Aspirin Derivative 5-(Bis(3-methylbut-2-enyl)amino)-2-hydroxybenzoic Acid Improves Thermotolerance via Stress Response Proteins in Caenorhabditis elegans
Abstract
:1. Induction
2. Results and Discussion
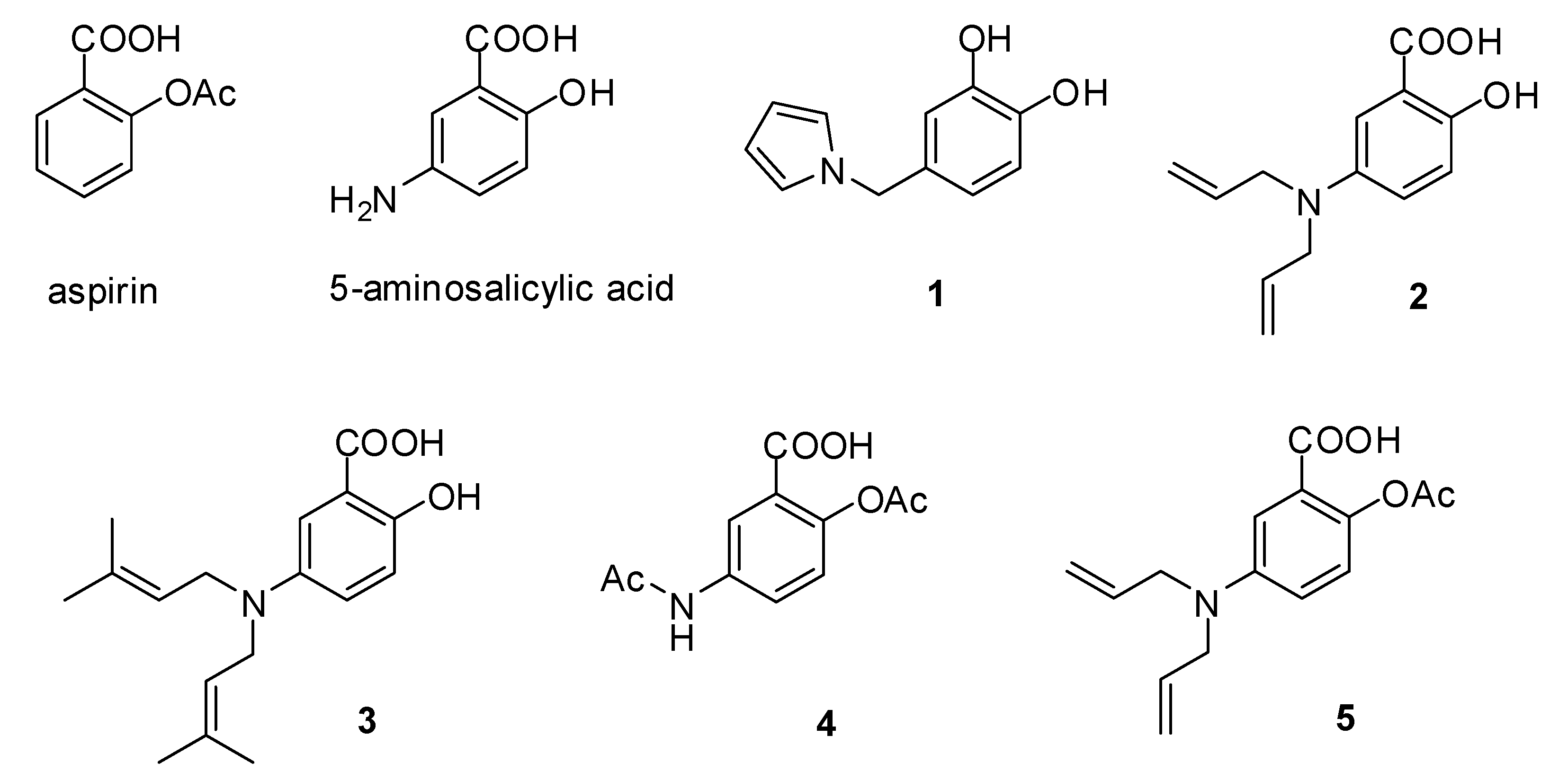
2.1. The Synthesis of Compounds
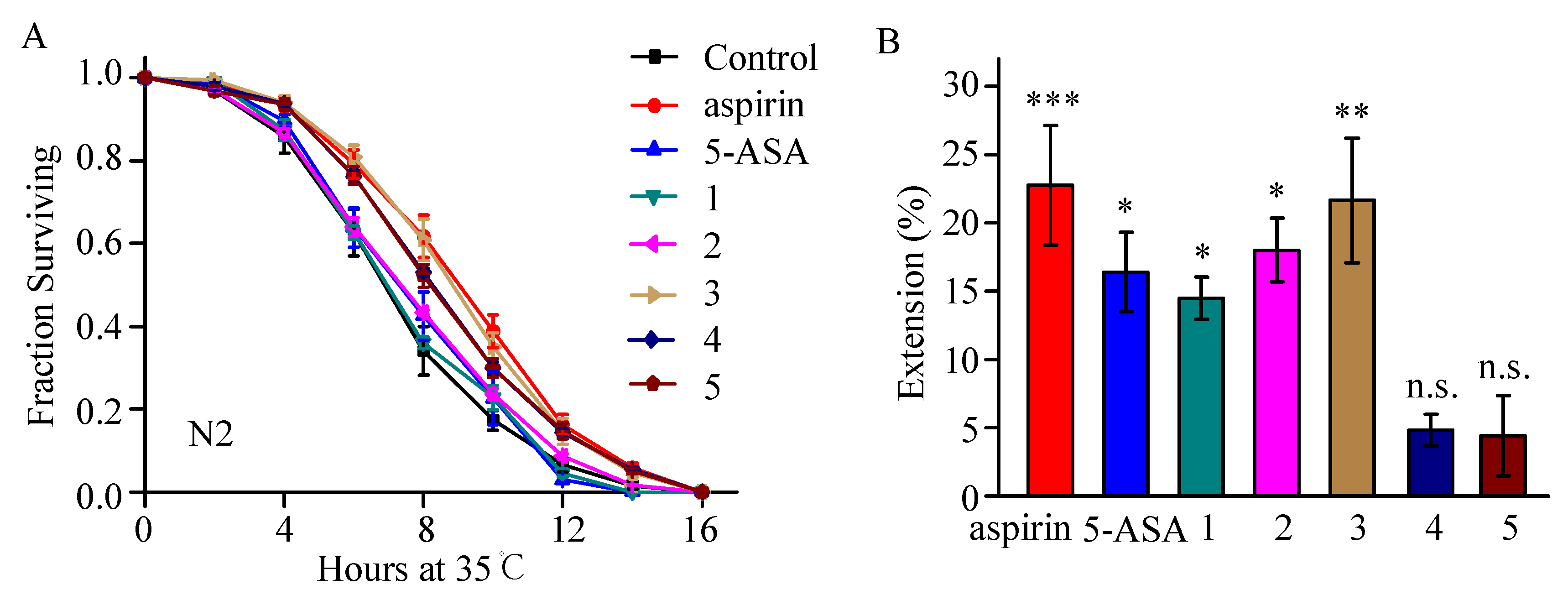
2.2. Thermotolerance Enhancement Activity of Aspirin Analogues Were Assayed in C. elegans
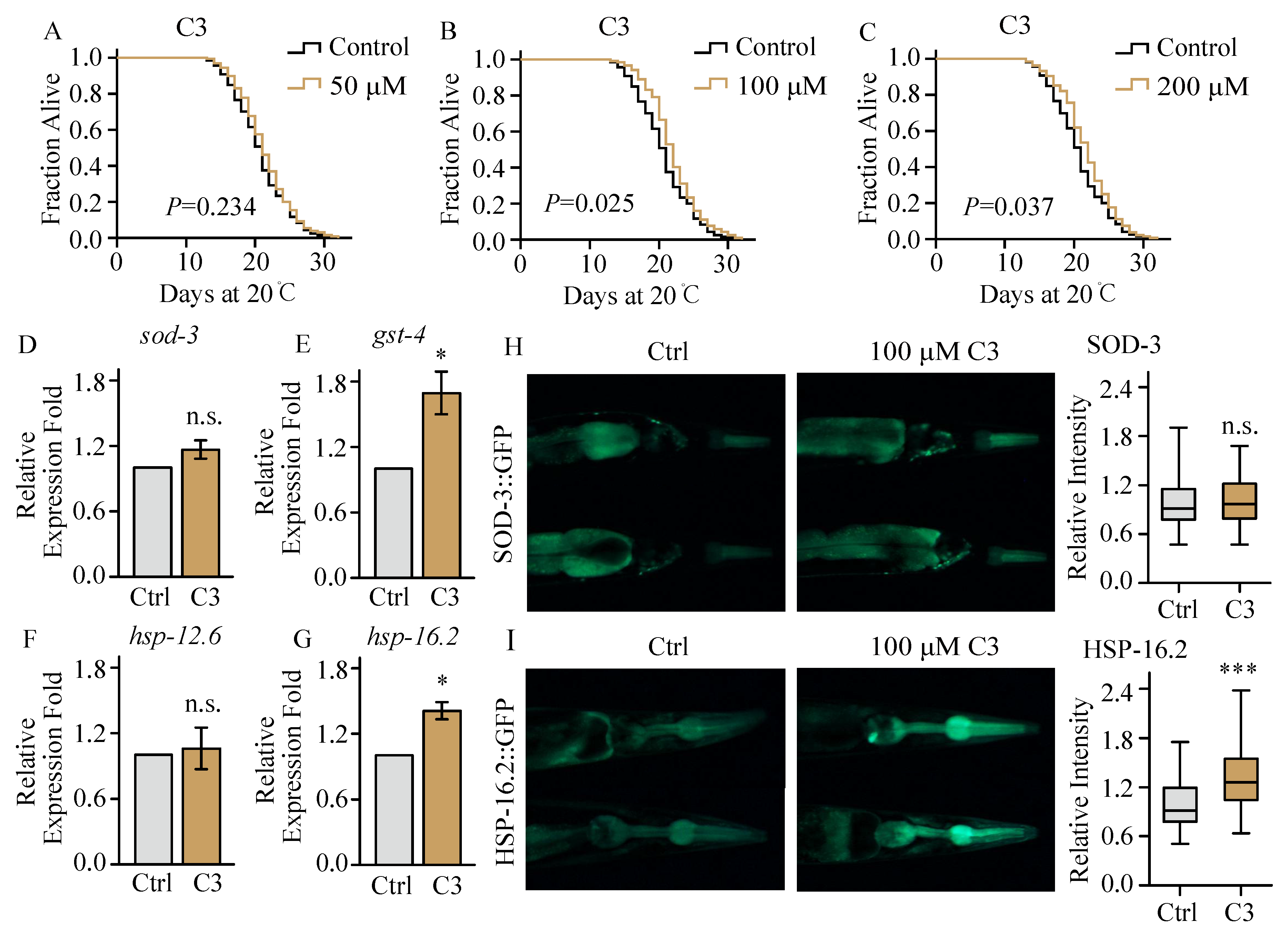
2.3. Compound 3 Barely Extends Lifespan under Normal Culture Condition
2.4. Compound 3 Increases the Expression of Stress Response Proteins in C. elegans under Heat Stress
2.5. Discussion
3. Materials and Methods
3.1. Chemistry and Synthesis
3.1.1. 4-((1H-Pyrrol-1-yl)methyl)benzene-1,2-diol (1)
3.1.2. 5-(Diallylamino)-2-hydroxybenzoic acid (2)
3.1.3. 5-(Bis(3-methylbut-2-enyl)amino)-2-hydroxybenzoic acid (3)
3.1.4. 5-Acetamido-2-acetoxybenzoic acid (4)
3.1.5. 2-Acetoxy-5-(diallylamino)benzoic acid (5)
3.2. Culture Conditions and Worm Strains
3.3. Thermotolerance Assay
3.4. Lifespan Assay
3.5. Green Fluorescent Protein Quantification and Visualization
3.6. DAF-16::GFP Localization Assay
3.7. Gene Expression Assay
- cdc-42 F 5′-CTGCTGGACAGGAAGATTACG-3′; R 5′-CTCGGACATTCTCGAATGAAG-3′;
- sod-3 F 5′-AGCATCATGCCACCTACGTGA-3′; R 5′-CACCACCATTGAATTTCAGCG-3′;
- gst-4 F 5′-TCCGTCAATTCACTTCTTCCG-3′; R 5′-AAGAAATCATCACGGGCTGG-3′;
- hsp-12.6 F 5′-GTGATGGCTGACGAAGGAAC-3′; R 5′-GGGAGGAAGTTATGGGCTTC-3′;
- hsp-16.2 F 5′-CTGCAGAATCTCTCCATCTGAGTC-3′; R 5′-AGATTCGAAGCAACTGCACC-3′.
3.8. Statistical Analyses
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Niccoli, T.; Partridge, L. Ageing as a risk factor for disease. Curr. Biol. 2012, 22, R741–R752. [Google Scholar] [CrossRef] [PubMed]
- Baur, J.A.; Pearson, K.J.; Price, N.L.; Jamieson, H.A.; Lerin, C.; Kalra, A.; Prabhu, V.V.; Allard, J.S.; Lopez-Lluch, G.; Lewis, K.; et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 2006, 444, 337–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.J.; Ahmad, F.; Philp, A.; Baar, K.; Williams, T.; Luo, H.; Ke, H.; Rehmann, H.; Taussig, R.; Brown, A.L.; et al. Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases. Cell 2012, 148, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.Q.; Huang, X.B.; Xing, T.K.; Ding, A.J.; Wu, G.S.; Luo, H.R. Chlorogenic acid extends the lifespan of Caenorhabditis elegans via Insulin/IGF-1 signaling pathway. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 464–472. [Google Scholar] [PubMed]
- Pietsch, K.; Saul, N.; Menzel, R.; Sturzenbaum, S.R.; Steinberg, C.E. Quercetin mediated lifespan extension in Caenorhabditis elegans is modulated by age-1, daf-2, sek-1 and unc-43. Biogerontology 2009, 10, 565–578. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.G.; Huang, J.A.; Li, J.; Yu, P.H.; Xiong, Z.; Zhang, J.W.; Gong, Y.S.; Liu, Z.H.; Chen, J.H. Black tea increased survival of Caenorhabditis elegans under stress. J. Agric. Food Chem. 2014, 62, 11163–11169. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.A.; Shukitt-Hale, B.; Kalt, W.; Ingram, D.K.; Joseph, J.A.; Wolkow, C.A. Blueberry polyphenols increase lifespan and thermotolerance in Caenorhabditis elegans. Aging Cell 2006, 5, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Smith, J.V.; Paramasivam, V.; Butko, P.; Khan, I.; Cypser, J.R.; Luo, Y. Ginkgo biloba extract EGb 761 increases stress resistance and extends life span of Caenorhabditis elegans. Cell. Mol. Biol. 2002, 48, 725–731. [Google Scholar] [PubMed]
- Cabreiro, F.; Au, C.; Leung, K.Y.; Vergara-Irigaray, N.; Cocheme, H.M.; Noori, T.; Weinkove, D.; Schuster, E.; Greene, N.D.; Gems, D. Metformin retards aging in C. elegans by altering microbial folate and methionine metabolism. Cell 2013, 153, 228–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Ou, Y.; Li, Y.; Hu, S.; Shao, L.-W.; Liu, Y. Metformin extends C. elegans lifespan through lysosomal pathway. eLife 2017, 6, e31268. [Google Scholar] [CrossRef] [PubMed]
- Ayyadevara, S.; Bharill, P.; Dandapat, A.; Hu, C.; Khaidakov, M.; Mitra, S.; Shmookler Reis, R.J.; Mehta, J.L. Aspirin inhibits oxidant stress, reduces age-associated functional declines, and extends lifespan of Caenorhabditis elegans. Antioxid. Redox Signal. 2013, 18, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Ayyadevara, S.; Balasubramaniam, M.; Kakraba, S.; Alla, R.; Mehta, J.L.; Shmookler Reis, R.J. Aspirin mediated acetylation protects against multiple neurodegenerative pathologies by impeding protein aggregation. Antioxid. Redox Signal. 2017, 27, 1383–1396. [Google Scholar] [CrossRef] [PubMed]
- Harrison, D.E.; Strong, R.; Sharp, Z.D.; Nelson, J.F.; Astle, C.M.; Flurkey, K.; Nadon, N.L.; Wilkinson, J.E.; Frenkel, K.; Carter, C.S.; et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 2009, 460, 392–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sutphin, G.L.; Bishop, E.; Yanos, M.E.; Moller, R.M.; Kaeberlein, M. Caffeine extends life span, improves healthspan, and delays age-associated pathology in Caenorhabditis elegans. Longev. Healthspan 2012, 1, 9. [Google Scholar] [CrossRef] [PubMed]
- Lucanic, M.; Lithgow, G.J.; Alavez, S. Pharmacological lifespan extension of invertebrates. Ageing Res. Rev. 2013, 12, 445–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, A.J.; Zheng, S.Q.; Huang, X.B.; Xing, T.K.; Wu, G.S.; Sun, H.Y.; Qi, S.H.; Luo, H.R. Current perspective in the discovery of anti-aging agents from natural products. Nat. Prod. Bioprospect. 2017, 7, 335–404. [Google Scholar] [CrossRef] [PubMed]
- Barardo, D.; Thornton, D.; Thoppil, H.; Walsh, M.; Sharifi, S.; Ferreira, S.; Anzic, A.; Fernandes, M.; Monteiro, P.; Grum, T.; et al. The DrugAge database of aging-related drugs. Aging Cell 2017, 16, 594–597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaeberlein, M.; Rabinovitch, P.S.; Martin, G.M. Healthy aging: The ultimate preventative medicine. Science 2015, 350, 1191–1193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frieden, T.R.; Berwick, D.M. The “Million Hearts” initiative—Preventing heart attacks and strokes. N. Engl. J. Med. 2011, 365, e27. [Google Scholar] [CrossRef] [PubMed]
- Rothwell, P.M.; Algra, A.; Chen, Z.; Diener, H.-C.; Norrving, B.; Mehta, Z. Effects of aspirin on risk and severity of early recurrent stroke after transient ischaemic attack and ischaemic stroke: Time-course analysis of randomised trials. Lancet 2016, 388, 365–375. [Google Scholar] [CrossRef]
- Grancher, A.; Michel, P.; Di Fiore, F.; Sefrioui, D. Aspirin and colorectal cancer. Bull. Cancer 2018, 105, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Wan, Q.L.; Zheng, S.Q.; Wu, G.S.; Luo, H.R. Aspirin extends the lifespan of Caenorhabditis elegans via AMPK and DAF-16/FOXO in dietary restriction pathway. Exp. Gerontol. 2013, 48, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Strong, R.; Miller, R.A.; Astle, C.M.; Floyd, R.A.; Flurkey, K.; Hensley, K.L.; Javors, M.A.; Leeuwenburgh, C.; Nelson, J.F.; Ongini, E.; et al. Nordihydroguaiaretic acid and aspirin increase lifespan of genetically heterogeneous male mice. Aging Cell 2008, 7, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.-B.; Mu, X.-H.; Wan, Q.-L.; He, X.-M.; Wu, G.-S.; Luo, H.-R. Aspirin increases metabolism through germline signalling to extend the lifespan of Caenorhabditis elegans. PLoS ONE 2017, 12, e0184027. [Google Scholar] [CrossRef] [PubMed]
- Pietrocola, F.; Castoldi, F.; Markaki, M.; Lachkar, S.; Chen, G.; Enot, D.P.; Durand, S.; Bossut, N.; Tong, M.; Malik, S.A.; et al. Aspirin recapitulates features of caloric restriction. Cell Rep. 2018, 22, 2395–2407. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, T.; Kodera, Y.; Hirata, D.; Blackwell, T.K.; Mizunuma, M. Natural thioallyl compounds increase oxidative stress resistance and lifespan in Caenorhabditis elegans by modulating SKN-1/Nrf. Sci. Rep. 2016, 6, 21611. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, M.J. Longevity and heat stress regulation in Caenorhabditis elegans. Mech. Ageing Dev. 2003, 124, 43–48. [Google Scholar] [CrossRef]
- Rea, S.L.; Wu, D.; Cypser, J.R.; Vaupel, J.W.; Johnson, T.E. A stress-sensitive reporter predicts longevity in isogenic populations of Caenorhabditis elegans. Nat. Genet. 2005, 37, 894–898. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.-M.; Xu, Z.-A.; Xu, H.; Luo, T.; Yu, X.-H.; Deng, Z.-J.; Zou, Z.-Q. Method for Preparing Pyrrole. Derivative. Patent CN102180824 A, 14 September 2011. [Google Scholar]
- Barrett, T.N.; Braddock, D.C.; Monta, A.; Webb, M.R.; White, A.J. Total synthesis of the marine metabolite (+/−)-polysiphenol via highly regioselective intramolecular oxidative coupling. J. Nat. Prod. 2011, 74, 1980–1984. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Xiong, C.; Kornfeld, K. Measurements of age-related changes of physiological processes that predict lifespan of Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 2004, 101, 8084–8089. [Google Scholar] [CrossRef] [PubMed]
- Hauso, O.; Martinsen, T.C.; Waldum, H. 5-Aminosalicylic acid, a specific drug for ulcerative colitis. Scand. J. Gastroenterol. 2015, 50, 933–941. [Google Scholar] [CrossRef] [PubMed]
- Douglas, P.M.; Baird, N.A.; Simic, M.S.; Uhlein, S.; McCormick, M.A.; Wolff, S.C.; Kennedy, B.K.; Dillin, A. Heterotypic signals from neural HSF-1 separate thermotolerance from longevity. Cell Rep. 2015, 12, 1196–1204. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.; Hsin, H.; Libina, N.; Kenyon, C. Regulation of the Caenorhabditis elegans longevity protein DAF-16 by insulin/IGF-1 and germline signaling. Nat. Genet. 2001, 28, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Ihara, A.; Uno, M.; Miyatake, K.; Honjoh, S.; Nishida, E. Cholesterol regulates DAF-16 nuclear localization and fasting-induced longevity in C. elegans. Exp. Gerontol. 2017, 87, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zeng, G.; Huang, X.; Wang, Z.; Tan, N. 1,4-Naphthoquinone triggers nematode lethality by inducing oxidative stress and activating Insulin/IGF signaling pathway in Caenorhabditis elegans. Molecules 2017, 22, 798. [Google Scholar] [CrossRef] [PubMed]
- Kenyon, C. The plasticity of aging: Insights from long-lived mutants. Cell 2005, 120, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Tullet, J.M.; Hertweck, M.; An, J.H.; Baker, J.; Hwang, J.Y.; Liu, S.; Oliveira, R.P.; Baumeister, R.; Blackwell, T.K. Direct inhibition of the longevity-promoting factor SKN-1 by insulin-like signaling in C. elegans. Cell 2008, 132, 1025–1038. [Google Scholar] [CrossRef] [PubMed]
- Mark, K.A.; Dumas, K.J.; Bhaumik, D.; Schilling, B.; Davis, S.; Oron, T.R.; Sorensen, D.J.; Lucanic, M.; Brem, R.B.; Melov, S.; et al. Vitamin D promotes protein homeostasis and longevity via the stress response pathway genes skn-1, ire-1, and xbp-1. Cell Rep. 2016, 17, 1227–1237. [Google Scholar] [CrossRef] [PubMed]
- Rathor, L.; Pant, A.; Awasthi, H.; Mani, D.; Pandey, R. An antidiabetic polyherbal phytomedicine confers stress resistance and extends lifespan in Caenorhabditis elegans. Biogerontology 2017, 18, 131–147. [Google Scholar] [CrossRef] [PubMed]
- Asthana, J.; Mishra, B.N.; Pandey, R. Acacetin promotes healthy aging by altering stress response in Caenorhabditis elegans. Free Radic. Res. 2016, 50, 861–874. [Google Scholar] [CrossRef] [PubMed]
- Libina, N.; Berman, J.R.; Kenyon, C. Tissue-specific activities of C. elegans DAF-16 in the regulation of lifespan. Cell 2003, 115, 489–502. [Google Scholar] [CrossRef]
- Hsin, H.; Kenyon, C. Signals from the reproductive system regulate the lifespan of C. elegans. Nature 1999, 399, 362–366. [Google Scholar] [CrossRef] [PubMed]
- Durieux, J.; Wolff, S.; Dillin, A. The cell-non-autonomous nature of electron transport chain-mediated longevity. Cell 2011, 144, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.C.; Dillin, A. XBP-1 is a cell-nonautonomous regulator of stress resistance and longevity. Cell 2013, 153, 1435–1447. [Google Scholar] [CrossRef] [PubMed]
- Prahlad, V.; Cornelius, T.; Morimoto, R.I. Regulation of the cellular heat shock response in Caenorhabditis elegans by thermosensory neurons. Science 2008, 320, 811–814. [Google Scholar] [CrossRef] [PubMed]
- Reinke, V.; Krause, M.; Okkema, P. Transcriptional Regulation of Gene Expression in C. elegans; WormBook: Pasadena, CA, USA, 2013; pp. 1–34. [Google Scholar]
- Walker, G.A.; Lithgow, G.J. Lifespan extension in C. elegans by a molecular chaperone dependent upon insulin-like signals. Aging Cell 2003, 2, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Walker, G.A.; White, T.M.; McColl, G.; Jenkins, N.L.; Babich, S.; Candido, E.P.; Johnson, T.E.; Lithgow, G.J. Heat shock protein accumulation is upregulated in a long-lived mutant of Caenorhabditis elegans. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, B281–B287. [Google Scholar] [CrossRef] [PubMed]
- Ghavami, A.; Nutt, M.P.; Hardy, S.P. Heat shock protein and high-dose aspirin: Effects on random skin flap survival in a rat model. Ann. Plast. Surg. 2002, 48, 60–67. [Google Scholar] [CrossRef] [PubMed]
- An, J.H.; Vranas, K.; Lucke, M.; Inoue, H.; Hisamoto, N.; Matsumoto, K.; Blackwell, T.K. Regulation of the Caenorhabditis elegans oxidative stress defense protein SKN-1 by glycogen synthase kinase-3. Proc. Natl. Acad. Sci. USA 2005, 102, 16275–16280. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, X.-B.; Wu, G.-S.; Ke, L.-Y.; Zhou, X.-G.; Wang, Y.-H.; Luo, H.-R. Aspirin Derivative 5-(Bis(3-methylbut-2-enyl)amino)-2-hydroxybenzoic Acid Improves Thermotolerance via Stress Response Proteins in Caenorhabditis elegans. Molecules 2018, 23, 1359. https://doi.org/10.3390/molecules23061359
Huang X-B, Wu G-S, Ke L-Y, Zhou X-G, Wang Y-H, Luo H-R. Aspirin Derivative 5-(Bis(3-methylbut-2-enyl)amino)-2-hydroxybenzoic Acid Improves Thermotolerance via Stress Response Proteins in Caenorhabditis elegans. Molecules. 2018; 23(6):1359. https://doi.org/10.3390/molecules23061359
Chicago/Turabian StyleHuang, Xiao-Bing, Gui-Sheng Wu, Lei-Yu Ke, Xiao-Gang Zhou, Yue-Hu Wang, and Huai-Rong Luo. 2018. "Aspirin Derivative 5-(Bis(3-methylbut-2-enyl)amino)-2-hydroxybenzoic Acid Improves Thermotolerance via Stress Response Proteins in Caenorhabditis elegans" Molecules 23, no. 6: 1359. https://doi.org/10.3390/molecules23061359



