Polyphenols from Tamarix nilotica: LC–ESI-MSn Profiling and In Vivo Antifibrotic Activity
Abstract
:1. Introduction
2. Results
2.1. Polyphenols Metabolic Profiling
2.2. Total Phenolic Content
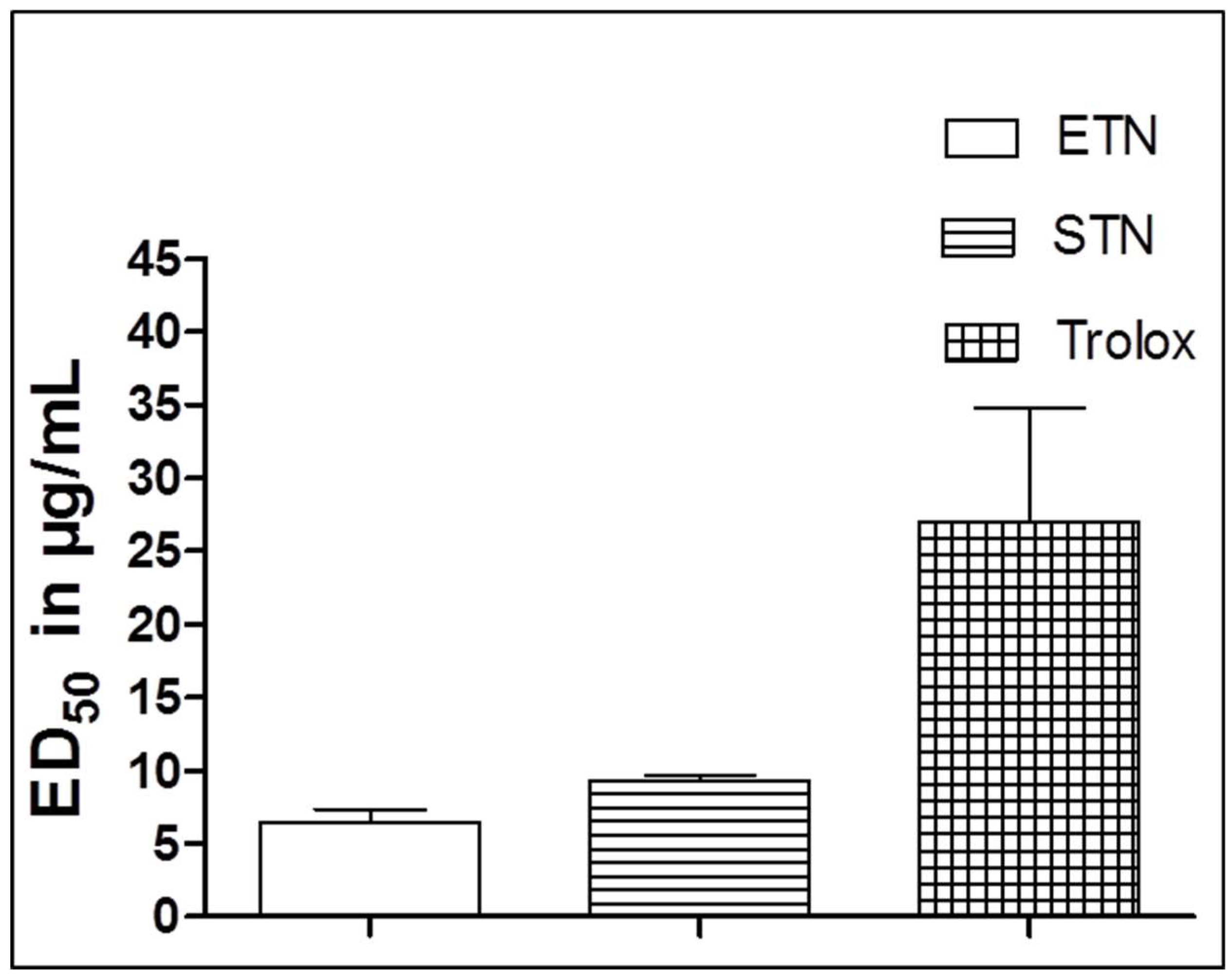
2.3. Oxygen Radical Absorbance Capacity (ORAC Assay)
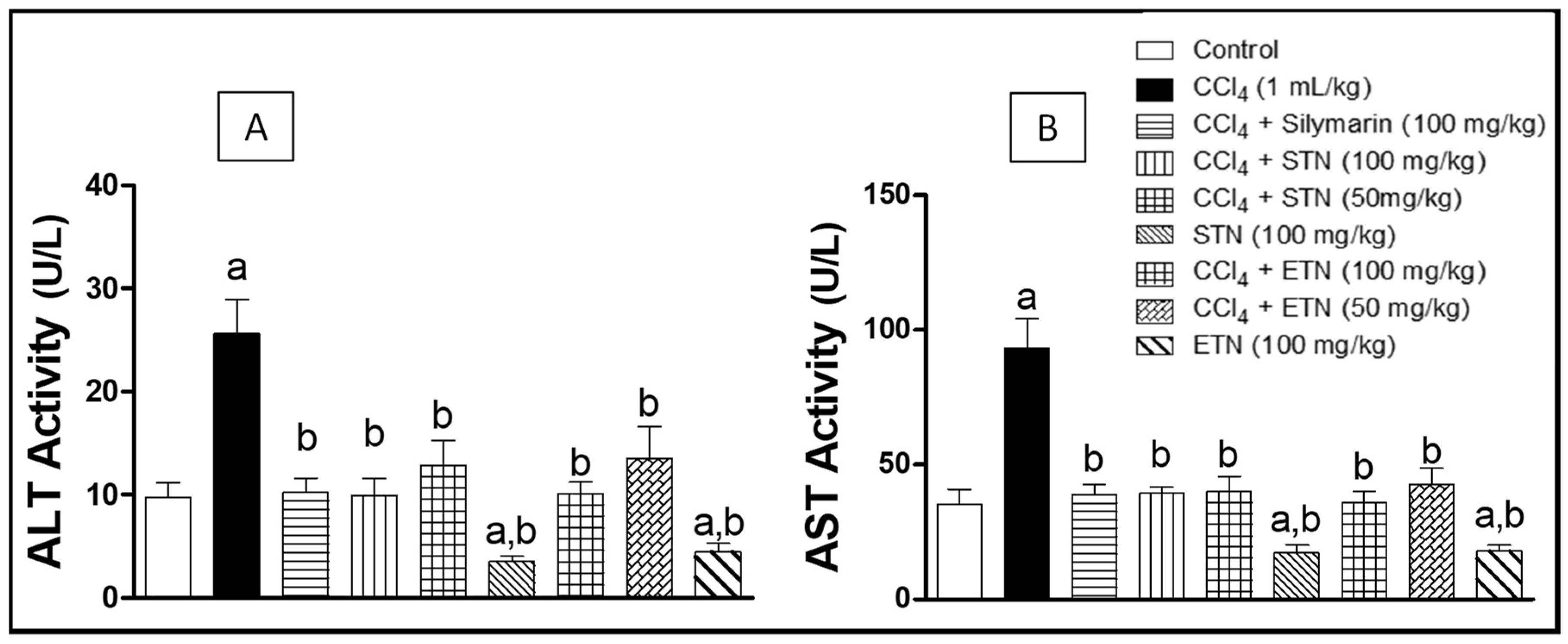
2.4. Hepatotoxicity Indices
2.5. Histopathological Examination with Hematoxylin and Eosin (H and E) Stain
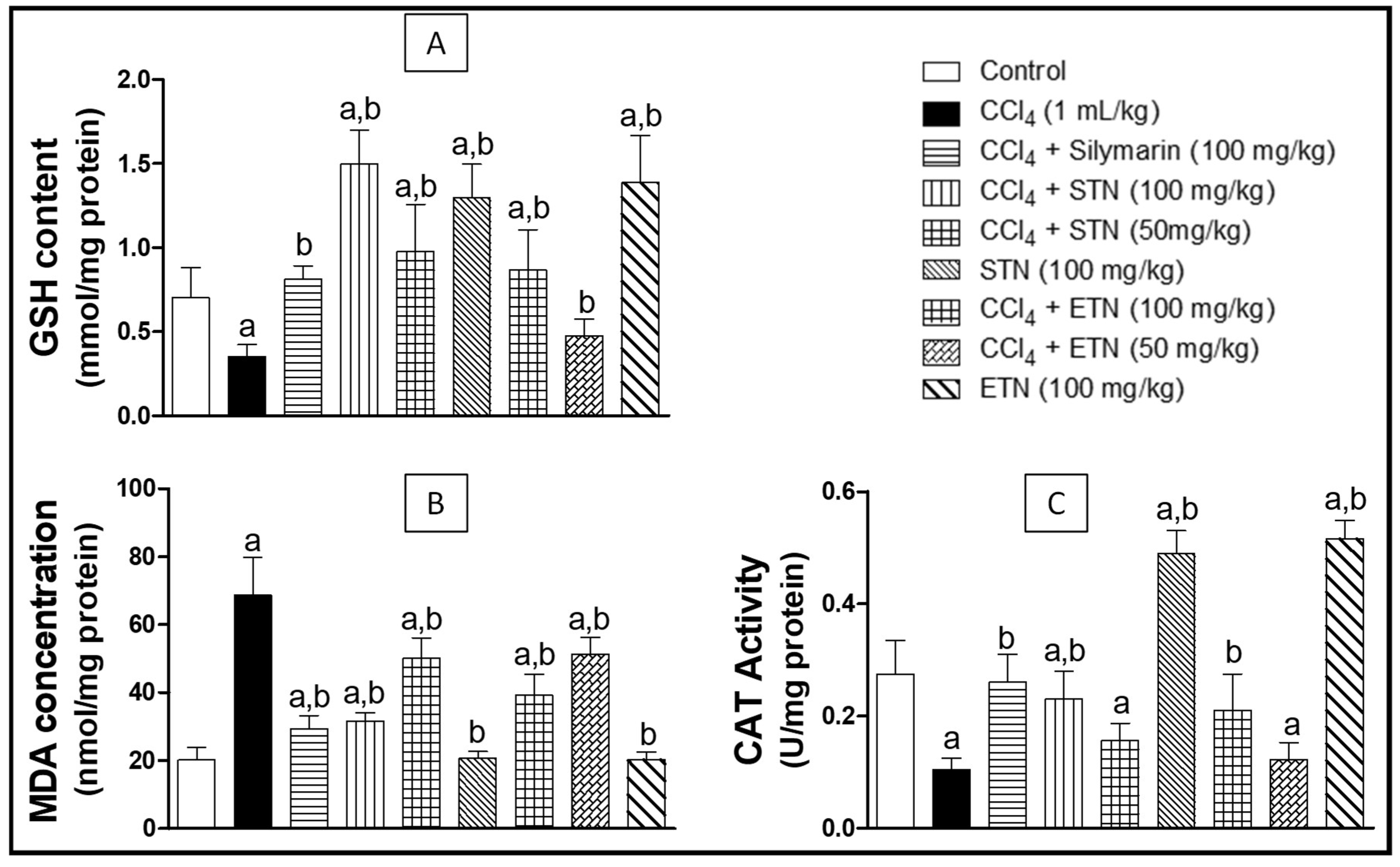
2.6. Oxidative Stress Parameters
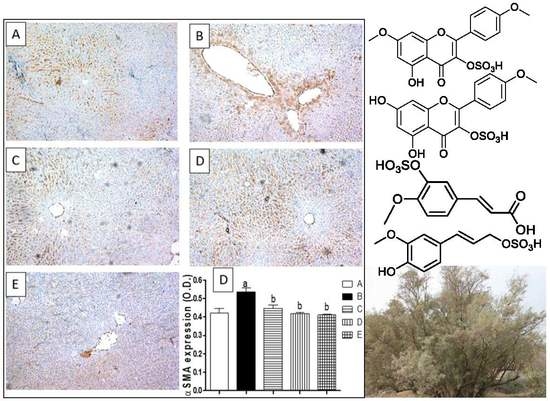
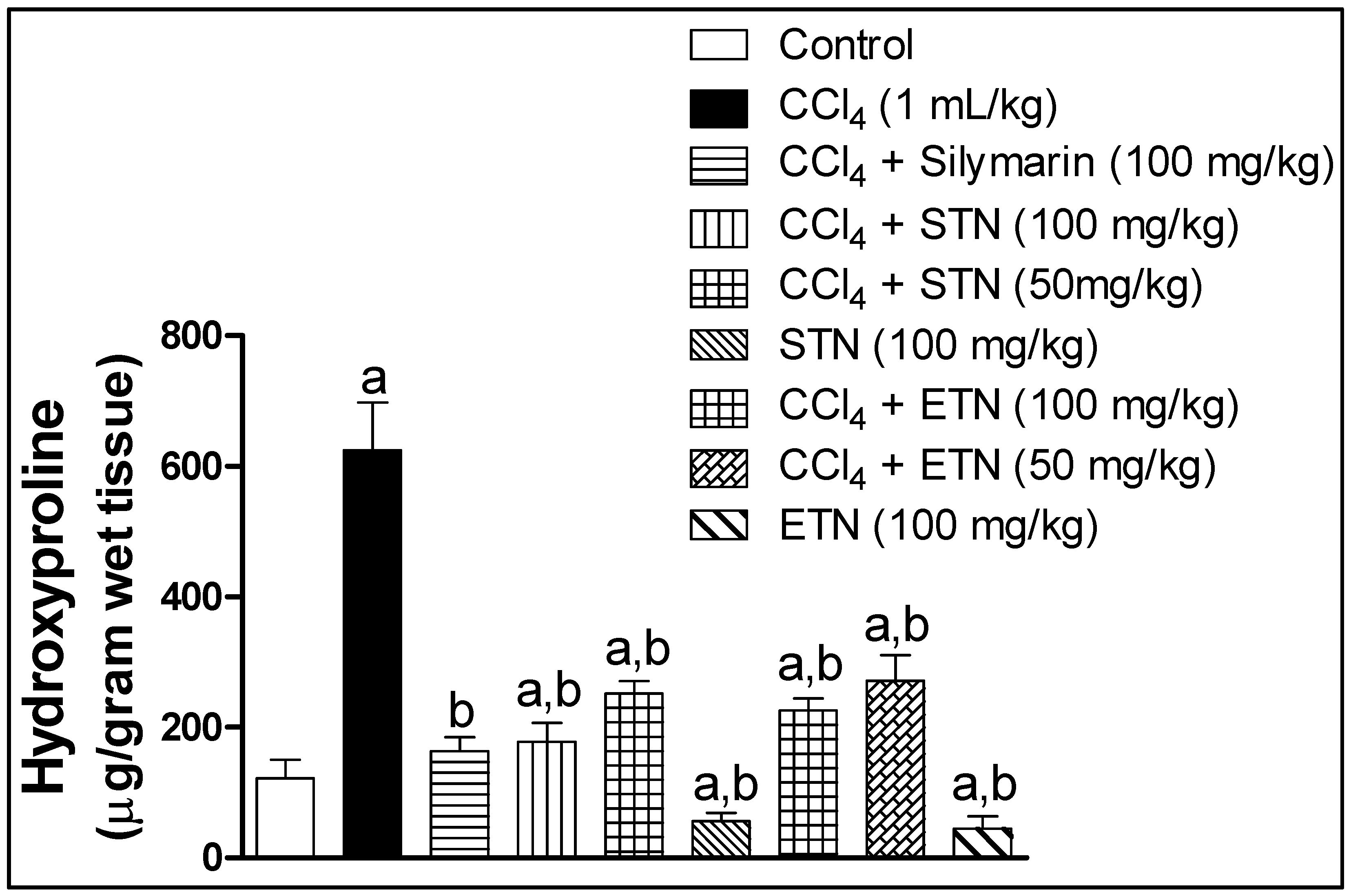
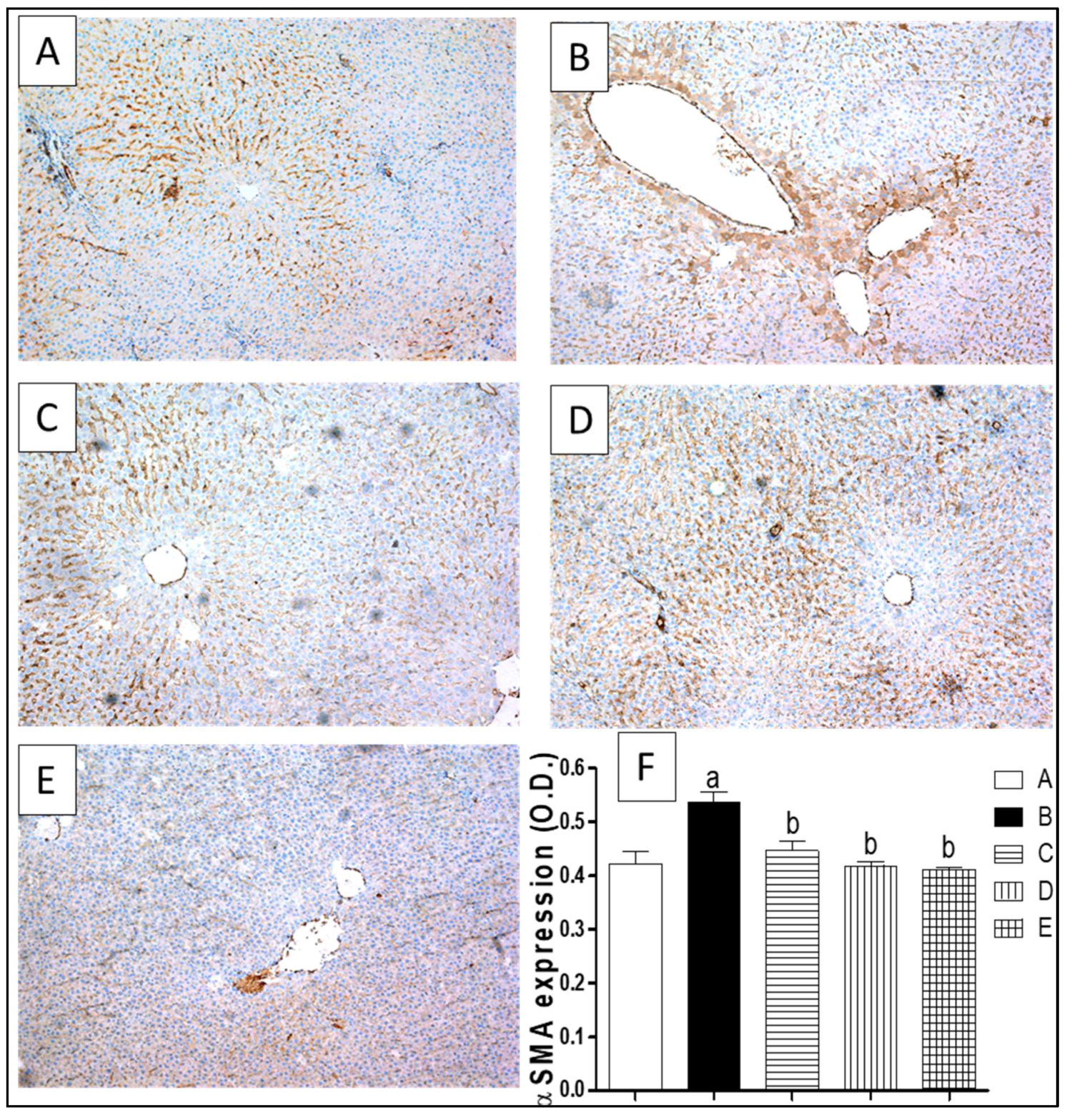
2.7. Liver Fibrosis Markers
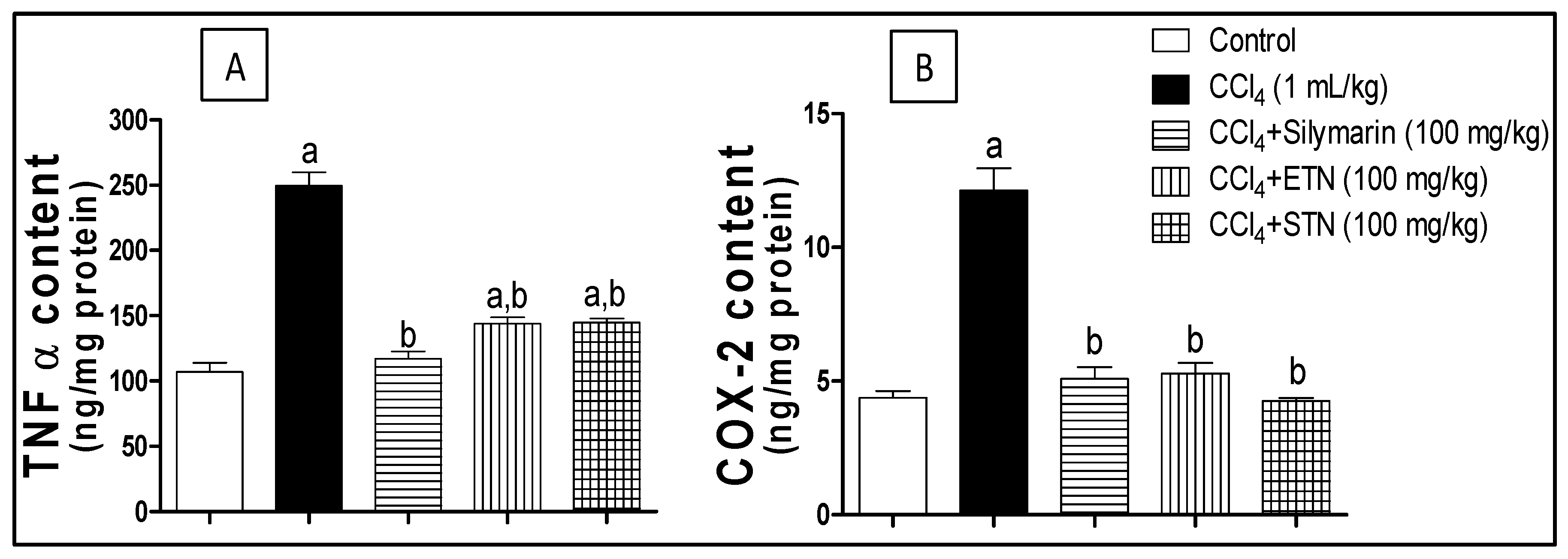
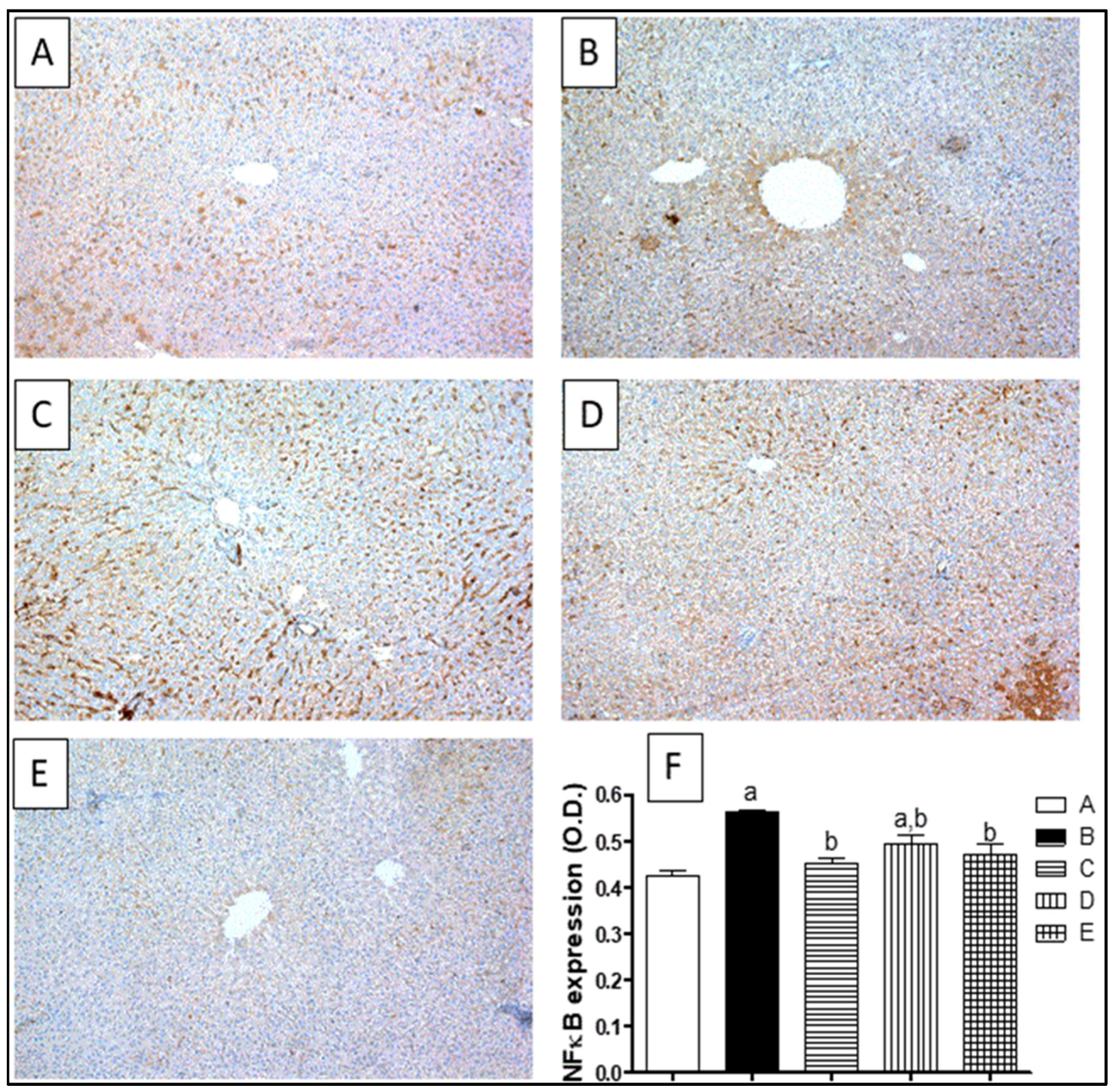
2.8. Inflammatory Markers
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Animals
4.3. Plant Extraction
4.4. Phenolic Content
4.5. LC–HRESI-MS–MS Analysis
4.6. Oxygen Radical Absorbance Capacity (ORAC Assay)
4.7. In-vivo Experimental Design
4.8. Assessment of Hepatotoxicity Indices
4.9. Assessment of Oxidative Stress Markers
4.10. Assessment of Liver Fibrosis
4.11. Assessment of Inflammatory Markers
4.12. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bataller, R.; Brenner, D.A. Liver fibrosis. J. Clin. Investig. 2005, 115, 209. [Google Scholar] [CrossRef] [PubMed]
- Trautwein, C.; Friedman, S.L.; Schuppan, D.; Pinzani, M. Hepatic fibrosis: Concept to treatment. J. Hepatol. 2015, 62, S15–S24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benyon, R.; Iredale, J. Is liver fibrosis reversible? Gut 2000, 46, 443–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Asmari, A.K.; Al-Elaiwi, A.M.; Athar, M.T.; Tariq, M.; Al Eid, A.; Al-Asmary, S.M. A review of hepatoprotective plants used in Saudi traditional medicine. Evid.-Based Complement. Altern. Med. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Migahid, A.M. Flora of Saudi Arabia; King Saud University Press: Riyadh, Saudi Arabia, 1978. [Google Scholar]
- Ghazanfar, S.A. Handbook of Arabian Medicinal Plants; CRC Press: Boca Raton, FL, USA, 1994. [Google Scholar]
- Boulos, L. Medicinal plants of north africa. Med. Plants N. Afr. 1983. [Google Scholar] [CrossRef]
- AbouZid, S.; Sleem, A. Hepatoprotective and antioxidant activities of Tamarix nilotica flowers. Pharm. Biol. 2011, 49, 392–395. [Google Scholar] [CrossRef] [PubMed]
- Abouzid, S.F.; Ali, S.A.; Choudhary, M.I. A new ferulic acid ester and other constituents from Tamarix nilotica leaves. Chem. Pharm. Bull. 2009, 57, 740–742. [Google Scholar] [CrossRef] [PubMed]
- Souleman, A.A.; Barakat, H.H.; Hussein, S.A.; El-Mousallamy, A.M.; Nawwar, M.A. Unique phenolic sulphate conjugates from the flowers of Tamarix amplexicaulis. Nat. Prod. Sci. 1998, 4, 245–252. [Google Scholar]
- Tomás-Barberán, F.A.; Iniesta-Sanmartín, E.; Ferreres, F.; Tomas-Lorente, F.; Trowitzsch-Kienastt, W.; Wray, V. Trans-coniferyl alcohol 4-O-sulphate and flavonoid sulphates from some Tamarix species. Phytochemistry 1990, 29, 3050–3051. [Google Scholar] [CrossRef]
- Song, R.; Xu, L.; Zhang, Z.; Tian, Y.; Xu, F.; Dong, H. Determination of gallic acid in rat plasma by LC-MS-MS. Chromatographia 2010, 71, 1107–1111. [Google Scholar] [CrossRef]
- Nawwar, M.; Buddrus, J.; Bauer, H. Dimeric phenolic constituents from the roots of Tamarix nilotica. Phytochemistry 1982, 21, 1755–1758. [Google Scholar] [CrossRef]
- Nawwar, M.; Souleman, A.; Buddrus, J.; Bauer, H.; Linscheid, M. Polyphenolic constituents of the flowers of Tamarix nilotica: The structure of nilocitin, a new digalloylglucose. Tetrahedron Lett. 1984, 25, 49–52. [Google Scholar] [CrossRef]
- Nawwar, M.; Souleman, A.; Buddrus, J.; Linscheid, M. Flavonoids of the flowers of Tamarix nilotica. Phytochemistry 1984, 23, 2347–2349. [Google Scholar] [CrossRef]
- Umbetova, A.K.; Esirkegenova, S.Z.; Chaudri, I.M.; Omurkamzinova, V.B.; Abilov, Z.A. Flavonoids of plants from the genus Tamarix. Chem. Nat. Compd. 2004, 40, 297–298. [Google Scholar] [CrossRef]
- Nawwar, M.; Hussein, S.; Ayoub, N.; Hofmann, K.; Linscheid, M.; Harms, M.; Wende, K.; Lindequist, U. Aphyllin, the first isoferulic acid glycoside and other phenolics from Tamarix aphylla flowers. Die Pharm. Int. J. Pharm. Sci. 2009, 64, 342–347. [Google Scholar]
- Chen, Y.; Yu, H.; Wu, H.; Pan, Y.; Wang, K.; Jin, Y.; Zhang, C. Characterization and quantification by lc-ms/ms of the chemical components of the heating products of the flavonoids extract in pollen typhae for transformation rule exploration. Molecules 2015, 20, 18352–18366. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Sun, Y.; Liang, W.; Fitzloff, J.F.; Van Breemen, R.B. Identification of caffeic acid derivatives in Actea racemosa (Cimicifuga racemosa, black cohosh) by liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2003, 17, 978–982. [Google Scholar] [CrossRef] [PubMed]
- Park, J.B. Isolation and characterization of n-feruloyltyramine as the p-selectin expression suppressor from garlic (Allium sativum). J. Agric. Food Chem. 2009, 57, 8868–8872. [Google Scholar] [CrossRef] [PubMed]
- Orfali, R.S.; Ebada, S.S.; El-Shafae, A.M.; Al-Taweel, A.M.; Lin, W.H.; Wray, V.; Proksch, P. 3-O-trans-Caffeoylisomyricadiol: A new triterpenoid from Tamarix nilotica growing in Saudi Arabia. Z. Für. Naturforschung C 2009, 64, 637–643. [Google Scholar] [CrossRef]
- Spitaler, R.; Schlorhaufer, P.D.; Ellmerer, E.P.; Merfort, I.; Bortenschlager, S.; Stuppner, H.; Zidorn, C. Altitudinal variation of secondary metabolite profiles in flowering heads of Arnica montana cv. Arbo. Phytochemistry 2006, 67, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.C.; Huxtable, R.J. Effect of taurine and guanidinoethane sulfonate on glutathione metabolism in the rat. In Taurine 3; Springer: Berlin, Germany, 1998; pp. 33–39. [Google Scholar]
- Martins, N.; Barros, L.; Ferreira, I.C. In vivo antioxidant activity of phenolic compounds: Facts and gaps. Trends Food Sci. Technol. 2016, 48, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Poli, G. Pathogenesis of liver fibrosis: Role of oxidative stress. Mol. Asp. Med. 2000, 21, 49–98. [Google Scholar] [CrossRef]
- Abdelgawad, A. Tamarix nilotica (ehrenb) bunge: A review of phytochemistry and pharmacology. J. Microb. Biochem. Technol. 2017, 9, 544–553. [Google Scholar]
- Johnston, D.E.; Kroening, C. Mechanism of early carbon tetrachloride toxicity in cultured rat hepatocytes. Basic Clin. Pharmacol. Toxicol. 1998, 83, 231–239. [Google Scholar] [CrossRef]
- Goeptar, A.R.; Scheerens, H.; Vermeulen, N.P. Oxygen and xenobiotic reductase activities of cytochrome p450. Crit. Rev. Toxicol. 1995, 25, 25–65. [Google Scholar] [CrossRef] [PubMed]
- Recknagel, R.O.; Glende, E.A.; Dolak, J.A.; Waller, R.L. Mechanisms of carbon tetrachloride toxicity. Pharmacol. Ther. 1989, 43, 139–154. [Google Scholar] [CrossRef]
- Bahcecioglu, I.H.; Koca, S.S.; Poyrazoglu, O.K.; Yalniz, M.; Ozercan, I.H.; Ustundag, B.; Sahin, K.; Dagli, A.F.; Isik, A. Hepatoprotective effect of infliximab, an anti-TNF-α agent, on carbon tetrachloride-induced hepatic fibrosis. Inflammation 2008, 31, 215. [Google Scholar] [CrossRef] [PubMed]
- Saller, R.; Meier, R.; Brignoli, R. The use of silymarin in the treatment of liver diseases. Drugs 2001, 61, 2035–2063. [Google Scholar] [CrossRef] [PubMed]
- Lykkesfeldt, J. Malondialdehyde as biomarker of oxidative damage to lipids caused by smoking. Clin. Chim. Acta 2007, 380, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Matés, J.M.; Pérez-Gómez, C.; De Castro, I.N. Antioxidant enzymes and human diseases. Clin. Biochem. 1999, 32, 595–603. [Google Scholar] [CrossRef]
- Dong, Y.; Qu, Y.; Xu, M.; Wang, X.; Lu, L. Catalase ameliorates hepatic fibrosis by inhibition of hepatic stellate cells activation. Front. Biosci. 2014, 19, 535–541. [Google Scholar] [CrossRef]
- Fang, Y.-Z.; Yang, S.; Wu, G. Free radicals, antioxidants, and nutrition. Nutrition 2002, 18, 872–879. [Google Scholar] [CrossRef]
- Chang, H.-F.; Lin, Y.-H.; Chu, C.-C.; Wu, S.-J.; Tsai, Y.-H.; Chao, J.C.-J. Protective effects of Ginkgo biloba, Panax ginseng, and Schizandra chinensis extract on liver injury in rats. Am. J. Chin. Med. 2007, 35, 995–1009. [Google Scholar] [CrossRef] [PubMed]
- Galicia-Moreno, M.; Rodríguez-Rivera, A.; Reyes-Gordillo, K.; Segovia, J.; Shibayama, M.; Tsutsumi, V.; Vergara, P.; Moreno, M.G.; Muriel, P. N-Acetylcysteine prevents carbon tetrachloride-induced liver cirrhosis: Role of liver transforming growth factor-beta and oxidative stress. Eur. J. Gastroenterol. Hepatol. 2009, 21, 908–914. [Google Scholar] [CrossRef] [PubMed]
- Elsharkawy, A.M.; Mann, D.A. Nuclear factor-κb and the hepatic inflammation-fibrosis-cancer axis. Hepatology 2007, 46, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Racanelli, V.; Rehermann, B. The liver as an immunological organ. Hepatology 2006, 43, S54–S62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luedde, T.; Schwabe, R.F. Nf-κb in the liver—Linking injury, fibrosis and hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Dang, S.-S.; Wang, B.-F.; Cheng, Y.-A.; Song, P.; Liu, Z.-G.; Li, Z.-F. Inhibitory effects of saikosaponin-D on CCl4-induced hepatic fibrogenesis in rats. World J. Gastroenterol. WJG 2007, 13, 557. [Google Scholar] [CrossRef] [PubMed]
- Tipoe, G.L.; Leung, T.M.; Liong, E.C.; Lau, T.Y.H.; Fung, M.L.; Nanji, A.A. Epigallocatechin-3-gallate (EGCG) reduces liver inflammation, oxidative stress and fibrosis in carbon tetrachloride (CCl4)-induced liver injury in mice. Toxicology 2010, 273, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Simeonova, P.P.; Gallucci, R.M.; Hulderman, T.; Wilson, R.; Kommineni, C.; Rao, M.; Luster, M.I. The role of tumor necrosis factor-α in liver toxicity, inflammation, and fibrosis induced by carbon tetrachloride. Toxicol. Appl. Pharmacol. 2001, 177, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Oakley, F.; Mann, J.; Nailard, S.; Smart, D.E.; Mungalsingh, N.; Constandinou, C.; Ali, S.; Wilson, S.J.; Millward-Sadler, H.; Iredale, J.P. Nuclear factor-κb1 (p50) limits the inflammatory and fibrogenic responses to chronic injury. Am. J. Pathol. 2005, 166, 695–708. [Google Scholar] [CrossRef]
- Mohammed, N.; El-Aleem, S.; El-Hafiz, H.; McMahon, R. Distribution of constitutive (COX-1) and inducible (COX-2) cyclooxygenase in postviral human liver cirrhosis: A possible role for COX-2 in the pathogenesis of liver cirrhosis. J. Clin. Pathol. 2004, 57, 350–354. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Imanishi, H.; Iijima, H.; Shimomura, S.; Yamamoto, T.; Amuro, Y.; Kubota, A.; Hada, T. Expression of cyclooxygenase 2 and cytosolic phospholipase a 2 in the liver tissue of patients with chronic hepatitis and liver cirrhosis. Hepatol. Res. 2002, 23, 185–195. [Google Scholar] [CrossRef]
- Ala-Kokko, L.; Stenbäck, F.; Ryhänen, L. Preventive effect of malotilate on carbon tetrachloride-induced liver damage and collagen accumulation in the rat. Biochem. J. 1987, 246, 503–509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedman, S.L. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J. Biol. Chem. 2000, 275, 2247–2250. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Kang, L.; Zhang, J.; Shi, N.; Zhang, M.; Wu, T. Antifibrotic effects of genistein and quercetin in vitro. Yao Xue Xue Bao Acta Pharm. Sin. 2001, 36, 648–651. [Google Scholar]
- Lee, E.S.; Lee, H.E.; Shin, J.Y.; Yoon, S.; Moon, J.O. The flavonoid quercetin inhibits dimethylnitrosamine-induced liver damage in rats. J. Pharm. Pharmacol. 2003, 55, 1169–1174. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhang, S.; Cheng, D.; Li, H.; Tang, Z.; Xue, J.; Cai, W.; Dong, J.; Zhao, L. Preliminary exploration on anti-fibrosis effect of kaempferol in mice with Schistosoma japonicum infection. Eur. J. Inflamm. 2013, 11, 161–168. [Google Scholar] [CrossRef]
- El-Lakkany, N.M.; El-Maadawy, W.H.; el-Din, S.H.S.; Saleh, S.; Safar, M.M.; Ezzat, S.M.; Mohamed, S.H.; Botros, S.S.; Demerdash, Z.; Hammam, O.A. Antifibrotic effects of gallic acid on hepatic stellate cells: In vitro and in vivo mechanistic study. J. Tradit. Complement. Med. 2018. [Google Scholar] [CrossRef]
- Cheng, Y.; Mai, J.-Y.; Wang, M.-F.; Chen, G.-F.; Ping, J. Antifibrotic effect of total flavonoids of Astmgali radix on dimethylnitrosamine-induced liver cirrhosis in rats. Chin. J. Integr. Med. 2017, 23, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzym. 1999, 299, 152–178. [Google Scholar]
- Sobeh, M.; ElHawary, E.; Peixoto, H.; Labib, R.M.; Handoussa, H.; Swilam, N.; El-Khatib, A.H.; Sharapov, F.; Mohamed, T.; Krstin, S. Identification of phenolic secondary metabolites from Schotia brachypetala sond. (Fabaceae) and demonstration of their antioxidant activities in Caenorhabditis elegans. PeerJ 2016, 4, e2404. [Google Scholar] [CrossRef] [PubMed]
- Lucas-Abellán, C.; Mercader-Ros, M.; Zafrilla, M.; Fortea, M.; Gabaldón, J.; Núñez-Delicado, E. ORAC-fluorescein assay to determine the oxygen radical absorbance capacity of resveratrol complexed in cyclodextrins. J. Agric. Food Chem. 2008, 56, 2254–2259. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Norton, P.A. Animal models of liver fibrosis. Scand. J. Gastroenterol. 1996, 31, 1137–1143. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Uchiyama, M.; Mihara, M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal. Biochem. 1978, 86, 271–278. [Google Scholar] [CrossRef]
- Woessner, J.F. The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch. Biochem. Biophys. 1961, 93, 440–447. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [PubMed]

| # | Rt (Min) | [M − H]− | Error (ppm) | MS/MS Fragment | Formula | Identity | References |
|---|---|---|---|---|---|---|---|
| 1 | 3.9 | 169.0220 | −2.9 | 125.1086 | C7H6O5 | Gallic acid | [12,13] |
| 2 | 4.12 | 301.0421 | 1.6 | 273.0435, 179.0096, 150.9980 | C15H10O7 | Quercetin | [15,18] |
| 3 | 6.59 | 483.0859 | −1.2 | 331.1573, 313.1711, 169.1114 | C20H20O14 | di-Galloyl-glucose (nilocitin) | [14] |
| 4 | 14.72 | 285.0469 | 2.8 | 239.0530, 143.1847 | C15H10O6 | Kaempferol | [15,18] |
| 5 | 28.09 | 197.0531 | −1.5 | 183.2035, 182.1017, 168.1108, 167.1539 | C9H10O5 | Methyl gallate methyl ether | [14] |
| 6 | 30.61 | 259.0356 | −0.8 | 229.0872, 179.0628, | C10H12O6S | Coniferyl alcohol sulphate | [11] |
| 7 | 36.82 | 193.0574 | 2.6 | 178.1750, 149.1777, 134.0983 | C10H10O4 | Ferulic acid isomer | [17,19] |
| 8 | 42.4 | 477.1101 | 2.1 | 315.0990, 300.1015 | C22H22O12 | Methylquercetin hexoside (tamarixetin-3-O-hexoside) | [16] |
| 9 | 43.93 | 273.0145 | 0.7 | 229.0810, 193.1538, 178.0940 | C10H10O7S | Ferulic acid sulphate derivative | [10] |
| 10 | 53.62 | 314.1315 | −0.32 | 177.0162, 164.1282, 145.1110 | C18H19NO4 | n-Feruloyltyramine * | [20,21] |
| 11 | 57.08 | 287.0299 | 1.4 | 272.1937, 207.1997, 192.1747 | C11H12O7S | Methyl ferulate sulphate | [9] |
| 12 | 58.3 | 461.0806 | −1.7 | 285.1083, 257.3536 | C21H18O12 | Kaempferol glucuronide | [15] |
| 13 | 67.53 | 315.0587 | −1.3 | 300.1302, 193.1954 | C16H12O7 | Methylquercetin (tamarixetin) | [9,16,17] |
| 14 | 71.19 | 299.0624 | 3 | 284.1601, 271.2777 | C16H12O6 | Methylkaempferol (kaempferide) | [9] |
| 15 | 74.7 | 395.0154 | −0.8 | 315.1095, 300.2426, 217.0871 | C16H12O10S | Methylquercetin-sulphate (tamarixetin sulphate) | [11,17] |
| 16 | 75.12 | 379.0195 | 1.8 | 299.1220, 284.2777 | C16H12O9S | Kaempferol methyl ether sulphate | [11] |
| 17 | 75.89 | 393.0366 | −2 | 313.1219, 298.0152, 283.8303 | C17H14O9S | Kaempferol dimethyl ether sulphate | [15] |
| # | Rt (Min) | [M − H]− | Error (ppm) | MS/MS Fragment | Formula | Identity | References |
|---|---|---|---|---|---|---|---|
| 1 | 3.97 | 169.0213 | 1.18 | 125.1079 | C7H6O5 | Gallic acid | [12,13] |
| 2 | 4.17 | 301.0425 | 0.33 | 273.0441, 179.0084, 150.9916 | C15H10O7 | Quercetin | [15,18] |
| 3 | 6.47 | 483.0851 | 0.41 | 331.1561, 313.1709, 169.1116 | C20H20O14 | di-Galloylglucose (nilocitin) | [14] |
| 4 | 14.79 | 285.0483 | −2.10 | 239.0536, 143.1841 | C15H10O6 | Kaempferol | [15,18] |
| 5 | 28.13 | 197.0533 | −2.52 | 183.2027, 182.1022, 168.1119, 167.1531 | C9H10O5 | Methyl gallate methyl ether | [14] |
| 6 | 30.45 | 259.0351 | 1.15 | 229.0876, 179.0632 | C10H12O6S | Coniferyl alcohol sulphate | [11] |
| 7 | 43.98 | 273.0151 | −1.46 | 229.0806, 193.1527, 178.0947 | C10H10O7S | Ferulic acid sulphate derivative | [10] |
| 8 | 53.72 | 314.1319 | −1.60 | 177.0161, 164.12874, 145.1103 | C18H19NO4 | n-Feruloyltyramine * | [20,21] |
| 9 | 56.93 | 287.0301 | 0.69 | 272.1925, 207.2002, 192.1733 | C11H12O7S | Methyl ferulate sulphate | [9] |
| 10 | 58.20 | 461.0795 | 0.65 | 285.1096, 257.3548 | C21H18O12 | Kaempferol glucruonide | [15] |
| 11 | 67.77 | 315.0589 | −1.90 | 300.1311, 193.1967 | C16H12O7 | Methyl-quercetin (Tamarixetin) | [9,16,17] |
| 12 | 71.17 | 299.0629 | 1.33 | 284.1607, 271.2769 | C16H12O6 | Methyl-kaempferol (Kaempferide) | [9] |
| 13 | 74.65 | 395.0159 | −2.02 | 315.1085, 300.2417, 217.0865 | C16H12O10S | Methyl-quercetin-sulphate (Tamarixetin-sulphate) | [11,17] |
| 14 | 75.14 | 379.0191 | 2.89 | 299.1221, 284.2782 | C16H12O9S | Kaempferol-methyl ether-sulphate | [11] |
| 15 | 75.60 | 393.0368 | −2.54 | 313.1212, 298.0147, 283.8312 | C17H14O9S | Kaempferol-dimethyl ether-sulphate | [15] |
| Total Phenolic Content (mg GA/gm Dry Extract) | |
|---|---|
| ETN | 95.1 |
| STN | 111.8 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sekkien, A.; Swilam, N.; Ebada, S.S.; Esmat, A.; El-Khatib, A.H.; Linscheid, M.W.; Singab, A.N. Polyphenols from Tamarix nilotica: LC–ESI-MSn Profiling and In Vivo Antifibrotic Activity. Molecules 2018, 23, 1411. https://doi.org/10.3390/molecules23061411
Sekkien A, Swilam N, Ebada SS, Esmat A, El-Khatib AH, Linscheid MW, Singab AN. Polyphenols from Tamarix nilotica: LC–ESI-MSn Profiling and In Vivo Antifibrotic Activity. Molecules. 2018; 23(6):1411. https://doi.org/10.3390/molecules23061411
Chicago/Turabian StyleSekkien, Ahmed, Noha Swilam, Sherif S. Ebada, Ahmed Esmat, Ahmed H. El-Khatib, Michael W. Linscheid, and Abdel Nasser Singab. 2018. "Polyphenols from Tamarix nilotica: LC–ESI-MSn Profiling and In Vivo Antifibrotic Activity" Molecules 23, no. 6: 1411. https://doi.org/10.3390/molecules23061411