6) | 62.683 (6) | 90 | 90 |
| U/A3 | 1501.5 (3) | 2940.9 (4) | 5250.6 (4) | 1818.5 (2) | 3595.3 (11) |
| Z | 2 | 4 | 6 | 4 | 4 |
| µ/cm−1 | 15.493 | 25.389 | 11.207 | 20.608 | 10.868 |
| Reflections collected | 27118 | 23358 | 64396 | 13393 | 42195 |
| Independent reflections | 6636 | 10652 | 19067 | 1718 | 6570 |
| Rint | 0.0389 | 0.0979 | 0.0618 | 0.1034 | 0.0613 |
| R1 [I > 2σ (I)] | 0.0294 | 0.0658 | 0.0614 | 0.0403 | 0.0429 |
| wR2 | 0.0819 | 0.1741 | 0.1713 | 0.1218 | 0.1066 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hua, G.; Cordes, D.B.; Du, J.; Slawin, A.M.Z.; Woollins, J.D. Diverse Derivatives of Selenoureas: A Synthetic and Single Crystal Structural Study. Molecules 2018, 23, 2143. https://doi.org/10.3390/molecules23092143
Hua G, Cordes DB, Du J, Slawin AMZ, Woollins JD. Diverse Derivatives of Selenoureas: A Synthetic and Single Crystal Structural Study. Molecules. 2018; 23(9):2143. https://doi.org/10.3390/molecules23092143
Chicago/Turabian StyleHua, Guoxiong, David B. Cordes, Junyi Du, Alexandra M. Z. Slawin, and J. Derek Woollins. 2018. "Diverse Derivatives of Selenoureas: A Synthetic and Single Crystal Structural Study" Molecules 23, no. 9: 2143. https://doi.org/10.3390/molecules23092143