Effect on White Grape Must of Multiflora Bee Pollen Addition during the Alcoholic Fermentation Process
Abstract
:1. Introduction
2. Results
2.1. Influence of Bee Pollen Addition on Grape Must Composition
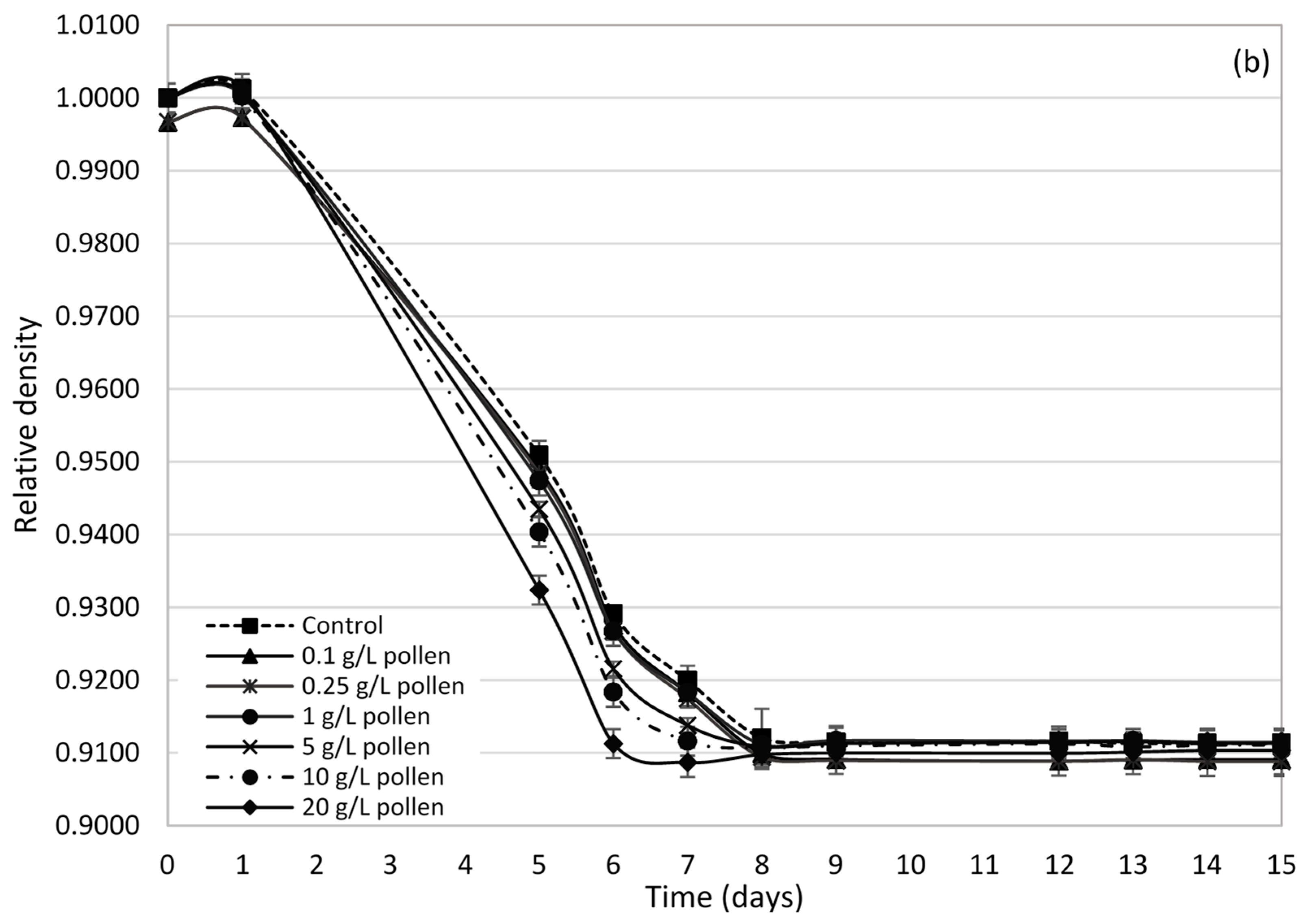
2.2. Influence of Bee Pollen Values on Alcoholic Fermentation Kinetics
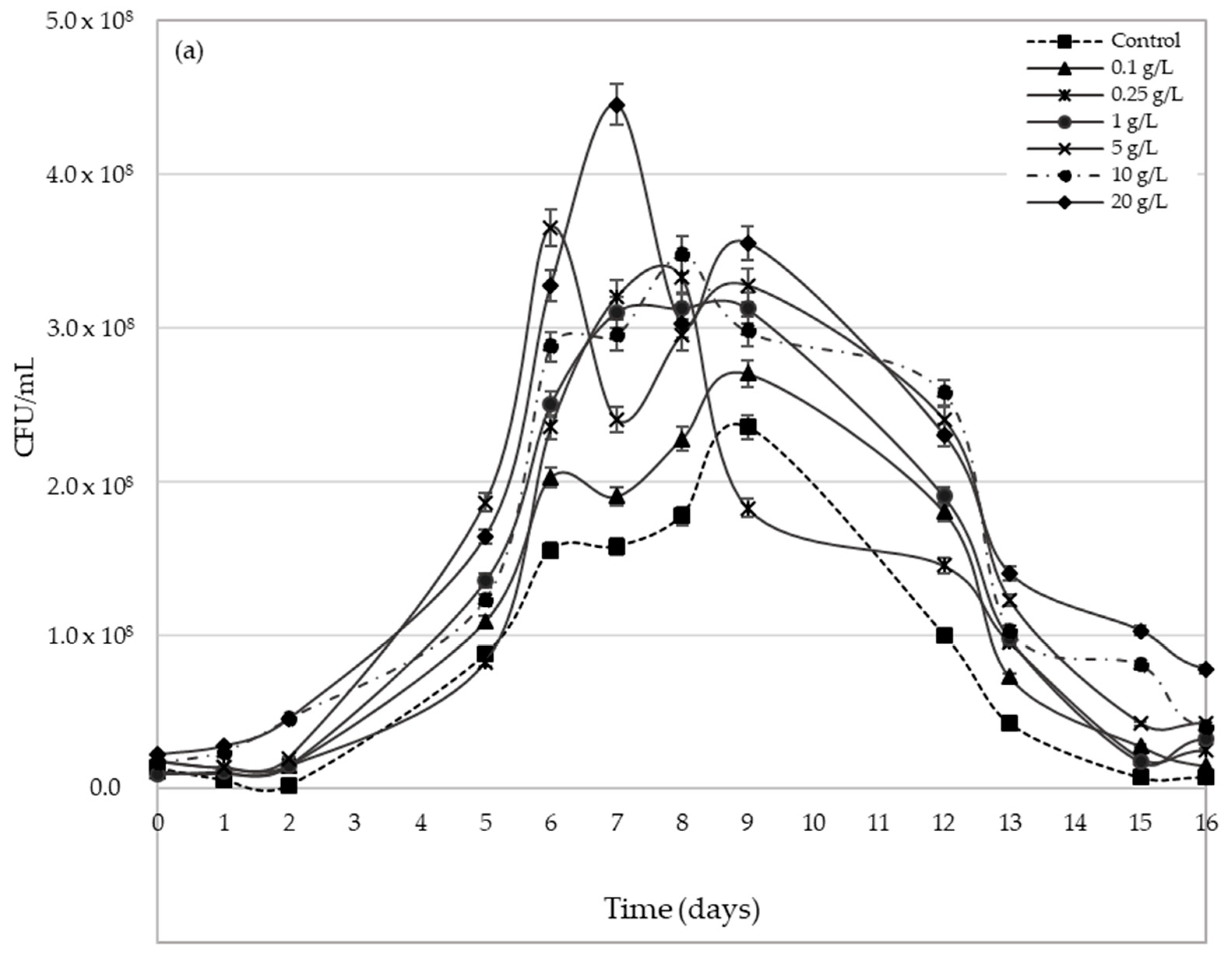
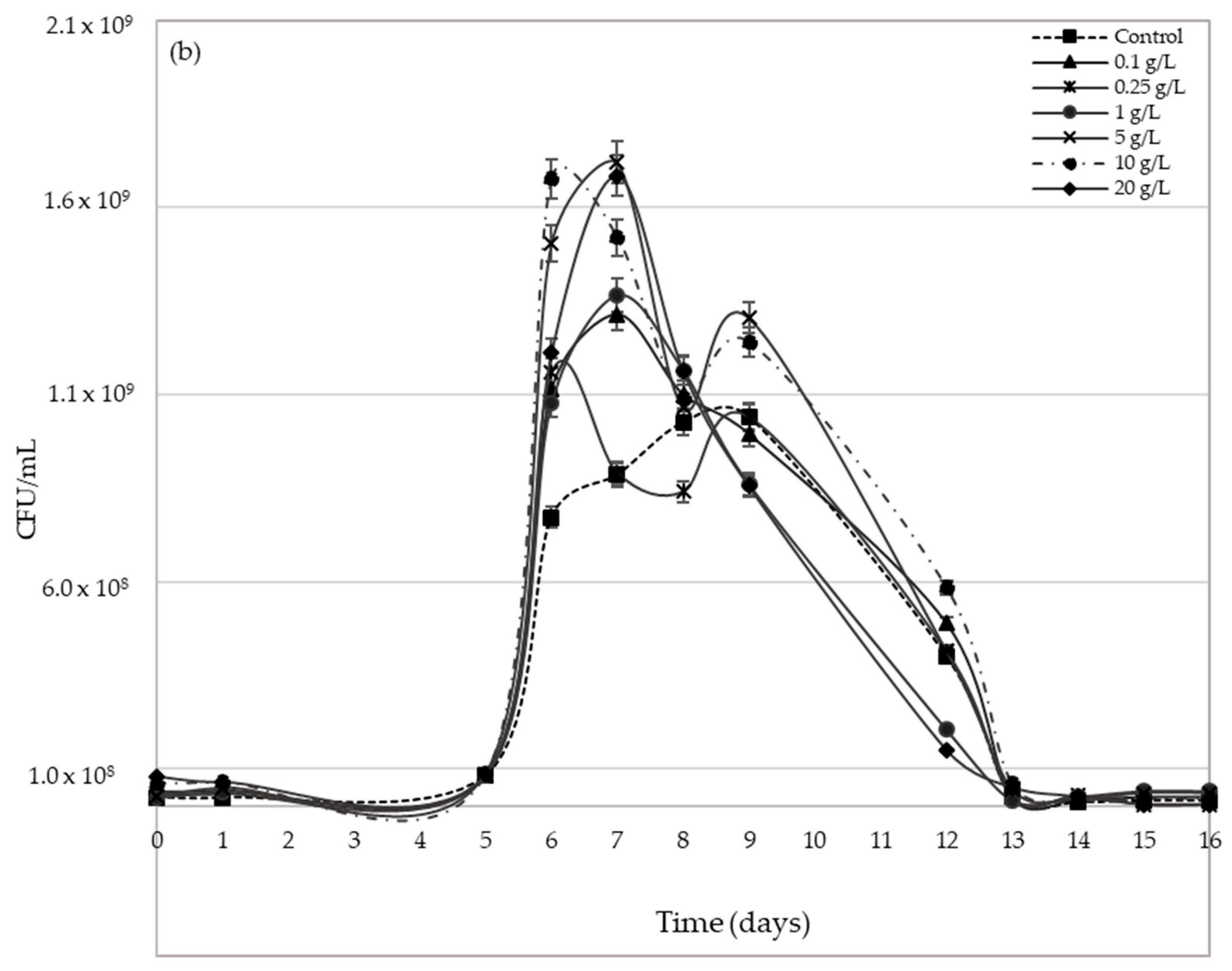
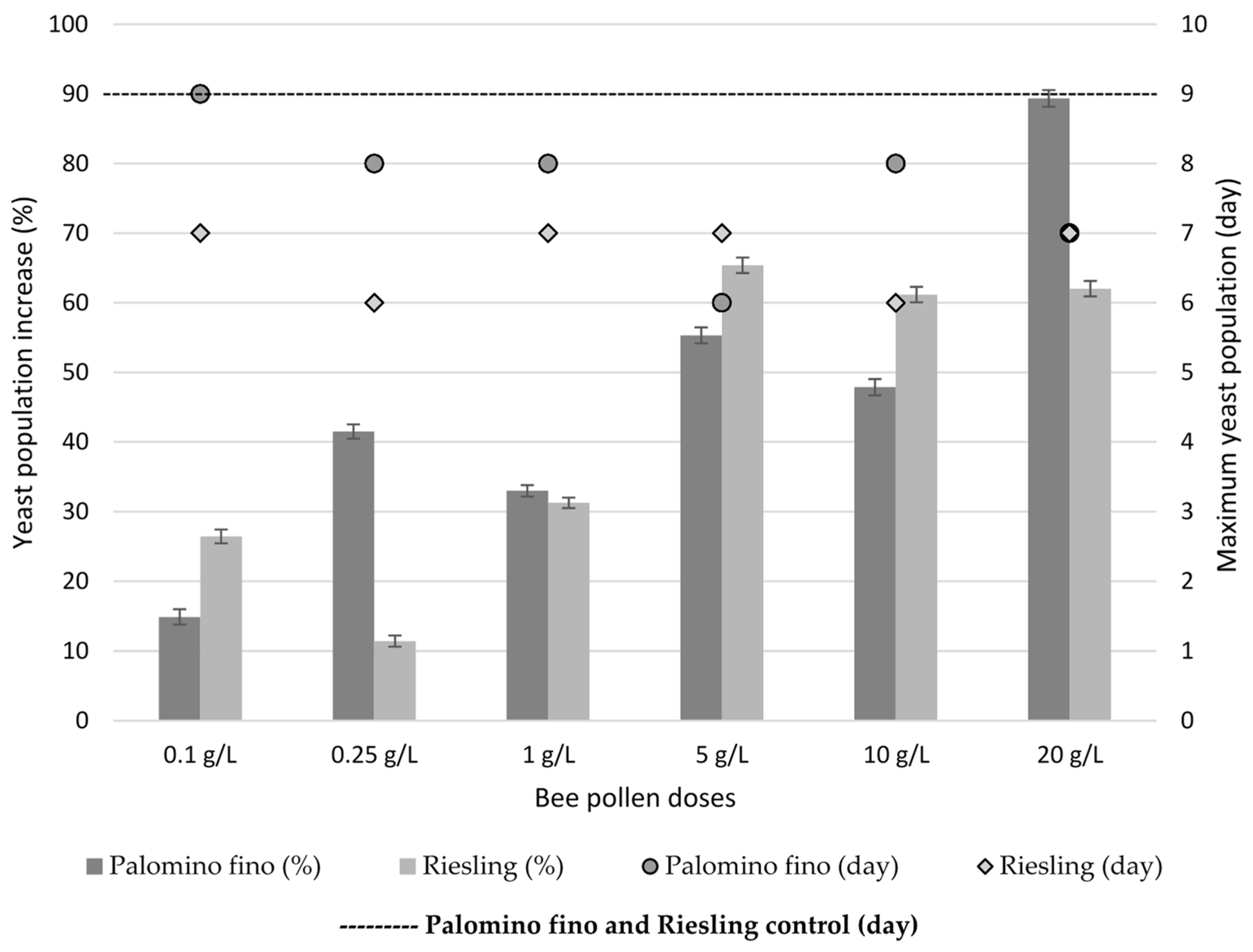
2.3. Yeast Population Development during AF
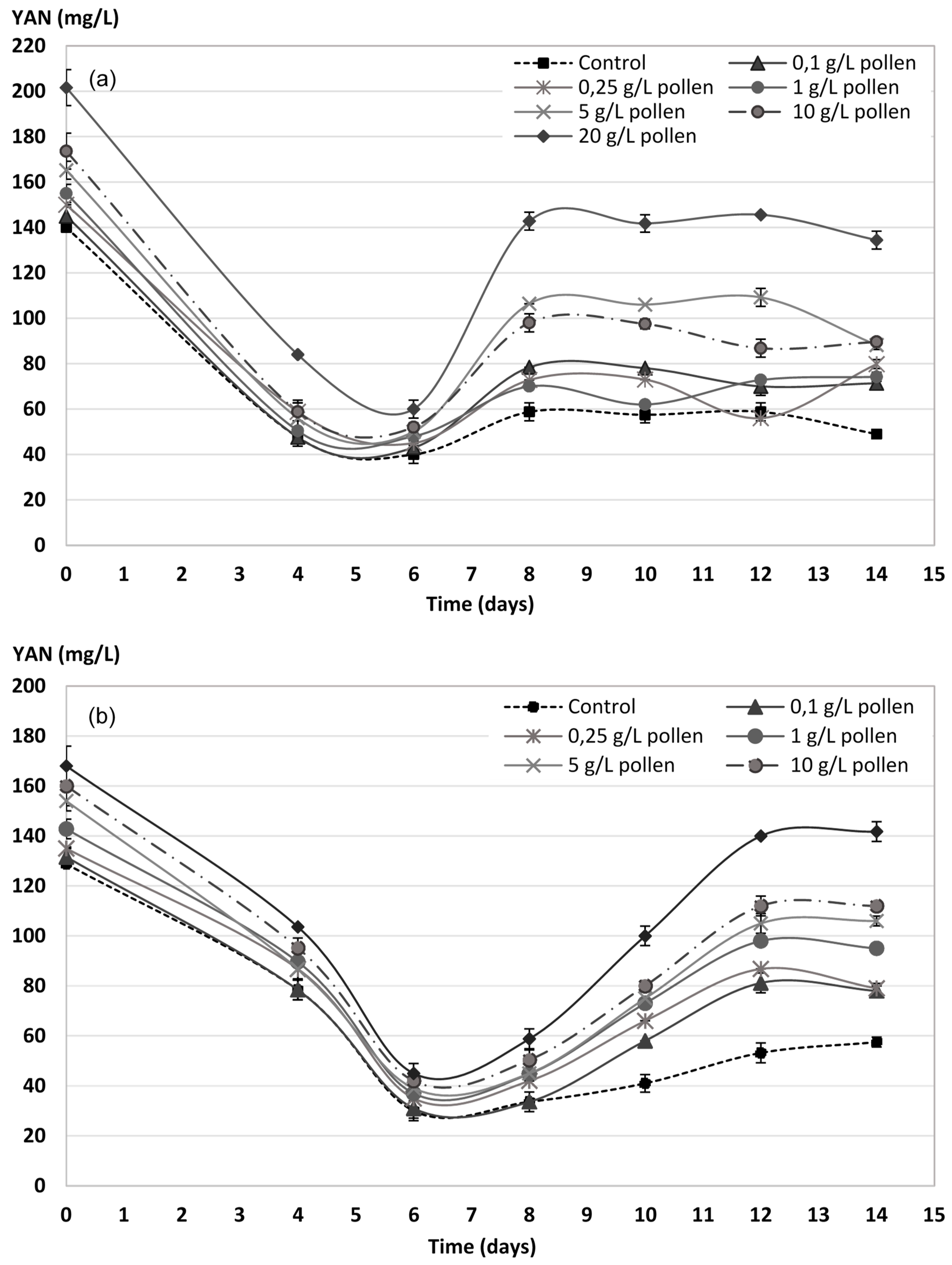
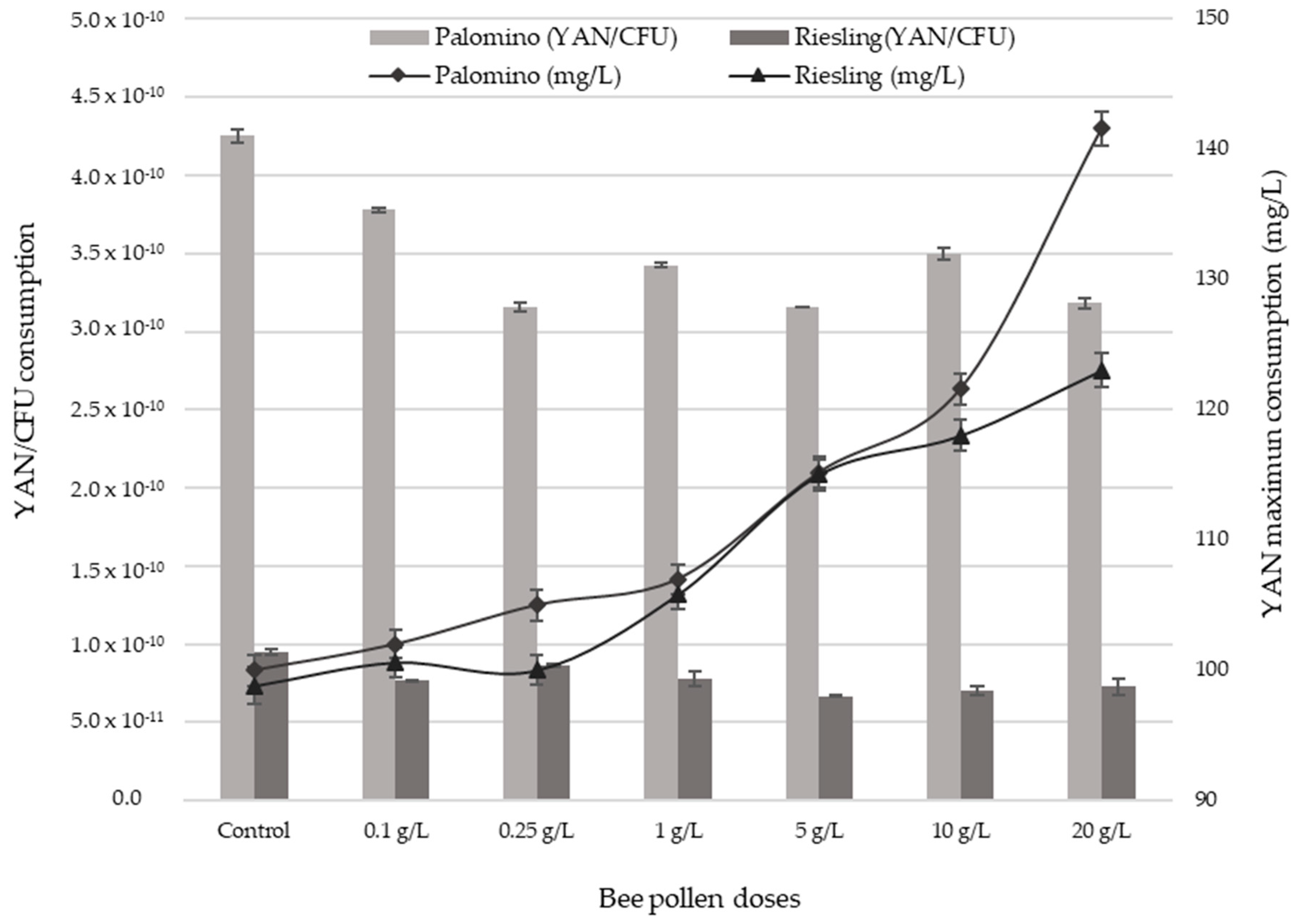
2.4. YAN Evolution during AF
2.5. Physico-Chemical and Colour Parameters in Final Wines
3. Discussion
4. Materials and Methods
4.1. Winemaking Conditions
4.2. Experimental Layout
4.3. Analytical Measurements
4.4. Yeast (Saccharomyces cerevisiae)
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Suárez-Lepe, J.A. Levaduras Vínicas. Funcionalidad y uso en Bodega; Mundi-Prensa: Madrid, Spain, 1997; pp. 110–111. ISBN 8471146851. [Google Scholar]
- Bisson, L.F. Stuck and sluggish fermentations. Am. J. Enol. Vitic. 1999, 50, 107–119. [Google Scholar]
- Fleet, G.H. Wine Microbiology and Biotechnology; CRC Press: Boca Raton, FL, USA, 1993; ISBN 0415278503. [Google Scholar]
- Webb, L.B.; Whetton, P.H.; Barlow, E.W.R. Modelled impact of future climate change on the phenology of winegrapes in Australia. Aust. J. Grape Wine Res. 2007, 13, 165–175. [Google Scholar] [CrossRef]
- Briche, E.; Beltrando, G.; Somot, S.; Quenol, H. Critical analysis of simulated daily temperature data from the ARPEGE-climate model: Application to climate change in the Champagne wine-producing region. Clim. Chang. 2014, 123, 241–254. [Google Scholar] [CrossRef]
- Sacchelli, S.; Fabbrizzi, S.; Menghini, S. Climate change effects and adaptation strategies in the wine sector: A quantitative literature review. Wine Econ. Policy 2016, 5, 114–126. [Google Scholar] [CrossRef]
- Jones, G.V.; White, M.A.; Cooper, O.R.; Storchmann, K. Climate change and global wine quality. Clim. Chang. 2005, 73, 319–343. [Google Scholar] [CrossRef]
- Bely, M.; Sablayrolles, J.M.; Barre, P. Description of alcoholic fermentation kinetics: Its variability and significance. Am. J. Enol. Vitic. 1990, 41, 319–324. [Google Scholar]
- Ough, C.; Davenport, M.; Joseph, K. Effects of Certain Vitamins on Growth and Fermentation Rate of Several Commercial Active Dry Wine Yeasts. Am. J. Enol. Vitic. 1989, 40, 208–213. [Google Scholar]
- Bataillon, M.; Rico, A.; Sablayrolles, J.M.; Salmon, J.M.; Barre, P. Early thiamin assimilation by yeasts under enological conditions: Impact on alcoholic fermentation kinetics. J. Ferment. Bioeng. 1996, 82, 145–150. [Google Scholar] [CrossRef]
- Reboredo-Rodríguez, P.; González-Barreiro, C.; Rial-Otero, R.; Cancho-Grande, B.; Simal-Gándara, J. Effects of Sugar Concentration Processes in Grapes and Wine Aging on Aroma Compounds of Sweet Wines—A Review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1051–1071. [Google Scholar] [CrossRef] [PubMed]
- Mira de Orduña Heidinger, R. Climate change associated effects on grape and wine quality and production. Food Res. Int. 2010, 7, 1844–1855. [Google Scholar] [CrossRef]
- González-Barreiro, C.; Rial-Otero, R.; Cancho-Grande, B.; Simal-Gándara, J. Wine aroma compounds in grapes: A critical review. Crit. Rev. Food Sci. Nutr. 2015, 55, 202–218. [Google Scholar] [CrossRef] [PubMed]
- Henschke, P.A.; Jiranek, V. Metabolism of nitrogen compounds. Wine Microbiol. Biotechnol. 1993, 77–164. [Google Scholar]
- Barre, P.; Blondin, P.; Dequin, S.; Feuillat, M.; Sablayrolles, J.-M.; Salmon, J.-M. La levure de Fermentation Alcoolique; Oenologie Technique et Documentation; Lavoisier: Paris, France, 1998; pp. 414–495. [Google Scholar]
- Bell, S.-J.; Henschke, P. Implications of nitrogen nutrition for grapes, fermentation and wine. Aust. J. Grape Wine Res. 2005, 11, 242–295. [Google Scholar] [CrossRef]
- Ribéreau-Gayon, P.; Dubourdieu, D.; Donéche, B.; Lonvaud, A. Tratado de Enologia: Microbiología del vino. Vinificaciones; Hemisferio Sur: Buenos Aires, Argentina, 2003. [Google Scholar]
- Gutiérrez, A.; Chiva, R.; Sancho, M.; Beltran, G.; Arroyo-López, F.N.; Guillamon, J.M. Nitrogen requirements of commercial wine yeast strains during fermentation of a synthetic grape must. Food Microbiol. 2012, 31, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, P.; Roldán, A.; Caro, I.; Pérez, L. Kinetic study of the velum formation by Saccharomyces cerevisiae (beticus ssp.) during the biological aging of wines. Process Biochem. 2010, 45, 493–499. [Google Scholar] [CrossRef]
- Albers, E.; Larsson, C.; Lidén, G.; Niklasson, C.; Gustafsson, L. Influence of the nitrogen source on Saccharomyces cerevisiae anaerobic growth and product formation. Appl. Environ. Microbiol. 1996, 62, 3187–3195. [Google Scholar] [PubMed]
- Garde-Cerdán, T.; Ancín-Azpilicueta, C. Effect of the addition of different quantities of amino acids to nitrogen-deficient must on the formation of esters, alcohols, and acids during wine alcoholic fermentation. LWT Food Sci. Technol. 2008, 41, 501–510. [Google Scholar] [CrossRef]
- Hernández-Orte, P.; Ibarz, M.J.; Cacho, J.; Ferreira, V. Effect of the addition of ammonium and amino acids to musts of Airen variety on aromatic composition and sensory properties of the obtained wine. Food Chem. 2005, 89, 163–174. [Google Scholar] [CrossRef]
- Arias-Gil, M.; Garde-Cerdán, T.; Ancín-Azpilicueta, C. Influence of addition of ammonium and different amino acid concentrations on nitrogen metabolism in spontaneous must fermentation. Food Chem. 2007, 103, 1312–1318. [Google Scholar] [CrossRef]
- Kotarska, K.; Czupryński, B.; Kłosowski, G. Effect of various activators on the course of alcoholic fermentation. J. Food Eng. 2006, 77, 965–971. [Google Scholar] [CrossRef]
- Almeida-Muradian, L.B.; Pamplona, L.C.; Coimbra, S.; Barth, O.M. Chemical composition and botanical evaluation of dried bee pollen pellets. J. Food Compos. Anal. 2005, 18, 105–111. [Google Scholar] [CrossRef]
- Human, H.; Nicolson, S.W. Nutritional content of fresh, bee-collected and stored pollen of Aloe greatheadii var. davyana (Asphodelaceae). Phytochemistry 2006, 67, 1486–1492. [Google Scholar] [CrossRef] [PubMed]
- Paramás, A.M.G.; Bárez, J.A.G.; Marcos, C.C.; García-Villanova, R.J.; Sánchez, J.S. HPLC-fluorimetric method for analysis of amino acids in products of the hive (honey and bee-pollen). Food Chem. 2006, 95, 148–156. [Google Scholar] [CrossRef]
- Xu, X.; Sun, L.; Dong, J.; Zhang, H. Breaking the cells of rape bee pollen and consecutive extraction of functional oil with supercritical carbon dioxide. Innov. Food Sci. Emerg. Technol. 2009, 10, 42–46. [Google Scholar] [CrossRef]
- Campos, M.G.R.; Bogdanov, S.; de Almeida-Muradian, L.B.; Szczesna, T.; Mancebo, Y.; Frigerio, C.; Ferreira, F. Pollen composition and standardisation of analytical methods. J. Apic. Res. 2008, 47, 154–161. [Google Scholar] [CrossRef]
- Roldán, A.; Van Muiswinkel, G.C.J.; Lasanta, C.; Palacios, V.; Caro, I. Influence of pollen addition on mead elaboration: Physicochemical and sensory characteristics. Food Chem. 2011, 126, 574–582. [Google Scholar] [CrossRef]
- Roldán, A.; van Muiswinkel, G.C.J.; Lasanta, C.; Caro, I. Effect of the Addition of Fermentative Activators on Honey-Wine (Mead) Fermentation. In Proceedings of the 18th International Congress of Chemical and Process Engineering, Praha, Praha, Czech Republic, 24–28 August 2008. [Google Scholar]
- Amores-Arrocha, A.; Roldán, A.; Jiménez-Cantizano, A.; Caro, I.; Palacios, V. Evaluation of the use of multiflora bee pollen on the volatile compounds and sensorial profile of Palomino fino and Riesling white young wines. Food Res. Int. 2018, 105, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Torija, M.J.; Beltran, G.; Novo, M.; Poblet, M.; Rozès, N.; Mas, A.; Guillamón, J.M. Effect of organic acids and nitrogen source on alcoholic fermentation: Study of their buffering capacity. J. Agric. Food Chem. 2003, 51, 916–922. [Google Scholar] [CrossRef] [PubMed]
- Ter Schure, E.G.; Van Riel, N.A.W.; Verrips, C.T. The role of ammonia metabolism in nitrogen catabolite repression in Saccharomyces cerevisiae. FEMS Microbiol. Rev. 2000, 24, 67–83. [Google Scholar] [CrossRef] [PubMed]
- Arroyo-López, F.N.; Querol, A.; Barrio, E. Application of a substrate inhibition model to estimate the effect of fructose concentration on the growth of diverse Saccharomyces cerevisiae strains. J. Ind. Microbiol. Biotechnol. 2009, 36, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Szczêsna, T. Study on the Sugar Composition of Honeybee-collected Pollen. J. Apic. Sci. 2007, 51, 15–22. [Google Scholar]
- Oliveira, C.M.; Ferreira, A.C.S.; De Freitas, V.; Silva, A.M.S. Oxidation mechanisms occurring in wines. Food Res. Int. 2011, 44, 1115–1126. [Google Scholar] [CrossRef]
- Lasanta, C.; Roldán, A.; Caro, I.; Pérez, L.; Palacios, V. Use of lysozyme for the prevention and treatment of heterolactic fermentation in the biological aging of sherry wines. Food Control 2010, 21, 1442–1447. [Google Scholar] [CrossRef]
- Alexandre, H.; Plourde, L.; Charpentier, C.; François, J. Lack of correlation between trehalose accumulation, cell viability and intracellular acidification as induced by various stresses in Saccharomyces cerevisiae. Microbiology 1998, 144, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Bisson, L.F.; Butzke, C.E. Diagnosis and rectification of stuck and sluggish fermentations. Am. J. Enol. Vitic. 2000, 51, 168–177. [Google Scholar]
- Rabie, A.L.; Wells, J.D.; Dent, L.K. The Nitrogen Content of Pollen Protein. J. Apic. Res. 1983, 22, 119–123. [Google Scholar] [CrossRef]
- Sroka, P.; Tuszyński, T. Changes in organic acid contents during mead wort fermentation. Food Chem. 2007, 104, 1250–1257. [Google Scholar] [CrossRef]
- Dizy, M.; Polo, M.C. Changes in concentration of nitrogenous compounds during fermentation of white grape musts at pilot plant scale. Food Sci. Technol. Int. 1996, 2, 87–93. [Google Scholar] [CrossRef]
- Camarasa, C.; Moutounet, M.; Salmon, J.M. New trends on yeast autolysis and wine ageing on lees: A bibliographical review. Int. J. Vine Wine Sci. 2001, 35. [Google Scholar] [CrossRef]
- Moreno-Arribas, M.V.; Polo, M.C. Wine Chemistry and Biochemistry; Springer: Berlin, Germany, 2009; ISBN 9780387741161. [Google Scholar]
- Alexandre, H.; Guilloux-Benatier, M. Yeast autolysis in sparkling wine—A review. Aust. J. Grape Wine Res. 2006, 12, 119–127. [Google Scholar] [CrossRef]
- Komosinska-Vassev, K.; Olczyk, P.; Kaźmierczak, J.; Mencner, L.; Olczyk, K. Bee pollen: Chemical composition and therapeutic application. Evid.-Based Complement. Altern. Med. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- OIV Office International de la Vigne; du Vin. Recueil des Méthodes Internationales D’analyse Des Vins Et Des Moûts; Édition Officielle: Paris, France, 1990. [Google Scholar]
- Aerny, J. Composés azotés des moûts et des vins. Rev. Suisse Vitic. Arboric. Hortic. 1997, 28, 161–168. [Google Scholar]
- Almela, L.; Javaloy, S.; Fernández-López, J.A.; López-Roca, J.M. Varietal classification of young red wines in terms of chemical and colour parameters. J. Sci. Food Agric. 1996, 70, 173–180. [Google Scholar] [CrossRef]
- Heredia, F.J.; Troncoso, A.M.; Guzmán-Chozas, M. Multivariate characterization of aging status in red wines based on chromatic parameters. Food Chem. 1997, 60, 103–108. [Google Scholar] [CrossRef]
- Pérez-Magariño, S.; González-San José, M.L. Polyphenols and colour variability of red wines made from grapes harvested at different ripeness grade. Food Chem. 2006, 96, 197–208. [Google Scholar] [CrossRef]
Sample Availability: Not available. |


| Parameters | Palomino Fino Grape Must Bee Pollen Doses | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | 0.1 g/L | 0.25 g/L | 1 g/L | 5 g/L | 10 g/L | 20 g/L | |||||||||||||||
| °Bè | 10.03 | ± | 0.10 a,b | 9.70 | ± | 0.02 a | 9.95 | ± | 0.04 a | 10.00 | ± | 0.03 a | 10.05 | ± | 0.05 a,b | 10.10 | ± | 0.05 a,b | 10.55 | ± | 0.03 b |
| Density (g/cm3) at 20 °C | 1.070 | ± | 0.000 a | 1.070 | ± | 0.000 a | 1.070 | ± | 0.000 a | 1.070 | ± | 0.000 a | 1.071 | ± | 0.000 a | 1.071 | ± | 0.000 a | 1.073 | ± | 0.000 a |
| Sugars (g/L) | 165.17 | ± | 0.64 a | 159.30 | ± | 0.37 a | 163.70 | ± | 0.34 a | 164.80 | ± | 0.25 a | 164.80 | ± | 0.21 a | 168.10 | ± | 0.18 a,b | 175.67 | ± | 0.58 b |
| Total acidity (g/L TH2) | 7.09 | ± | 0.07 a,b,c | 6.87 | ± | 0.12 a | 6.97 | ± | 0.03 a,b | 7.22 | ± | 0.07 a,b,c | 7.24 | ± | 0.05 a,b,c | 7.26 | ± | 0.05 b,c | 7.46 | ± | 0.10 c |
| pH | 3.11 | ± | 0.01 a | 3.10 | ± | 0.01 a | 3.10 | ± | 0.01 a | 3.11 | ± | 0.01 a | 3.11 | ± | 0.01 a | 3.11 | ± | 0.01 a | 3.18 | ± | 0.01 a |
| Free SO2 | 24 | ± | 1 a | 23 | ± | 1 a | 24 | ± | 1 a | 24 | ± | 1 a | 24 | ± | 1 a | 24 | ± | 2 a | 27 | ± | 1 b |
| Total SO2 | 70 | ± | 2 a | 68 | ± | 1 a | 70 | ± | 2 a | 70 | ± | 2 a | 70 | ± | 1 a | 70 | ± | 3 a | 74 | ± | 1 b |
| YAN (mg/L) | 140 | ± | 1 a | 144 | ± | 3 a,b,c | 151 | ± | 0 b,c | 154 | ± | 2 c | 165 | ± | 4 d | 184 | ± | 8 e | 202 | ± | 8 f |
| Parameters | Riesling Grape Must Bee Pollen Doses | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | 0.1 g/L | 0.25 g/L | 1 g/L | 5 g/L | 10 g/L | 20 g/L | |||||||||||||||
| °Bè | 11.07 | ± | 0.06 a | 10.87 | ± | 0.06 a | 11.02 | ± | 0.06 a | 11.27 | ± | 0.06 a | 11.27 | ± | 0.01 a | 11.37 | ± | 0.06 a | 11.62 | ± | 0.06 a |
| Density (g/cm3) at 20 °C | 1.081 | ± | 0.000 a | 1.081 | ± | 0.000 a | 1.080 | ± | 0.000 a | 1.082 | ± | 0.000 a | 1.082 | ± | 0.000 a | 1.083 | ± | 0.000 a | 1.084 | ± | 0.000 a |
| Sugars (g/L) | 189.00 | ± | 0.00 a | 189.70 | ± | 0.00 a | 187.40 | ± | 0.00 a | 191.80 | ± | 0.00 a,b | 191.80 | ± | 0.00 a,b | 195.13 | ± | 0.06 a,b | 204.83 | ± | 0.06 b |
| Total acidity (g/L TH2) | 4.83 | ± | 0.24 a | 4.80 | ± | 0.26 a | 4.91 | ± | 0.21 a | 4.89 | ± | 0.24 a | 4.99 | ± | 0.23 a | 4.81 | ± | 0.22 a | 4.76 | ± | 0.21 a |
| pH | 3.34 | ± | 0.01 a | 3.34 | ± | 0.01 a | 3.34 | ± | 0.01 a | 3.34 | ± | 0.00 a | 3.35 | ± | 0.00 a | 3.33 | ± | 0.01 a | 3.33 | ± | 0.01 a |
| Free SO2 | 17 | ± | 0 a | 17 | ± | 1 a | 17 | ± | 1 a | 18 | ± | 1 a | 17 | ± | 1 a | 17 | ± | 1 a | 17 | ± | 1 a |
| Total SO2 | 60 | ± | 2 a | 62 | ± | 1 a | 61 | ± | 1 a | 60 | ± | 1 a | 61 | ± | 1 a | 60 | ± | 2 a | 61 | ± | 1 a |
| YAN (mg/L) | 128 | ± | 0 a | 132 | ± | 1 a,b | 140 | ± | 1 b,c | 146 | ± | 4 c,d | 154 | ± | 2 d,e | 160 | ± | 3 e,f | 168 | ± | 3 f |
| Parameters | Palomino Fino Wines Bee Pollen Doses | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | 0.1 g/L | 0.25 g/L | 1 g/L | 5 g/L | 10 g/L | 20 g/L | |||||||||||||||
| % Alcohol v/v | 9.81 | ± | 0.03 a | 9.78 | ± | 0.01 a | 9.72 | ± | 0.01 a | 9.73 | ± | 0.02 a | 9.52 | ± | 0.02 a | 9.91 | ± | 0.02 a | 10.08 | ± | 0.02 a |
| pH | 2.99 | ± | 0.01 a | 3.01 | ± | 0.02 a | 3.01 | ± | 0.01 a | 3.01 | ± | 0.01 a | 3.04 | ± | 0.01 a | 3.04 | ± | 0.01 a | 3.13 | ± | 0.01 a |
| Total acidity (g/L) | 6.24 | ± | 0.16 a | 6.01 | ± | 0.03 a | 6.08 | ± | 0.01 a | 6.27 | ± | 0.03 a | 6.44 | ± | 0.02 a | 6.21 | ± | 0.02 a | 5.97 | ± | 0.02 a |
| Volatile acidity (g/L) | 0.10 | ± | 0.01 a | 0.11 | ± | 0.01 a | 0.10 | ± | 0.01 a | 0.10 | ± | 0.01 a | 0.10 | ± | 0.01 a | 0.15 | ± | 0.01 b | 0.24 | ± | 0.01 c |
| Glucose (g/L) | 0.214 | ± | 0.016 a | 0.187 | ± | 0.017 b | 0.190 | ± | 0.020 b | 0.170 | ± | 0.020 c | 0.110 | ± | 0.001 d | 0.110 | ± | 0.001 d | 0.130 | ± | 0.002 e |
| Fructose (g/L) | 0.468 | ± | 0.019 a | 0.194 | ± | 0.007 b | 0.190 | ± | 0.010 b | 0.380 | ± | 0.040 c | 0.420 | ± | 0.002 d | 0.370 | ± | 0.001 c | 0.150 | ± | 0.010 e |
| L* | 98.97 | ± | 0.04 a | 98.85 | ± | 0.16 a | 98.79 | ± | 0.07 a | 98.63 | ± | 0.10 a | 98.51 | ± | 0.08 a | 98.47 | ± | 0.06 a | 97.84 | ± | 0.05 a |
| a* | 1.02 | ± | 0.03 a | 1.06 | ± | 0.03 a | 1.07 | ± | 0.02 a,b | 1.16 | ± | 0.03 b | 1.28 | ± | 0.01 c,d | 1.35 | ± | 0.02 d | 1.65 | ± | 0.02 e |
| b* | 5.92 | ± | 0.04 a | 6.05 | ± | 0.08 a | 6.17 | ± | 0.03 a | 6.86 | ± | 0.05 b | 7.50 | ± | 0.07 c,d | 7.83 | ± | 0.01 d | 10.09 | ± | 0.01 e |
| H* | 99.71 | ± | 0.32 a | 99.92 | ± | 0.40 a | 99.57 | ± | 0.15 a | 99.55 | ± | 0.25 a | 99.64 | ± | 0.04 a | 99.81 | ± | 0.12 a | 99.28 | ± | 0.08 a |
| C* | 6.01 | ± | 0.04 a | 6.14 | ± | 0.07 a | 6.47 | ± | 0.03 a,b | 6.96 | ± | 0.05 b | 7.61 | ± | 0.07 c,d | 7.95 | ± | 0.01 d | 10.22 | ± | 0.02 e |
| Color Intensity | 0.11 | ± | 0.00 a | 0.11 | ± | 0.01 a | 0.12 | ± | 0.01 a | 0.14 | ± | 0.00 b | 0.16 | ± | 0.01 c | 0.18 | ± | 0.01 d | 0.21 | ± | 0.01 e |
| Abs. 420 | 0.09 | ± | 0.00 a | 0.09 | ± | 0.01 a | 0.10 | ± | 0.01 a,b | 0.11 | ± | 0.00 b,c | 0.12 | ± | 0.00 c,d | 0.13 | ± | 0.00 d | 0.17 | ± | 0.01 e |
| Parameters | Riesling Grape Wines Bee Pollen Doses | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | 0.1 g/L | 0.25 g/L | 1 g/L | 5 g/L | 10 g/L | 20 g/L | |||||||||||||||
| % Alcohol v/v | 12.47 | ± | 0.15 a | 12.67 | ± | 0.05 a | 12.67 | ± | 0.05 a | 12.63 | ± | 0.05 a | 12.57 | ± | 0.05 a | 12.70 | ± | 0.10 a | 12.77 | ± | 0.05 a |
| pH | 3.39 | ± | 0.01 a | 3.39 | ± | 0.01 a | 3.39 | ± | 0.01 a | 3.38 | ± | 0.01 a | 3.43 | ± | 0.01 a | 3.47 | ± | 0.05 a | 3.57 | ± | 0.02 a |
| Total acidity (g/L) | 3.98 | ± | 0.03 a | 3.94 | ± | 0.07 a | 4.06 | ± | 0.03 a | 4.04 | ± | 0.08 a | 4.09 | ± | 0.08 a | 3.96 | ± | 0.01 a | 3.90 | ± | 0.01 a |
| Volatile acidity (g/L) | 0.25 | ± | 0.01 a | 0.23 | ± | 0.01 b | 0.23 | ± | 0.01 b | 0.18 | ± | 0.00 c | 0.13 | ± | 0.01 d | 0.28 | ± | 0.01 e | 0.32 | ± | 0.01 f |
| Glucose (g/L) | 0.028 | ± | 0.001 a | 0.194 | ± | 0.003 b | 0.207 | ± | 0.003 c | 0.228 | ± | 0.002 d | 0.227 | ± | 0.000 d | 0.246 | ± | 0.002 d | 0.251 | ± | 0.002 d |
| Fructose (g/L) | 0.083 | ± | 0.004 a | 0.436 | ± | 0.005 b,c | 0.432 | ± | 0.003 c | 0.395 | ± | 0.002 d | 0.264 | ± | 0.002 e | 0.259 | ± | 0.000 e | 0.252 | ± | 0.024 e |
| L* | 98.98 | ± | 0.02 a | 99.16 | ± | 0.01 a | 99.19 | ± | 0.03 a | 99.16 | ± | 0.03 a | 98.69 | ± | 0.07 a | 97.49 | ± | 0.01 a | 97.52 | ± | 0.04 a |
| a* | 0.59 | ± | 0.02 a | 0.53 | ± | 0.01 a | 0.54 | ± | 0.02 a | 0.75 | ± | 0.02 b | 0.91 | ± | 0.01 c | 1.26 | ± | 0.01 d | 2.03 | ± | 0.01 e |
| b* | 4.10 | ± | 0.02 a | 4.20 | ± | 0.01 a | 4.30 | ± | 0.01 a | 4.95 | ± | 0.02 b | 6.54 | ± | 0.05 c | 8.58 | ± | 0.02 d | 12.74 | ± | 0.02 e |
| H* | 97.44 | ± | 0.29 a | 97.25 | ± | 0.25 a | 97.7 | ± | 0.36 a | 97.81 | ± | 0.26 a | 97.9 | ± | 0.16 a | 98.4 | ± | 0.08 a | 99.1 | ± | 0.08 a |
| C* | 4.53 | ± | 0.02 a | 4.24 | ± | 0.01 a | 4.39 | ± | 0.01 a | 4.80 | ± | 0.03 a | 6.61 | ± | 0.05 b | 8.58 | ± | 0.02 c | 12.9 | ± | 0.02 d |
| Color Intensity | 0.08 | ± | 0.01 a | 0.07 | ± | 0.01 a | 0.07 | ± | 0.01 a | 0.10 | ± | 0.01 b | 0.12 | ± | 0.01 c | 0.19 | ± | 0.01 d | 0.25 | ± | 0.01 e |
| Abs. 420 | 0.06 | ± | 0.01 a | 0.06 | ± | 0.01 b,c | 0.06 | ± | 0.01 c | 0.10 | ± | 0.01 d,e | 0.10 | ± | 0.01 e | 0.14 | ± | 0.01 f | 0.21 | ± | 0.01 g |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amores-Arrocha, A.; Roldán, A.; Jiménez-Cantizano, A.; Caro, I.; Palacios, V. Effect on White Grape Must of Multiflora Bee Pollen Addition during the Alcoholic Fermentation Process. Molecules 2018, 23, 1321. https://doi.org/10.3390/molecules23061321
Amores-Arrocha A, Roldán A, Jiménez-Cantizano A, Caro I, Palacios V. Effect on White Grape Must of Multiflora Bee Pollen Addition during the Alcoholic Fermentation Process. Molecules. 2018; 23(6):1321. https://doi.org/10.3390/molecules23061321
Chicago/Turabian StyleAmores-Arrocha, Antonio, Ana Roldán, Ana Jiménez-Cantizano, Ildefonso Caro, and Víctor Palacios. 2018. "Effect on White Grape Must of Multiflora Bee Pollen Addition during the Alcoholic Fermentation Process" Molecules 23, no. 6: 1321. https://doi.org/10.3390/molecules23061321






