In Situ Monitoring of the Effect of Ultrasound on the Sulfhydryl Groups and Disulfide Bonds of Wheat Gluten
Abstract
:1. Introduction
2. Results and Discussion
2.1. The Effects of Ultrasound Treatment on the SH and SS Content
2.2. Variable Selection
2.3. Development of the Si-PLS Model
2.4. Development of the BP-ANN Model
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. In-Situ Monitoring of the Ultrasonic Treatment Process
4.3. Determination of SH and SS Content by Off-Line Methods
4.4. Spectral Data Preprocessing
4.5. Multivariate Analysis
4.5.1. Si-PLS
4.5.2. BP-ANN
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Dadzie, R.G.; Ma, H.; Abano, E.E.; Qu, W.; Mao, S. Optimization of process conditions for production of angiotensin I-converting enzyme (ACE) inhibitory peptides from vital wheat gluten using response surface methodology. Food Sci. Biotechnol. 2013, 22, 1531–1537. [Google Scholar] [CrossRef]
- Qu, W.; Ma, H.; Jia, J.; He, R.; Luo, L.; Pan, Z. Enzymolysis kinetics and activities of ACE inhibitory peptides from wheat germ protein prepared with SFP ultrasound-assisted processing. Ultrason. Sonochem. 2012, 19, 1021–1026. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Ma, H.; Zhao, W.; Wang, Z.; Tian, W.; Luo, L.; He, R. The use of ultrasound for enzymatic preparation of ACE-inhibitory peptides from wheat germ protein. Food Chem. 2010, 119, 336–342. [Google Scholar] [CrossRef]
- Jin, J.; Ma, H.; Wang, B.; Yagoub, A.E.G.A.; Wang, K.; He, R.; Zhou, C. Effects and mechanism of dual-frequency power ultrasound on the molecular weight distribution of corn gluten meal hydrolysates. Ultrason. Sonochem. 2016, 30, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Ma, H.; Wang, K.; Yagoub, A.E.G.A.; Owusu, J.; Qu, W.; He, R.; Zhou, C.; Ye, X. Effects of multi-frequency power ultrasound on the enzymolysis and structural characteristics of corn gluten meal. Ultrason. Sonochem. 2015, 24, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Lateef, A.; Oloke, J.K.; Prapulla, S.G. The effect of ultrasonication on the release of fructosyltransferase from Aureobasidium pullulans CFR 77. Enzyme Microb. Technol. 2007, 40, 1067–1070. [Google Scholar] [CrossRef]
- Sang, H.L.; Nguyen, H.M.; Koo, Y.M.; Ha, S.H. Ultrasound-enhanced lipase activity in the synthesis of sugar ester using ionic liquids. Process Biochem. 2008, 43, 1009–1012. [Google Scholar] [CrossRef]
- Ren, X.; Ma, H.; Mao, S.; Zhou, H. Effects of sweeping frequency ultrasound treatment on enzymatic preparations of ACE-inhibitory peptides from zein. Eur. Food Res. Technol. 2014, 238, 435–442. [Google Scholar] [CrossRef]
- Zhou, C.; Hu, J.; Yu, X.; Yagoub, A.E.A.; Zhang, Y.; Ma, H.; Gao, X.; Otu, P.N.Y. Heat and/or ultrasound pretreatments motivated enzymolysis of corn gluten meal: Hydrolysis kinetics and protein structure. LWT-Food Sci. Technol. 2017, 77, 488–496. [Google Scholar] [CrossRef]
- Zhou, C.; Yu, X.; Zhang, Y.; He, R.; Ma, H. Ultrasonic degradation, purification and analysis of structure and antioxidant activity of polysaccharide from Porphyra yezoensis udea. Carbohydr. Polym. 2012, 87, 2046–2051. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, H.; Wang, B.; Qu, W.; Wali, A.; Zhou, C. Relationships between the structure of wheat gluten and ACE inhibitory activity of hydrolysate: Stepwise multiple linear regression analysis. J. Sci. Food Agric. 2015, 96, 3313–3320. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhao, J.; Liu, M.; Cai, J.; Liu, J. Determination of total polyphenols content in green tea using FT-NIR spectroscopy and different PLS algorithms. J. Pharm. Biomed. Anal. 2008, 46, 568–573. [Google Scholar] [CrossRef] [PubMed]
- Mandato, S.; Taliani, C.C.; Aïtkaddour, A.; Ruiz, T.; Cuq, B. In-line monitoring of durum wheat semolina wet agglomeration by near infrared spectroscopy for different water supply conditions and water addition levels. J. Food Eng. 2013, 119, 533–543. [Google Scholar] [CrossRef]
- Porep, J.U.; Kammerer, D.R.; Carle, R. On-line application of near infrared (NIR) spectroscopy in food production. Trends Food Sci. Technol. 2015, 42, 211–230. [Google Scholar] [CrossRef]
- Song, G.S.; Hu, J.; Shen, X.; Hu, S.; Li, L. Effects of ultrasound-assisted freeze on secondary structure of gluten protein. Mod. Food Sci. Technol. 2009, 25, 860–864. [Google Scholar]
- Krešić, G.; Lelas, V.; Jambrak, A.R.; Herceg, Z.; Brnčić, S.R. Influence of novel food processing technologies on the rheological and thermophysical properties of whey proteins. J. Food Eng. 2008, 87, 64–73. [Google Scholar] [CrossRef]
- Bruun, S.W.; Søndergaard, I.; Jacobsen, S. Analysis of protein structures and interactions in complex food by near-infrared spectroscopy: Part 2: Hydrated gluten. J. Agric. Food Chem. 2007, 55, 7244–7251. [Google Scholar] [CrossRef] [PubMed]
- Bruun, S.W.; Søndergaard, I.; Jacobsen, S. Analysis of protein structures and interactions in complex food by near-infrared spectroscopy. 1. Gluten powder. J. Agric. Food Chem. 2007, 55, 7234–7243. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, B.; Zhou, C.; Atungulu, G.G.; Xu, K.; Ma, H.; Ye, X.; Abdualrahman, M.A. Surface topography, nano-mechanics and secondary structure of wheat gluten pretreated by alternate dual-frequency ultrasound and the correlation to enzymolysis. Ultrason. Sonochem. 2016, 31, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Luo, L.; Li, J.; Li, S.; Qu, W.; Ma, H.; Oladejo, A.O.; Ye, X. In-situ and real-time monitoring of enzymatic process of wheat gluten by miniature fiber NIR spectrometer. Food Res. Int. 2017, 99, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Jin, J.; Ma, H.; Qu, W.; Wang, K.; Zhou, C.; He, R.; Luo, L.; Owusu, J. Effects of multi-frequency power ultrasound on the enzymolysis of corn gluten meal: Kinetics and thermodynamics study. Ultrason. Sonochem. 2015, 27, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.M.; Cai, J.R.; Sun, L.; Han, E.; Ernest, T. Nondestructive measurement of soluble solids content in apples by a portable fruit analyzer. Food Anal. Methods 2015, 16, 785–794. [Google Scholar] [CrossRef]
- Ouyang, Q.; Chen, Q.; Zhao, J.; Lin, H. Determination of amino acid nitrogen in soy sauce using near infrared spectroscopy combined with characteristic variables selection and extreme learning machine. Food Bioprocess Technol. 2013, 6, 2486–2493. [Google Scholar] [CrossRef]
- Chen, Q.; Hu, W.; Su, J.; Li, H.; Ouyang, Q.; Zhao, J. Nondestructively sensing of total viable count (TVC) in chicken using an artificial olfaction system based colorimetric sensor array. J. Food Eng. 2015, 25, 259–266. [Google Scholar] [CrossRef]
- Ouyang, Q.; Zhao, J.; Pan, W.; Chen, Q. Real-time monitoring of process parameters in rice wine fermentation by a portable spectral analytical system combined with multivariate analysis. Food Chem. 2016, 190, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Jiang, P.; Zhao, J. Measurement of total flavone content in snow lotus (Saussurea involucrate) using near infrared spectroscopy combined with interval PLS and genetic algorithm. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2010, 76, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Guo, Z.; Zhao, J.; Ouyang, Q. Comparisons of different regressions tools in measurement of antioxidant activity in green tea using near infrared spectroscopy. J. Pharm. Biomed. Anal. 2012, 60, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Thukaram, D.; Khincha, H.P.; Vijaynarasimha, H.P. Artificial neural network and support vector Machine approach for locating faults in radial distribution systems. Eng. Electr. Electron. 2005, 20, 710–721. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |



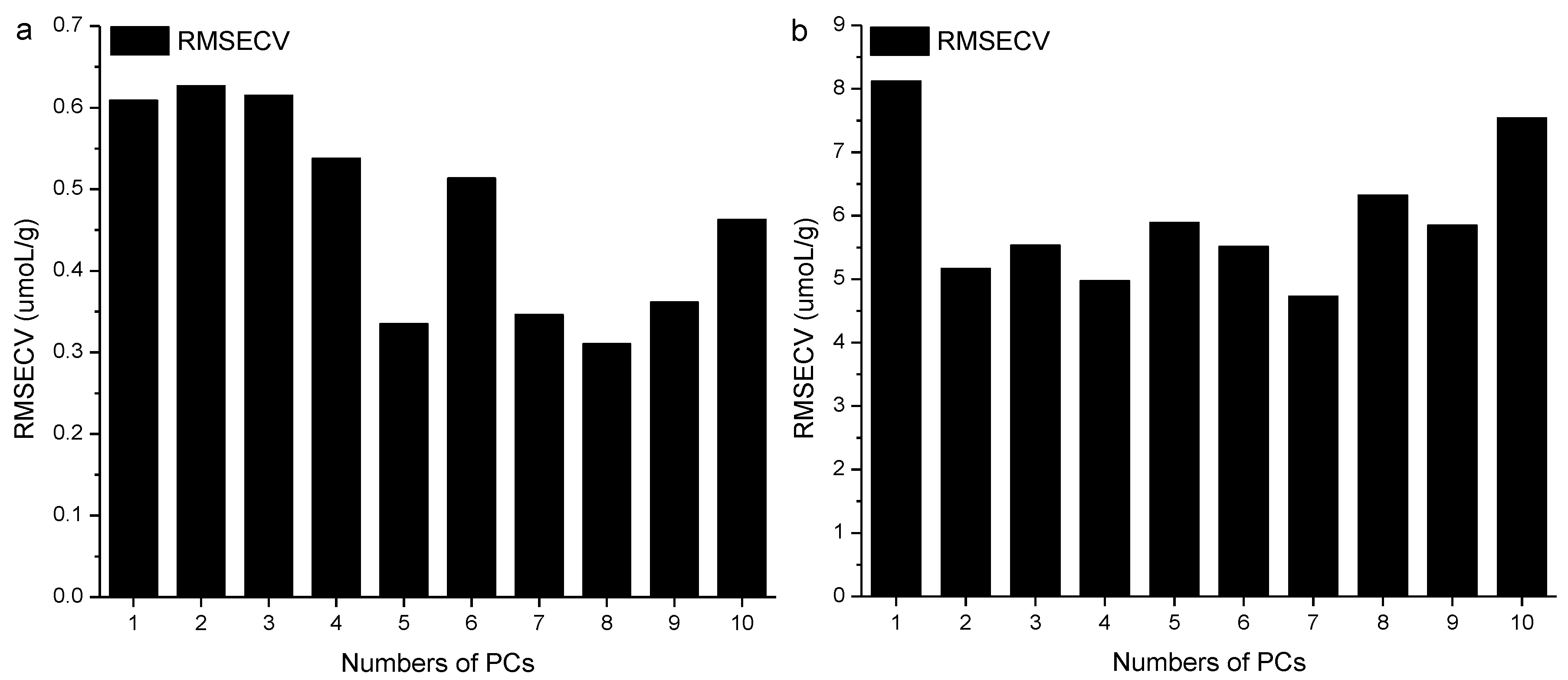

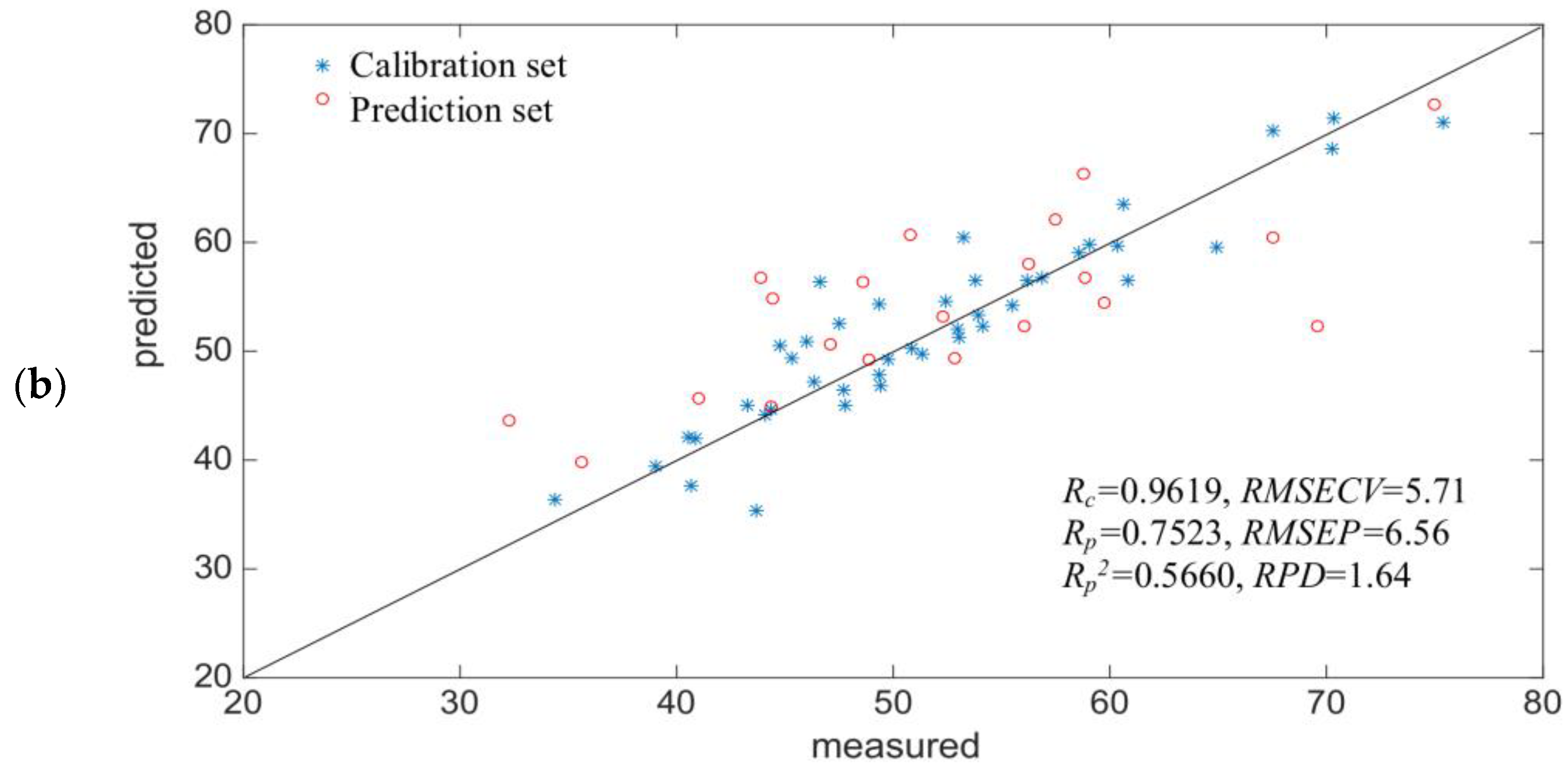

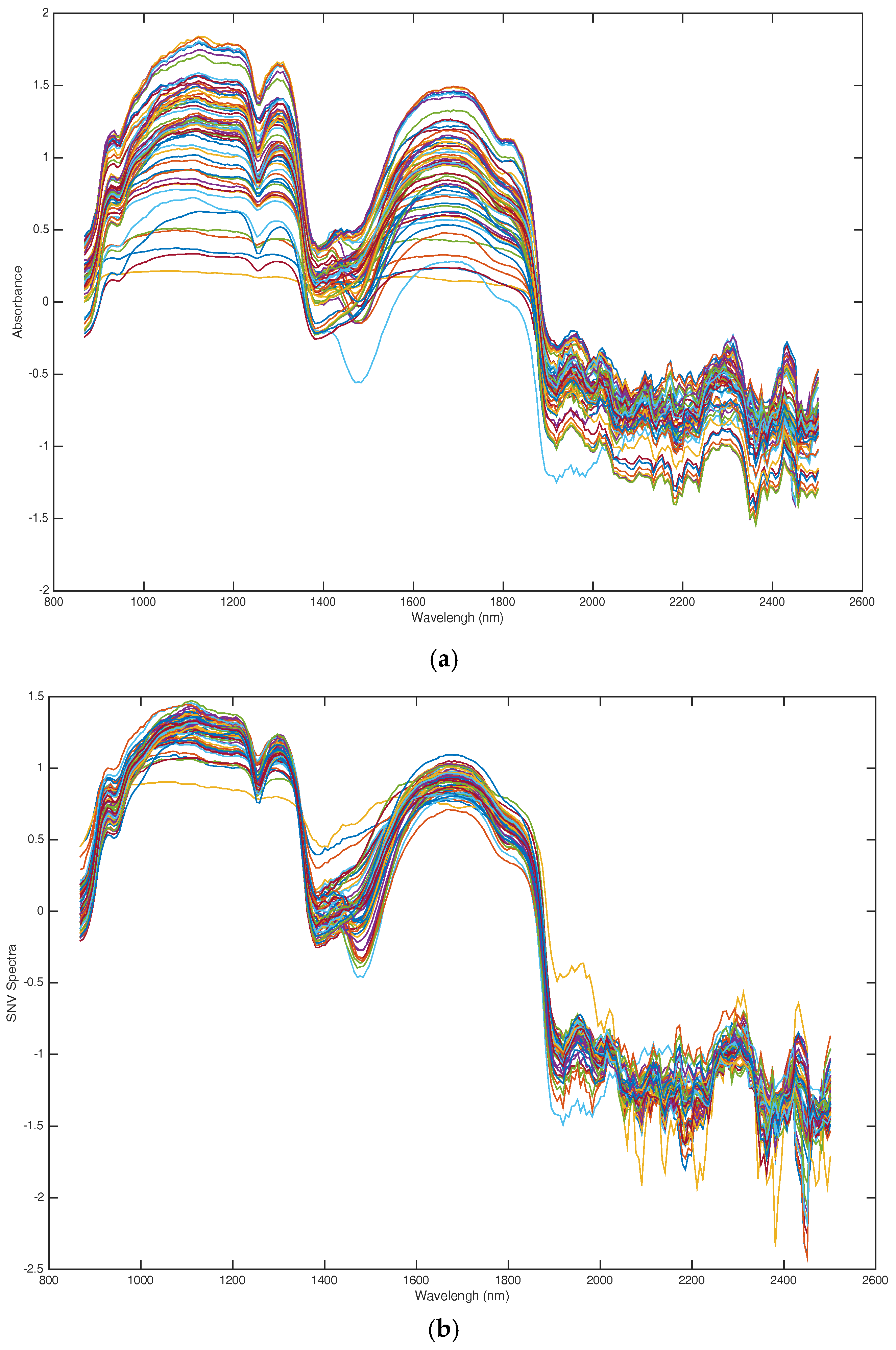
| Parameters | Number of Subintervals | Selected Subintervals | PCs | Rc | RMSEC | Rp | RMSEP |
|---|---|---|---|---|---|---|---|
| SH (μmol/g) | 15 | [4, 5, 6, 14] | 10 | 0.9302 | 0.39 | 0.7930 | 0.76 |
| 16 | [4, 5, 13, 15] | 9 | 0.9325 | 0.38 | 0.7162 | 0.94 | |
| 17 | [1, 4, 7] | 4 | 0.9226 | 0.40 | 0.8514 | 0.58 | |
| 18 | [1, 4, 11] | 5 | 0.9200 | 0.41 | 0.8662 | 0.57 | |
| 19 | [1, 3, 5] | 5 | 0.9192 | 0.41 | 0.8188 | 0.63 | |
| 20 | [1, 5, 8, 17] | 10 | 0.9405 | 0.36 | 0.8488 | 0.64 | |
| 21 | [4, 5, 13] | 6 | 0.9348 | 0.37 | 0.8930 | 0.53 | |
| 22 | [4, 9, 19] | 10 | 0.9334 | 0.38 | 0.7773 | 0.91 | |
| 23 | [5, 7, 18, 21] | 10 | 0.9381 | 0.37 | 0.7410 | 0.95 | |
| 24 | [2, 6, 9] | 8 | 0.9344 | 0.37 | 0.8021 | 0.66 | |
| 25 | [2, 4, 6, 7] | 7 | 0.9331 | 0.37 | 0.8441 | 0.60 | |
| 26 | [2, 4, 8, 23] | 10 | 0.9512 | 0.32 | 0.7423 | 0.93 | |
| 27 | [2, 4, 8, 24] | 10 | 0.9512 | 0.33 | 0.7423 | 0.93 | |
| 28 | [2, 5, 10, 25] | 10 | 0.9366 | 0.37 | 0.8122 | 0.76 | |
| 29 | [2, 5, 7] | 9 | 0.9380 | 0.36 | 0.8126 | 0.66 | |
| 30 | [2, 6, 11, 25] | 10 | 0.9447 | 0.35 | 0.7615 | 0.93 | |
| SS (μmol/g) | 15 | [1, 3, 8, 10] | 9 | 0.6298 | 8.28 | 0.6236 | 7.40 |
| 16 | [2, 3, 6, 7] | 6 | 0.6087 | 8.17 | 0.4910 | 8.17 | |
| 17 | [9, 10, 11, 16] | 2 | 0.5667 | 7.52 | 0.6756 | 9.16 | |
| 18 | [14, 17, 18] | 7 | 0.6153 | 7.40 | 0.5898 | 9.47 | |
| 19 | [4, 10, 22, 27] | 10 | 0.8056 | 5.52 | 0.5266 | 12.10 | |
| 20 | [1, 2, 5] | 5 | 0.5571 | 8.25 | 0.4175 | 8.53 | |
| 21 | [7, 14, 18] | 7 | 0.6481 | 6.99 | 0.4884 | 10.60 | |
| 22 | [9, 10, 20] | 2 | 0.5833 | 7.40 | 0.6731 | 7.95 | |
| 23 | [8, 17, 18, 21] | 10 | 0.7238 | 6.38 | 0.4840 | 11.40 | |
| 24 | [8, 10, 11, 20] | 10 | 0.4842 | 9.56 | 0.5399 | 9.33 | |
| 25 | [8, 15, 18] | 8 | 0.6665 | 6.83 | 0.3248 | 12.60 | |
| 26 | [2, 9, 20, 22] | 10 | 0.7045 | 6.54 | 0.5664 | 9.91 | |
| 27 | [2, 7, 24] | 8 | 0.6952 | 6.63 | 0.5358 | 9.53 | |
| 28 | [2, 14, 7, 26] | 9 | 0.6505 | 7.01 | 0.6660 | 7.90 | |
| 29 | [10, 17, 27] | 7 | 0.6683 | 6.72 | 0.6123 | 8.30 | |
| 30 | [10, 17, 28] | 7 | 0.6489 | 6.91 | 0.6186 | 8.25 |
| Parameters | Units | Subsets | S.N. a | Range | Mean | S.D. b |
|---|---|---|---|---|---|---|
| SH | μmol/g | Calibration set | 59 | 1.9508–5.4225 | 3.6189 | 1.0573 |
| Prediction set | 29 | 2.1976–5.3258 | 3.7203 | 1.1061 | ||
| SS | μmol/g | Calibration set | 59 | 34.3516–75.4038 | 51.9267 | 9.0196 |
| Prediction set | 29 | 32.2722–75.0061 | 52.4415 | 10.7267 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Li, Y.; Li, S.; Zhang, H.; Ma, H. In Situ Monitoring of the Effect of Ultrasound on the Sulfhydryl Groups and Disulfide Bonds of Wheat Gluten. Molecules 2018, 23, 1376. https://doi.org/10.3390/molecules23061376
Zhang Y, Li Y, Li S, Zhang H, Ma H. In Situ Monitoring of the Effect of Ultrasound on the Sulfhydryl Groups and Disulfide Bonds of Wheat Gluten. Molecules. 2018; 23(6):1376. https://doi.org/10.3390/molecules23061376
Chicago/Turabian StyleZhang, Yanyan, Yinli Li, Suyun Li, Hua Zhang, and Haile Ma. 2018. "In Situ Monitoring of the Effect of Ultrasound on the Sulfhydryl Groups and Disulfide Bonds of Wheat Gluten" Molecules 23, no. 6: 1376. https://doi.org/10.3390/molecules23061376





