Hydrogen-Bonded Organic–Inorganic Hybrid Based on Hexachloroplatinate and Nitrogen Heterocyclic Cations: Their Synthesis, Characterization, Crystal Structures, and Antitumor Activities In Vitro
Abstract
:1. Introduction
2. Results and Discussion
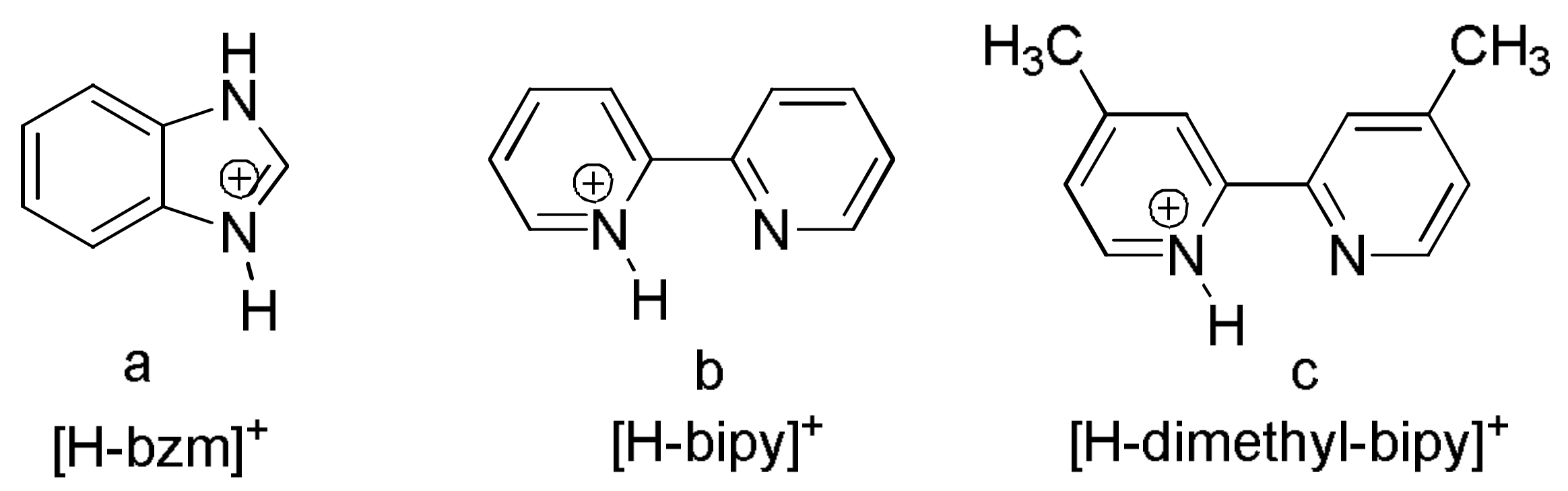
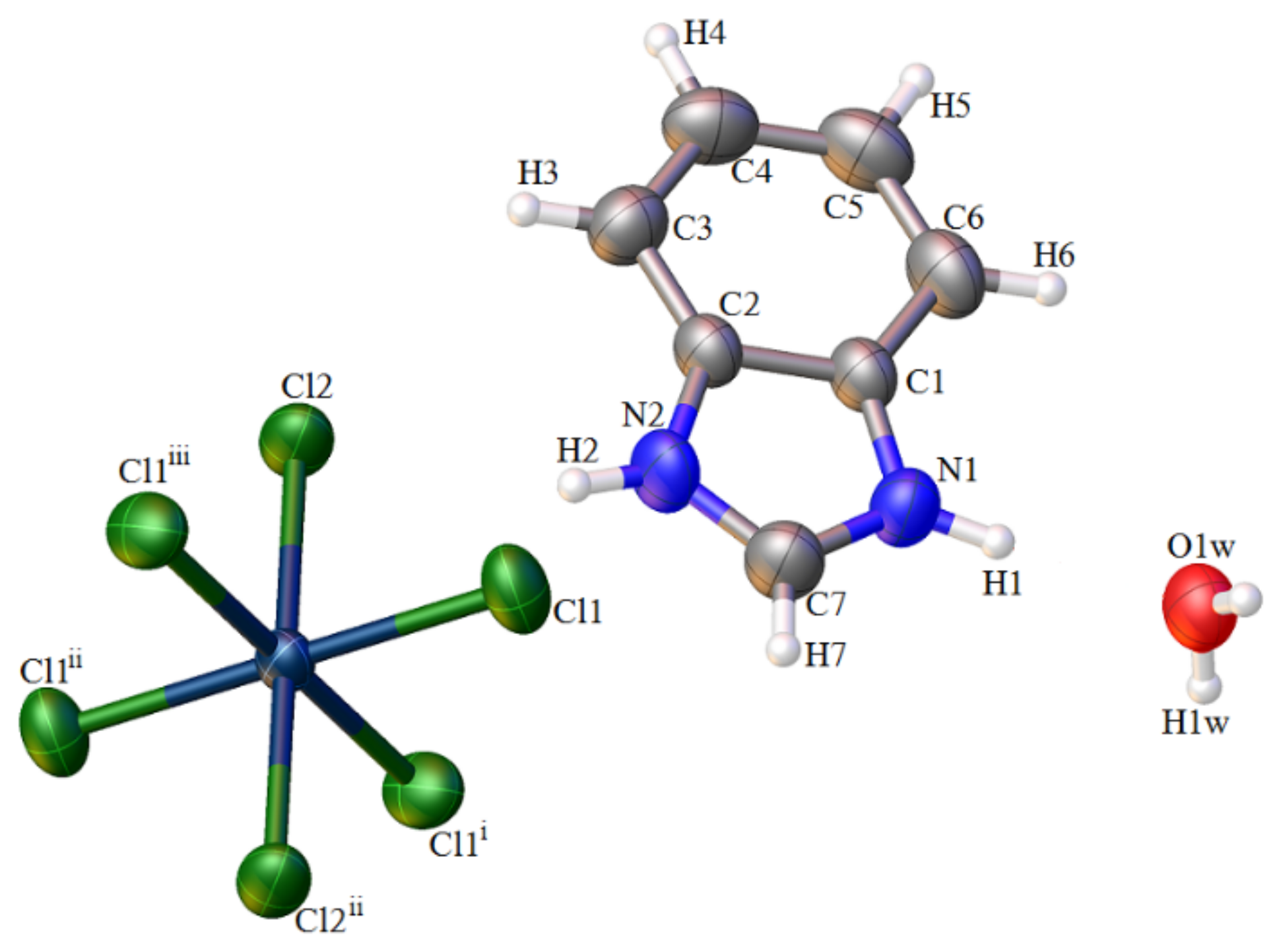
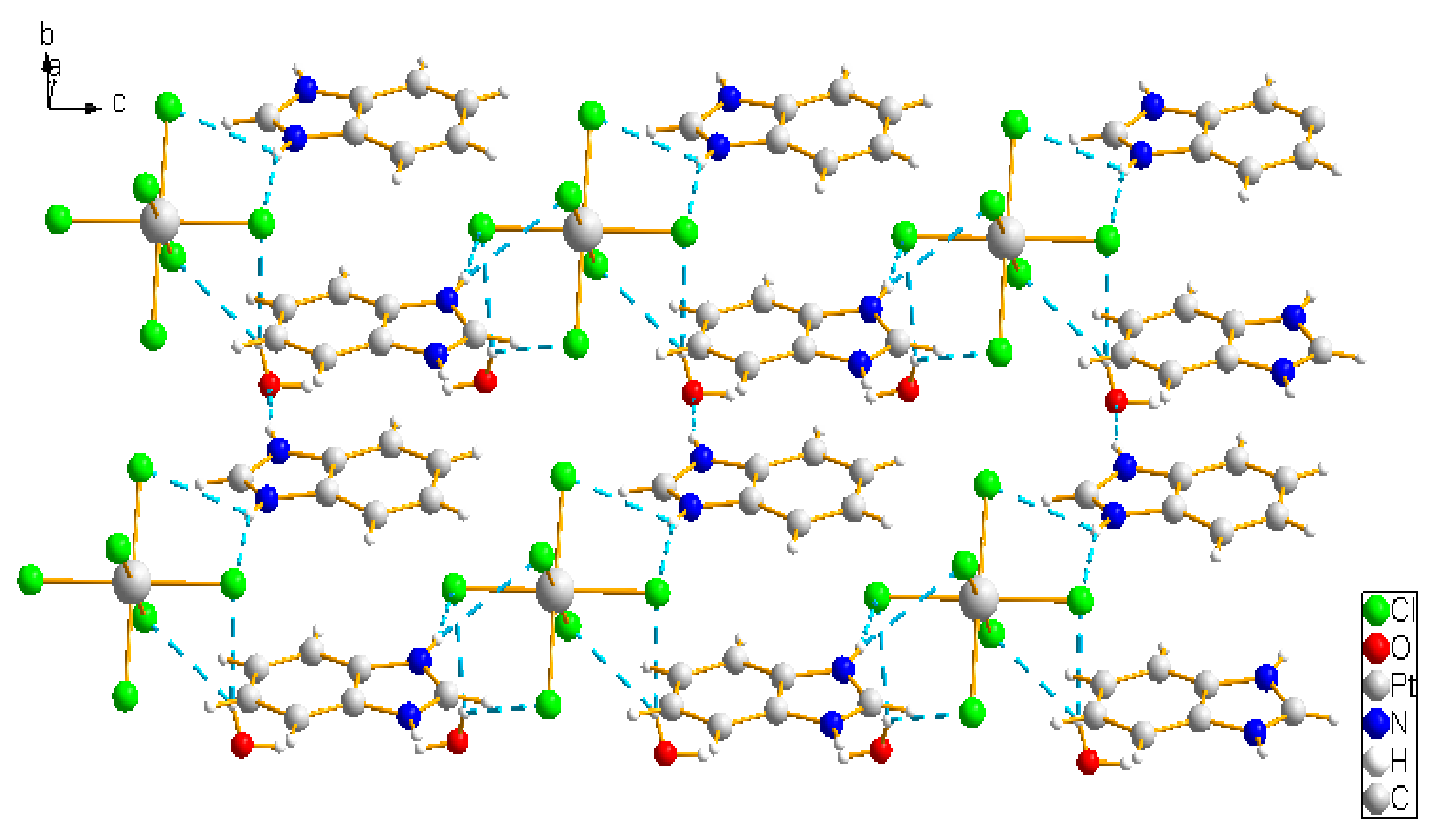
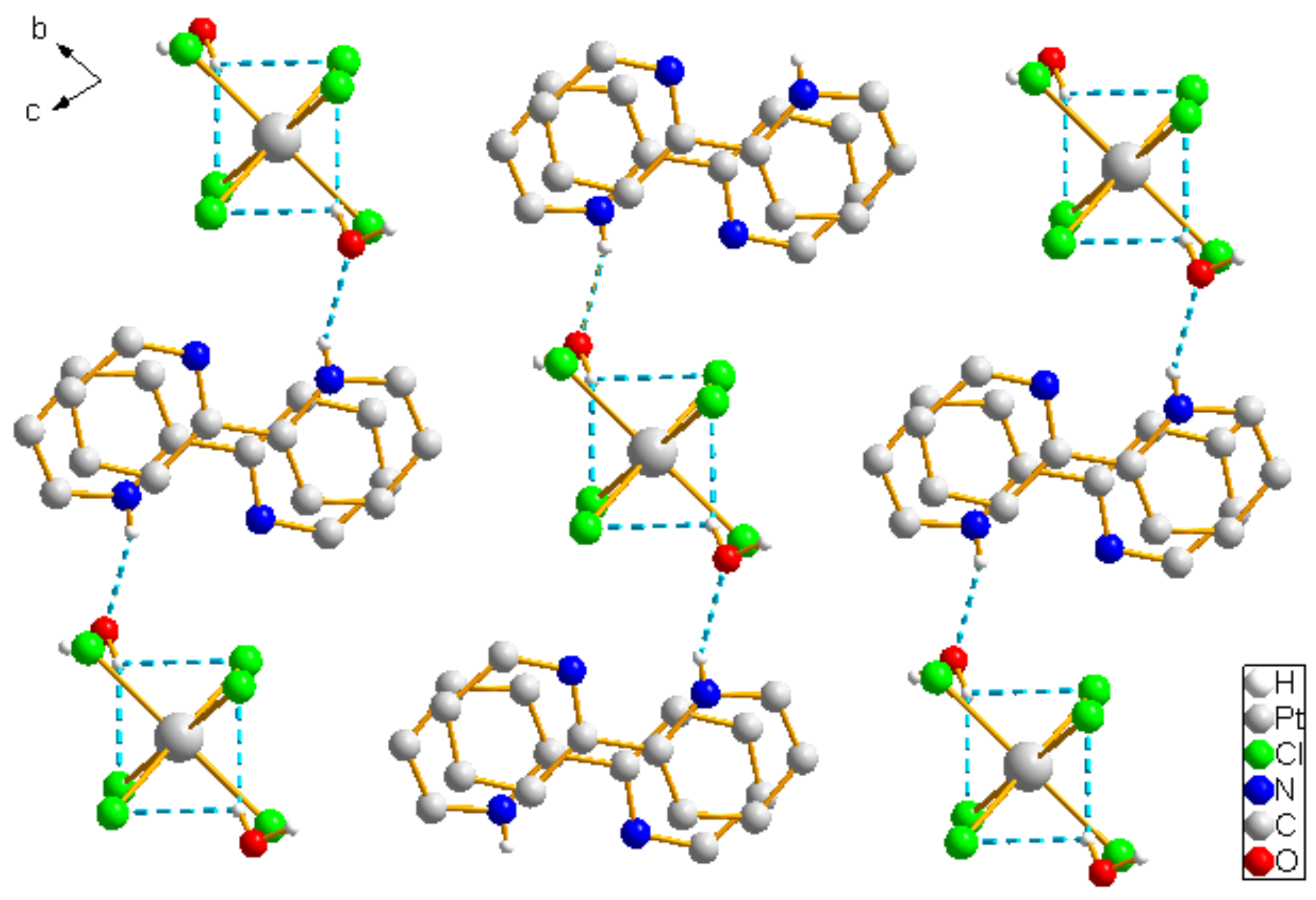
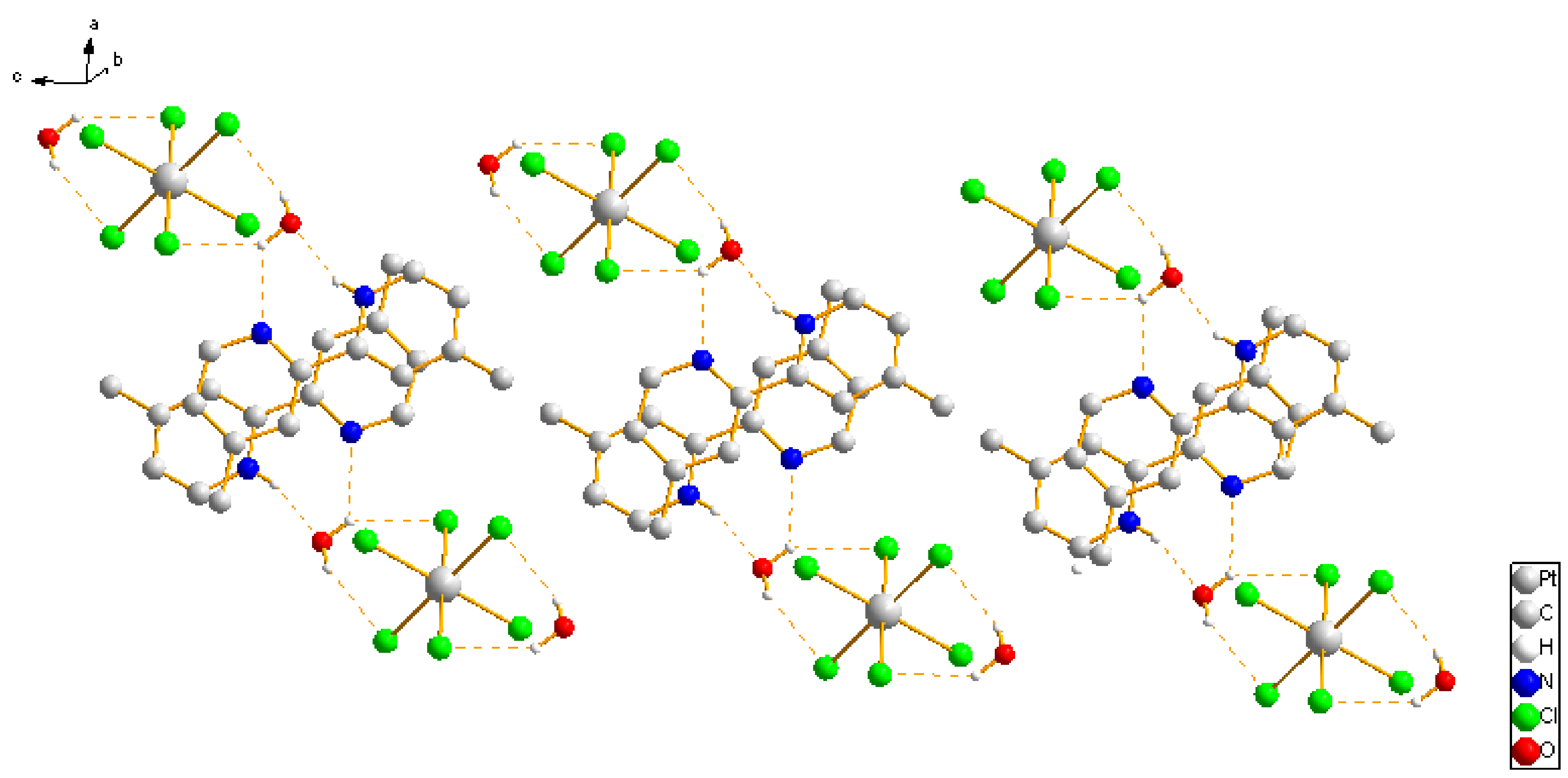
2.1. Analysis of the Structures of 1–3
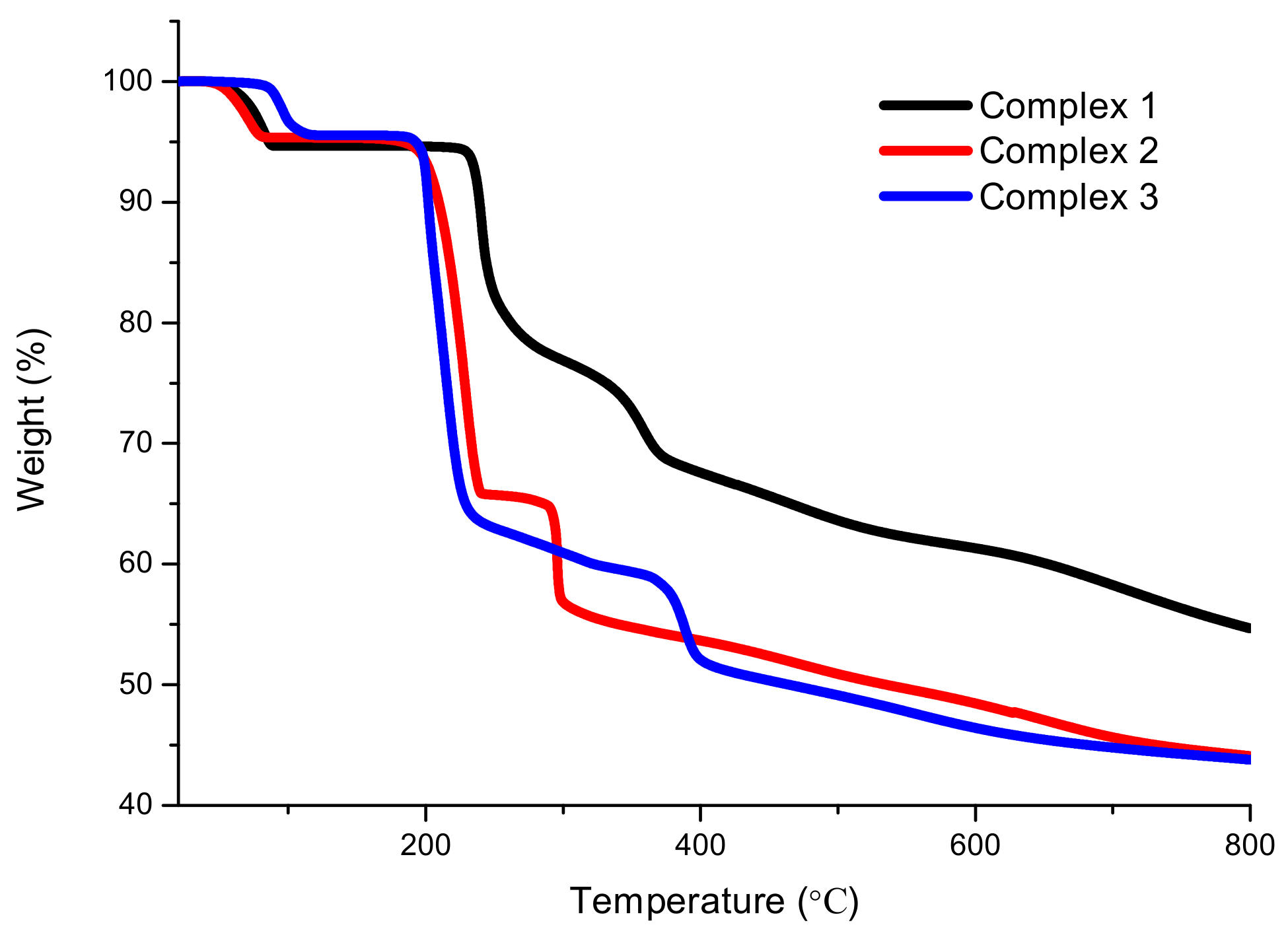
2.2. XRD and TG Analysis
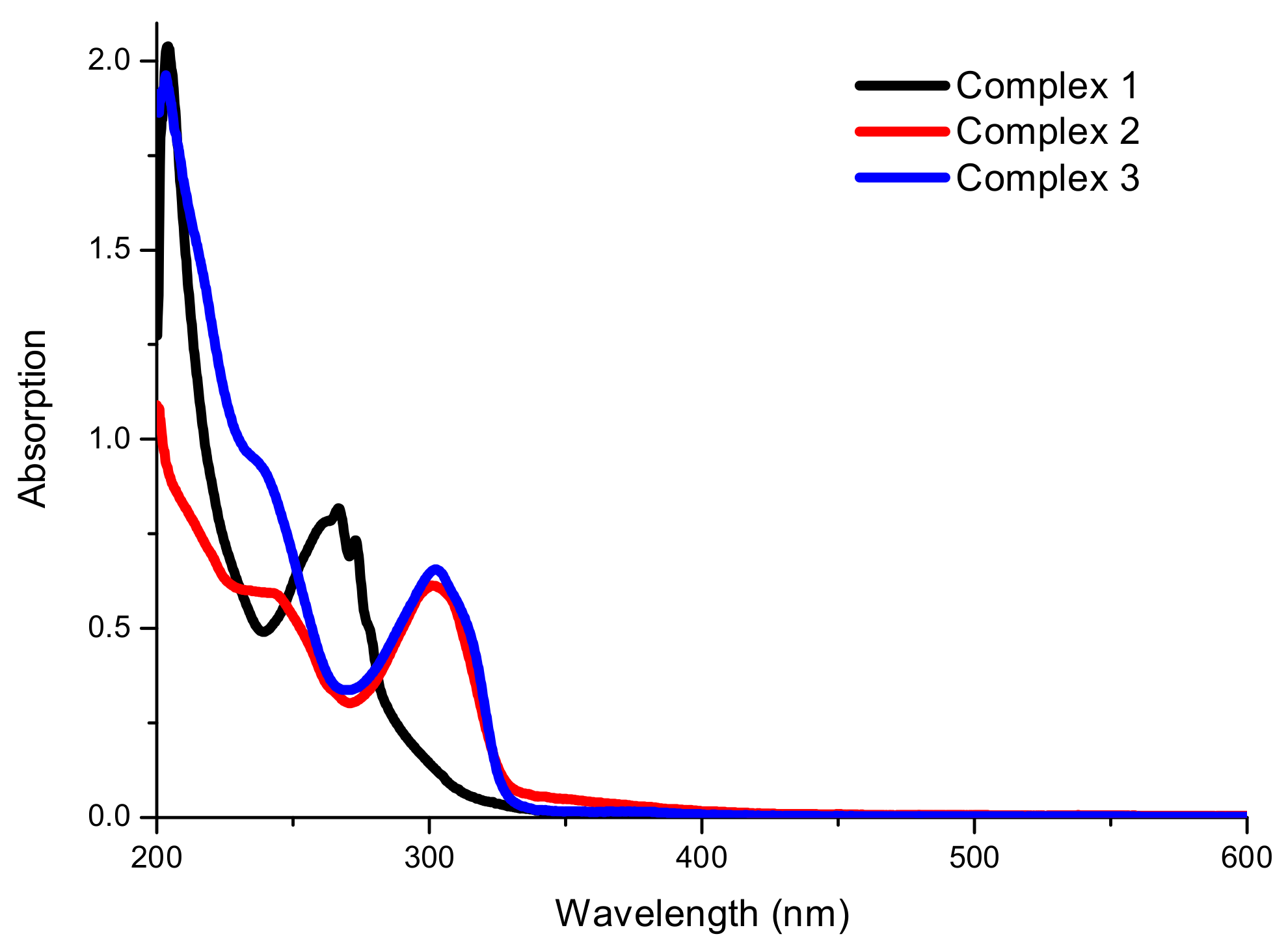
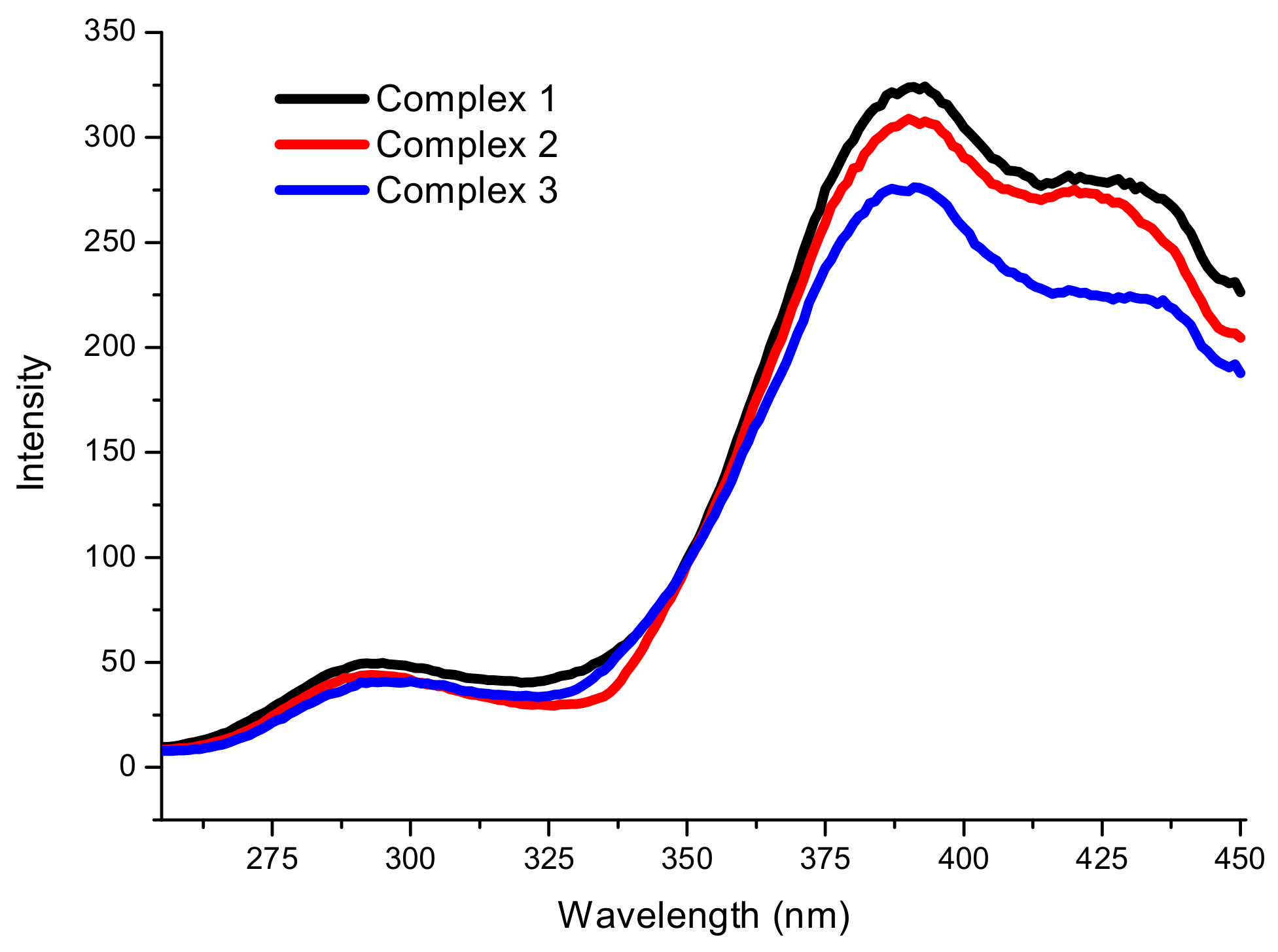
2.3. Spectroscopic Analysis
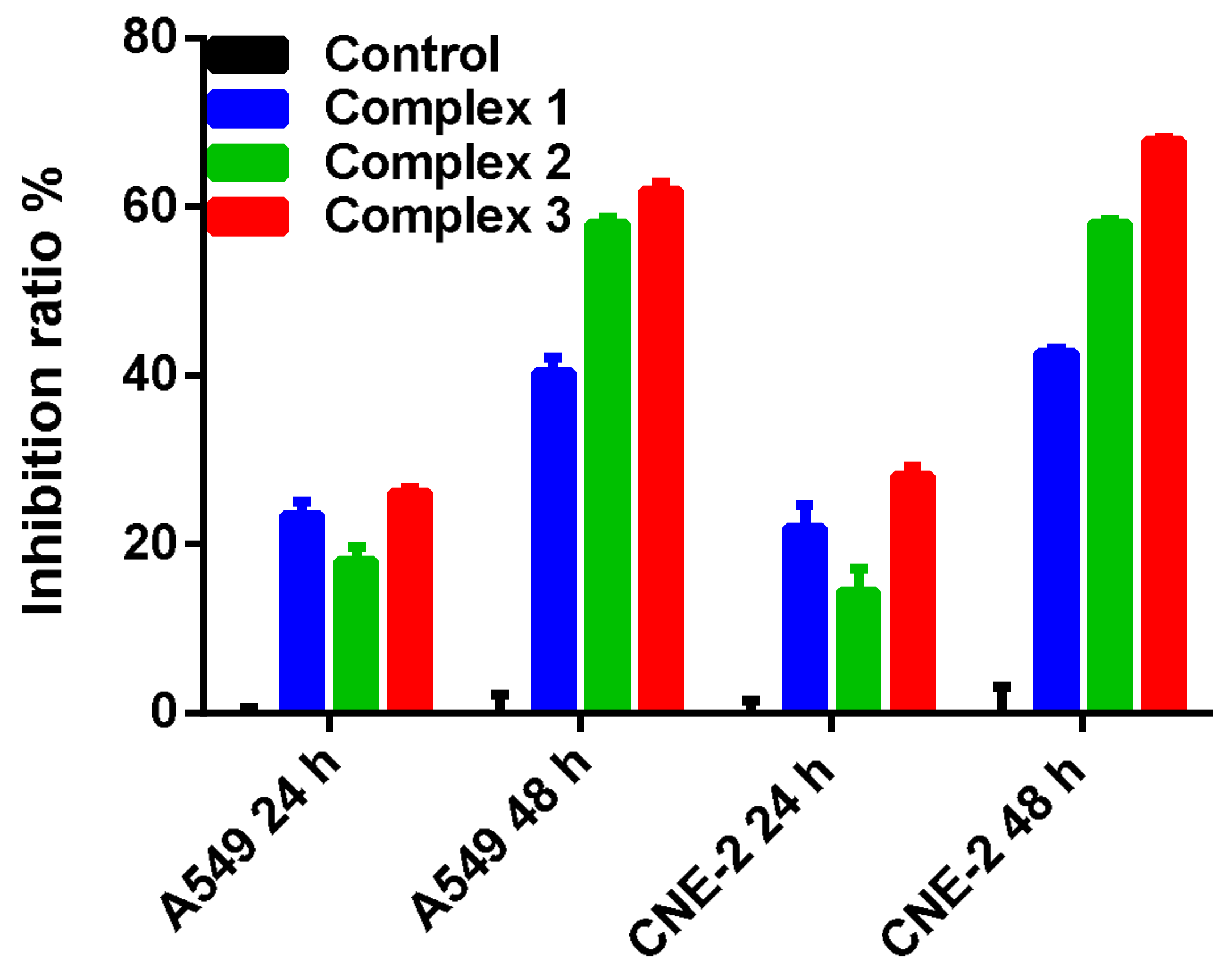
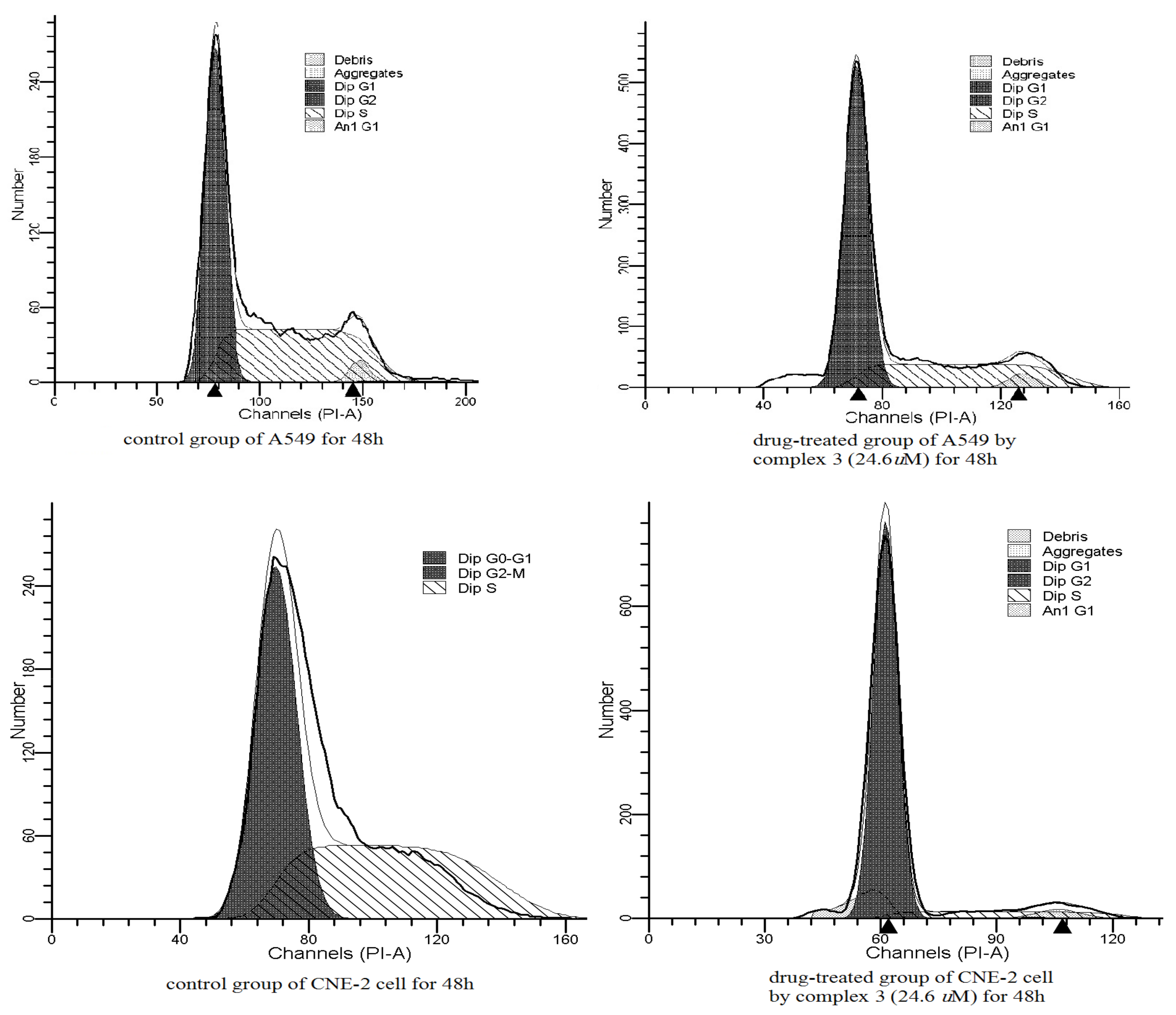
2.4. In Vitro Antitumor Activities
3. Experimental Section
3.1. Materials and Physical Measurements
3.2. Synthesis of the Three Complexes
3.3. X-ray Crystallography Measurement
3.4. In Vitro Antitumor Activity Assay
3.5. Cell Cycle Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Desiraju, G.R. Chemistry beyond the molecule. Nature 2001, 412, 397–400. [Google Scholar] [CrossRef] [PubMed]
- Moulton, B.; Zaworotko, M.J. From molecules to crystal engineering: Supramolecular isomerism and polymorphism in network solids. Chem. Rev. 2001, 101, 1629–1658. [Google Scholar] [CrossRef] [PubMed]
- Janiak, C. Engineering coordination polymers towards applications. Dalton Trans. 2003, 2781–2804. [Google Scholar] [CrossRef]
- Rowsell, J.L.C.; Yaghi, O.M. Strategies for hydrogen storage in metal-organic frameworks. Angew. Chem. Int. Ed. 2005, 44, 4670–4679. [Google Scholar] [CrossRef] [PubMed]
- Pitt, M.A.; Johnson, D.W. Main group supramolecular chemistry. Chem. Soc. Rev. 2007, 36, 1441–1453. [Google Scholar] [CrossRef] [PubMed]
- Aakeroy, C.B.; Champness, N.R.; Janiak, C. Recent advances in crystal engineering. Crystengcomm 2010, 12, 22–43. [Google Scholar] [CrossRef]
- Steiner, T. The hydrogen bond in the solid state. Angew. Chem. Int. Ed. 2002, 41, 48–76. [Google Scholar] [CrossRef]
- Del Bene, J.E.; Elguero, J.; Alkorta, I. Complexes of CO2 with the azoles: Tetrel bonds, hydrogen bonds and other secondary interactions. Molecules 2018, 23, 906. [Google Scholar] [CrossRef] [PubMed]
- Biradha, K. Crystal engineering: From weak hydrogen bonds to co-ordination bonds. Crystengcomm 2003, 5, 374–384. [Google Scholar] [CrossRef]
- Du, M.; Zhang, Z.H.; Zhao, X.J.; Cai, H. Synthons competition/prediction in cocrystallization of flexible dicarboxylic acids with bent dipyridines. Cryst. Growth Des. 2006, 6, 114–121. [Google Scholar] [CrossRef]
- Gillon, A.L.; Lewis, G.R.; Orpen, A.G.; Rotter, S.; Starbuck, J.; Wang, X.M.; Rodriguez-Martin, Y.; Ruiz-Perez, C. Organic-inorganic hybrid solids: Control of perhalometallate solid state structures. J. Chem. Soc. Dalton. 2000, 3897–3905. [Google Scholar] [CrossRef]
- Oksanen, E.; Chen, J.C.; Fisher, S.Z. Neutron crystallography for the study of hydrogen bonds in macromolecules. Molecules 2017, 22, 596. [Google Scholar] [CrossRef] [PubMed]
- Grabowski, S.J. New type of halogen bond: Multivalent halogen interacting with pi- and sigma-electrons. Molecules 2017, 22, 2150. [Google Scholar] [CrossRef] [PubMed]
- Jian, F.; Liu, E.; Xu, J. A water custer cnduit in cystal. Molecules 2017, 22, 2278. [Google Scholar] [CrossRef] [PubMed]
- Adams, C.J.; Angeloni, A.; Orpen, A.G.; Podesta, T.J.; Shore, B. Crystal synthesis of organic anorganic hybrid salts based on tetrachloroplatinate and -palladate salts of organic cations: Frmation of linear, two-, and three-dimensional NH center dot center dot center dot Cl hydrogen bond networks. Cryst. Growth Des. 2006, 6, 411–422. [Google Scholar] [CrossRef]
- Campos-Gaxiola, J.J.; Vega-Paz, A.; Roman-Bravo, P.; Hopfl, H.; Sanchez-Vazquez, M. Pyridineboronic acids as useful building blocks in combination with perchloroplatinate(II) and -(IV) salts: 1D, 2D, and 3D hydrogen-bonded networks containing X-H center dot center dot center dot Cl2Pt- (X = C, N+), B(OH)2 center dot center dot center dot Cl2Pt-, and B(OH)2 center dot center dot center dot(HO)2 B synthons. Cryst. Growth Des. 2010, 10, 3182–3190. [Google Scholar]
- Lewis, G.R.; Orpen, A.G. A metal-containing synthon for crystal engineering: Synthesis of the hydrogen bond ribbon polymer [4,4′-H2bipy] [MCl4] (M = Pd, Pt). Chem. Commun. 1998, 1873–1874. [Google Scholar] [CrossRef]
- Felloni, M.; Hubberstey, P.; Wilson, C.; Schroder, M. Conserved hydrogen-bonded supramolecular synthons in pyridinium tetrachlorometallates. Crystengcomm 2004, 6, 87–95. [Google Scholar] [CrossRef]
- Podesta, T.J.; Orpen, A.G. Tris(pyridinium)triazine in crystal synthesis of 3-fold symmetric structures. Cryst. Growth Des. 2005, 5, 681–693. [Google Scholar] [CrossRef]
- Baldovino-Pantaleon, O.; Morales-Morales, D.; Hernandez-Ortgega, S.; Toscano, R.A.; Valdes-Martinez, J. Pd-N-H center dot center dot center dot Cl-Pd hydrogen bonds and pi-pi interactions between fluorinated aromatic rings in trans-[PdCl2(NH2ArF)2]. Cryst. Growth Des. 2007, 7, 117–123. [Google Scholar] [CrossRef]
- Hall, M.D.; Alderden, R.A.; Zhang, M.; Beale, P.J.; Cai, Z.H.; Lai, B.; Stampfl, A.P.J.; Hambley, T.W. The fate of platinum(II) and platinum(IV) anti-cancer agents in cancer cells and tumours. J. Struct. Biol. 2006, 155, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Caires, A.C. Recent advances involving palladium(II) complexes for the cancer therapy. Anti-Cancer Agent. Med. Chem. 2007, 7, 484–491. [Google Scholar] [CrossRef]
- Li, L.J.; Yan, Q.Q.; Liu, G.J.; Yuan, Z.; Lv, Z.H.; Fu, B.; Han, Y.J.; Du, J.L. Synthesis characterization and cytotoxicity studies of platinum(II) complexes with reduced amino pyridine schiff base and its derivatives as ligands. J. Agric. Chem. Soc. Jpn. 2017, 419, 1081–1089. [Google Scholar] [CrossRef] [PubMed]
- Marin-Luna, M.; Sanchez-Sanz, G. Ootoxicity studies of platinum(II) complexes with reduced amino pyritical study. J. Phys. Chem. A 2014, 118, 5540–5547. [Google Scholar] [CrossRef] [PubMed]
- Bednarski, P.J.; Korpis, K.; Westendorf, A.F.; Perfahl, S.; Gruenert, R. Effects of light-activated diazido-Pt-IV complexes on cancer cells in vitro. Philos. T. Roy. Soc. A 2013, 371, 20120118. [Google Scholar] [CrossRef] [PubMed]
- Karakucuk-Iyidogan, A.; Tasdemir, D.; Oruc-Emre, E.E.; Balzarini, J. Novel platinum(II) and palladium(II) complexes of thiosemicarbazones derived from 5-substitutedthiophene-2-carboxaldehydes and their antiviral and cytotoxic activities. Eur. J. Med. Chem. 2011, 46, 5616–5624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Intini, F.P.; Zajac, J.; Novohradsky, V.; Saltarella, T.; Pacifico, C.; Brabec, V.; Natile, G.; Kasparkova, J. Novel antitumor platinum(II) conjugates containing the nonsteroidal anti-inflammatory agent diclofenac: Synthesis and dual mechanisms of antiproliferative effects. Inorg. Chem. 2017, 56, 1483. [Google Scholar] [CrossRef] [PubMed]
- Politzer, P.; Lane, P.; Murray, J. Electrostatic potentials, intralattice attractive forces and crystal densities of nitrogen-rich C, H, N, O salts. Crystals 2016, 6, 7. [Google Scholar] [CrossRef]
- Cavallo, G.; Murray, J.S.; Politzer, P.; Pilati, T.; Ursini, M.; Resnati, G. Halogen bonding in hypervalent iodine and bromine derivatives: Halonium salts. IUCrJ 2017, 4, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.S.; Shields, P.I.; Seybold, P.G.; Politzer, P. Intuitive and counterintuitive noncovalent interactions of aromatic π regions with the hydrogen and the nitrogen of HCN. J. Comput. Sci. 2015, 10, 209–216. [Google Scholar] [CrossRef]
- Angeloni, A.; Crawford, P.C.; Orpen, A.G.; Podesta, T.J.; Shore, B.J. Does hydrogen bonding matter in crystal engineering? Crystal structures of salts of isomeric ions. Chem.-Eur. J. 2004, 10, 3783–3791. [Google Scholar] [CrossRef] [PubMed]
- Desiraju, G.R. Supramolecular synthons in crystal engineering—A new organic-synthesis. Angew. Chem. Int. Ed. Eng. 1995, 34, 2311–2327. [Google Scholar] [CrossRef]
- Desiraju, G.R. Designer crystals: Intermolecular interactions, network structures and supramolecular synthons. Chem. Commun. 1997, 1475–1482. [Google Scholar] [CrossRef]
- Simard, M.; Su, D.; Wuest, J.D. Use of hydrogen-bonds to control molecular aggregation—Self-assembly of 3-dimensional networks with large chambers. J. Am. Chem. Soc. 1991, 113, 4696–4698. [Google Scholar] [CrossRef]
- Fournier, J.H.; Maris, T.; Wuest, J.D.; Guo, W.Z.; Galoppini, E. Molecular tectonics. Use of the hydrogen bonding of boronic acids to direct supramolecular construction. J. Am. Chem. Soc. 2003, 125, 1002–1006. [Google Scholar] [CrossRef] [PubMed]
- Garcia, H.C.; Diniz, R.; de Oliveira, L.F.C. An interesting pseudo-honeycomb supramolecular arrangement obtained from the interaction between 4-aminosalicylic acid, trans-1,2-bis (4-pyridyl) ethylene and transition metal ions. Cryst. Eng. Comm. 2012, 14, 1812–1818. [Google Scholar] [CrossRef]
- Akhbari, K.; Morsali, A.; Zhu, L.-G. Thermal, fluorescence, solution and structural studies of one-dimensional AgI coordination polymer with Ag–Ag and Ag–π interactions. J. Mol. Struct. 2008, 891, 132–137. [Google Scholar] [CrossRef]
- Aragoni, M.C.; Arca, M.; Demartin, F.; Devillanova, F.A.; Isaia, F.; Garau, A.; Lippolis, V.; Jalali, F.; Papke, U.; Shamsipur, M. Fluorometric chemosensors. Interaction of toxic heavy metal ions PbII, CdII, and HgII with novel mixed-donor phenanthroline-containing macrocycles: Spectrofluorometric, conductometric, and crystallographic studies. Inorg. Chem. 2002, 41, 6623–6632. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.-L.; Gembicky, M.; Messerschmidt, M.; Dominiak, P.M.; Coppens, P. Effect of the environment on molecular properties: Synthesis, structure, and photoluminescence of Cu(I) bis (2, 9-dimethyl-1, 10-phenanthroline) nanoclusters in eight different supramolecular frameworks. Inorg. Chem. 2006, 45, 9281–9289. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.-L.; Coppens, P. Luminescence quenching and energy transfer in supramolecular solids. Cryst. Growth Des. 2005, 5, 2050–2059. [Google Scholar] [CrossRef]
- Zhao, G.-J.; Northrop, B.H.; Han, K.-L.; Stang, P.J. The effect of intermolecular hydrogen bonding on the fluorescence of a bimetallic platinum complex. J. Phys. Chem. A 2010, 114, 9007–9013. [Google Scholar] [CrossRef] [PubMed]
- Kizaka-Kondoh, S.; Konse-Nagasawa, H. Significance of nitroimidazole compounds and hypoxia-inducible factor-1 for imaging tumor hypoxia. Cancer Sci. 2009, 100, 1366–1373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Efthimiadou, E.K.; Thomadaki, H.; Sanakis, Y.; Raptopoulou, C.P.; Katsaros, N.; Scorilas, A.; Karaliota, A.; Psomas, G. Structure and biological properties of the copper(II) complex with the quinolone antibacterial drug N-propyl-norfloxacin and 2,2′-bipyridine. J. Inorg. Biochem. 2007, 101, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Mazuryk, O.; Maciuszek, M.; Stochel, G.; Suzenet, F.; Brindell, M. 2-Nitroimidazole-ruthenium polypyridyl complex as a new conjugate for cancer treatment and visualization. J. Inorg. Biochem. 2014, 134, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Pierroz, V.; Joshi, T.; Leonidova, A.; Mari, C.; Schur, J.; Ott, I.; Spiccia, L.; Ferrari, S.; Gasser, G. Molecular and Cellular Characterization of the Biological Effects of Ruthenium(II) Complexes Incorporating 2-Pyridyl-2-pyrimidine-4-carboxylic Acid. J. Am. Chem. Soc. 2012, 134, 20376–20387. [Google Scholar] [CrossRef] [PubMed]
- CrysAlisPro, Version 1.171.33.52; Oxford Diffraction Ltd.: Oxford, UK, 2009.
- Sheldrick, G.M. Shelxl-97, Program for Crystal Structure Refinement; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Farrugia, L.J. WinGX suite for small-molecule single-crystal crystallography. J. Appl. Crystallogr. 1999, 32, 837–838. [Google Scholar] [CrossRef]
- Macrae, C.F.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Shields, G.P.; Taylor, R.; Towler, M.; van de Streek, J. Mercury: Visualization and analysis of crystal structures. J. Appl. Cryst. 2006, 39, 453–457. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 1–3 are available from the authors. |



| Complex | 1 | 2 | 3 |
|---|---|---|---|
| empirical | C14 H18 Cl6 N4 O2 Pt | C20 H22 Cl6 N4 O2 Pt | C24 H30 Cl6 N4 O2 Pt |
| fw | 682.09 | 758.20 | 814.30 |
| Cryst syst | Monoclinic | Triclinic | Triclinic |
| Space group | C 2/m | P −1 | P −1 |
| a (Å) | 16.5752(11) | 7.6476(5) | 8.1295(4) |
| b (Å) | 7.0641(5) | 9.0089(6) | 8.6453(4) |
| c (Å) | 9.6557(7) | 10.4610(7) | 11.0288(5) |
| α (deg.) | 90 | 69.925(1) | 99.379(4) |
| β (deg.) | 91.043(2) | 73.123(1) | 99.347(4) |
| γ (deg.) | 90 | 80.402(2) | 103.042(4) |
| V (Å3) | 1130.39(14) | 645.98(7) | 728.86(6) |
| Z | 2 | 1 | 1 |
| Dc (g cm−3) | 2.004 | 1.949 | 1.855 |
| T (K) | 293 | 293 | 293 |
| μ (mm−1) | 6.933 | 6.077 | 5.393 |
| GOF | 1.093 | 1.055 | 1.028 |
| R1, wR2 (I > 2sigma) | 0.018, 0.042 | 0.025, 0.074 | 0.032, 0.069 |
| R1, wR2 (all data) | 0.018, 0.042 | 0.027, 0.085 | 0.034, 0.070 |
| D–H···A | D–H | H···A | D···A | D–H···A |
|---|---|---|---|---|
| Complex 1 | ||||
| O1w–H1w ∙∙Cl1 | 0.813(18) | 2.86(3) | 3.551(3) | 144(4) |
| O1w–H1w…Cl2 N1–H1 ∙∙O1w | 0.813(18) 0.860(19) | 2.84(3) 1.86(2) | 3.5374(3) 2.720(5) | 146(4) 177(6) |
| N2–H2 ∙∙Cl1 | 0.86 | 2.97 | 3.518(3) | 123.7 |
| N2–H2 ∙∙Cl2 | 0.86 | 2.50 | 3.316(4) | 159.7 |
| Complex 2 | ||||
| O1w–H1wb ∙∙Cl1i | 0.851(10) | 2.57(6) | 3.381(6) | 161(8) |
| N1–H1 ∙∙O1wii | 0.73(8) | 2.11(8) | 2.795(8) | 157(8) |
| C10–H10 ∙∙O1wiii | 0.93 | 2.66 | 3.295(10) | 125.6 |
| Complex 3 | ||||
| O1w–H1wa ∙∙Cl1i | 0.85 | 2.51 | 3.295(3) | 154.9 |
| O1w–H1wb ∙∙N2ii | 0.85 | 2.52 | 3.050(6) | 121.3 |
| O1w–H1B ∙∙Cl3i | 0.85 | 2.60 | 3.267(4) | 136.3 |
| N1–H1 ∙∙O1wii | 0.82 | 2.01 | 2.794(5) | 160.1 |
| Complex | λmax/nm (ε/dm3 mol−1 cm−1) | ||
|---|---|---|---|
| 1 | 273 (3.77 × 104) | 266 (4.17 × 104) | 204 (1.04 × 105) |
| 2 | 302 (2.47 × 104) | 243 (2.41 × 104) | 201 (4.35 × 105) |
| 3 | 302 (2.56 × 104) | 237 (3.64 × 104) | 202 (7.60 × 104) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Chen, F.; Han, Y.; Chen, H.; Luo, Z.; Tian, H.; Zhao, Y.; Ma, A.; Zhu, L. Hydrogen-Bonded Organic–Inorganic Hybrid Based on Hexachloroplatinate and Nitrogen Heterocyclic Cations: Their Synthesis, Characterization, Crystal Structures, and Antitumor Activities In Vitro. Molecules 2018, 23, 1397. https://doi.org/10.3390/molecules23061397
Zhao J, Chen F, Han Y, Chen H, Luo Z, Tian H, Zhao Y, Ma A, Zhu L. Hydrogen-Bonded Organic–Inorganic Hybrid Based on Hexachloroplatinate and Nitrogen Heterocyclic Cations: Their Synthesis, Characterization, Crystal Structures, and Antitumor Activities In Vitro. Molecules. 2018; 23(6):1397. https://doi.org/10.3390/molecules23061397
Chicago/Turabian StyleZhao, Jin, Fuming Chen, Yutong Han, Huaqing Chen, Zhidong Luo, Hao Tian, Yi Zhao, Aiqing Ma, and Longguan Zhu. 2018. "Hydrogen-Bonded Organic–Inorganic Hybrid Based on Hexachloroplatinate and Nitrogen Heterocyclic Cations: Their Synthesis, Characterization, Crystal Structures, and Antitumor Activities In Vitro" Molecules 23, no. 6: 1397. https://doi.org/10.3390/molecules23061397




