Characterization of Goat Milk Hydrolyzed by Cell Envelope Proteinases from Lactobacillus plantarum LP69: Proteolytic System Optimization, Bioactivity, and Storage Stability Evaluation
Abstract
:1. Introduction
2. Results
2.1. Optimization of Proteolytic Conditions of Goat Milk Catalyzed by CEP
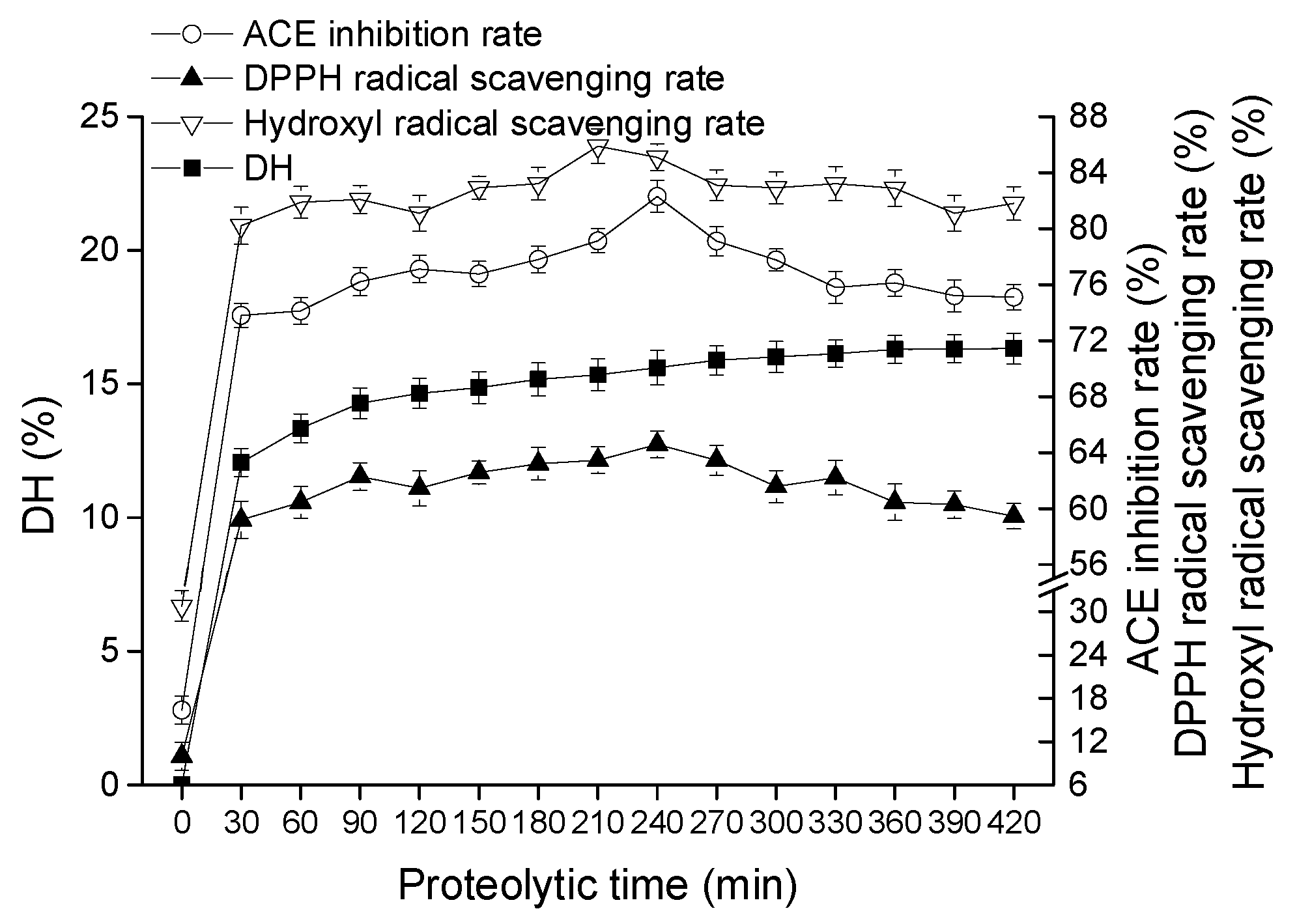
2.1.1. Effect of Proteolytic Time on Goat Milk Catalyzed by CEP
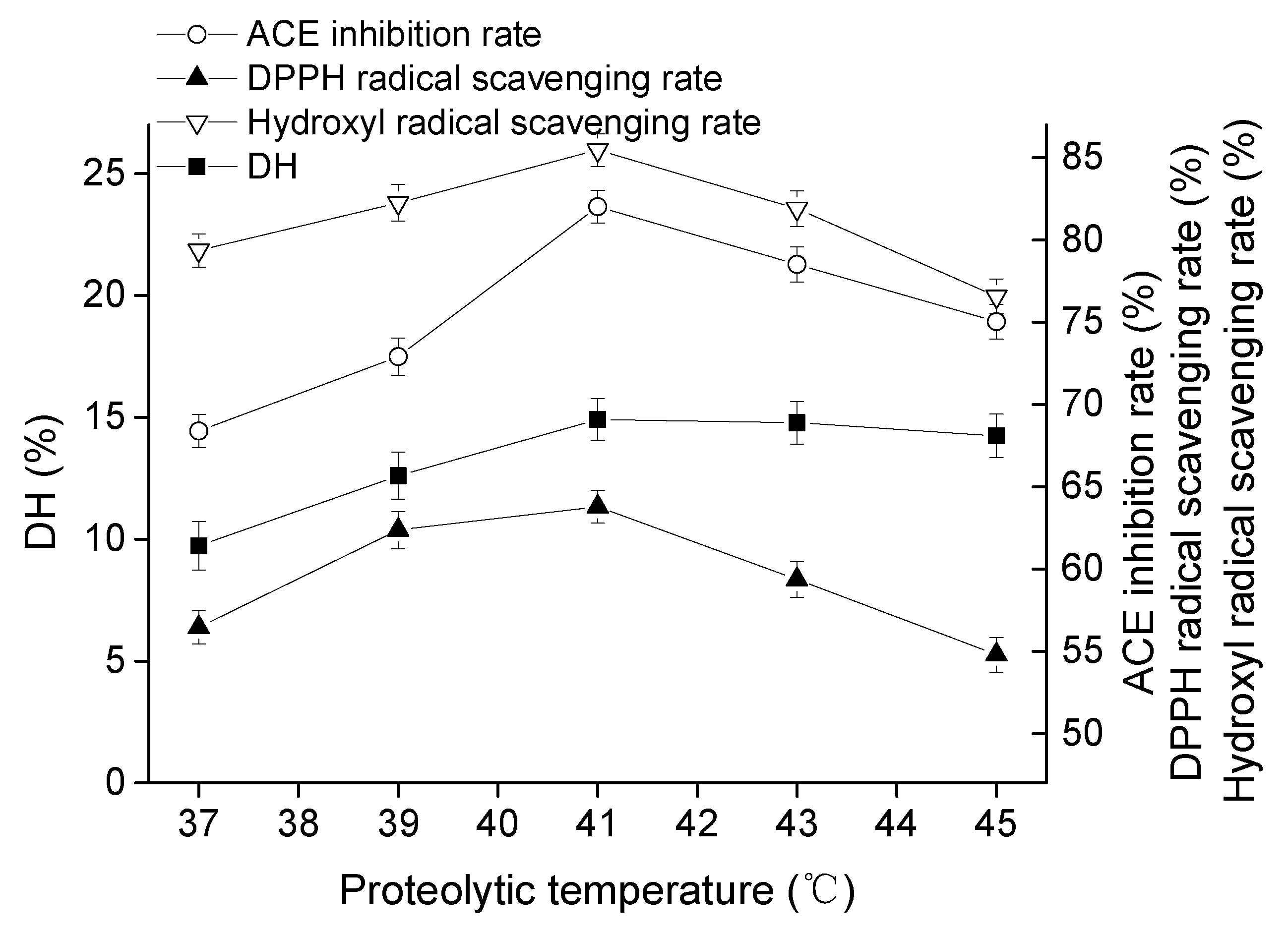
2.1.2. Effect of Proteolytic Temperature on Goat Milk Catalyzed by CEP
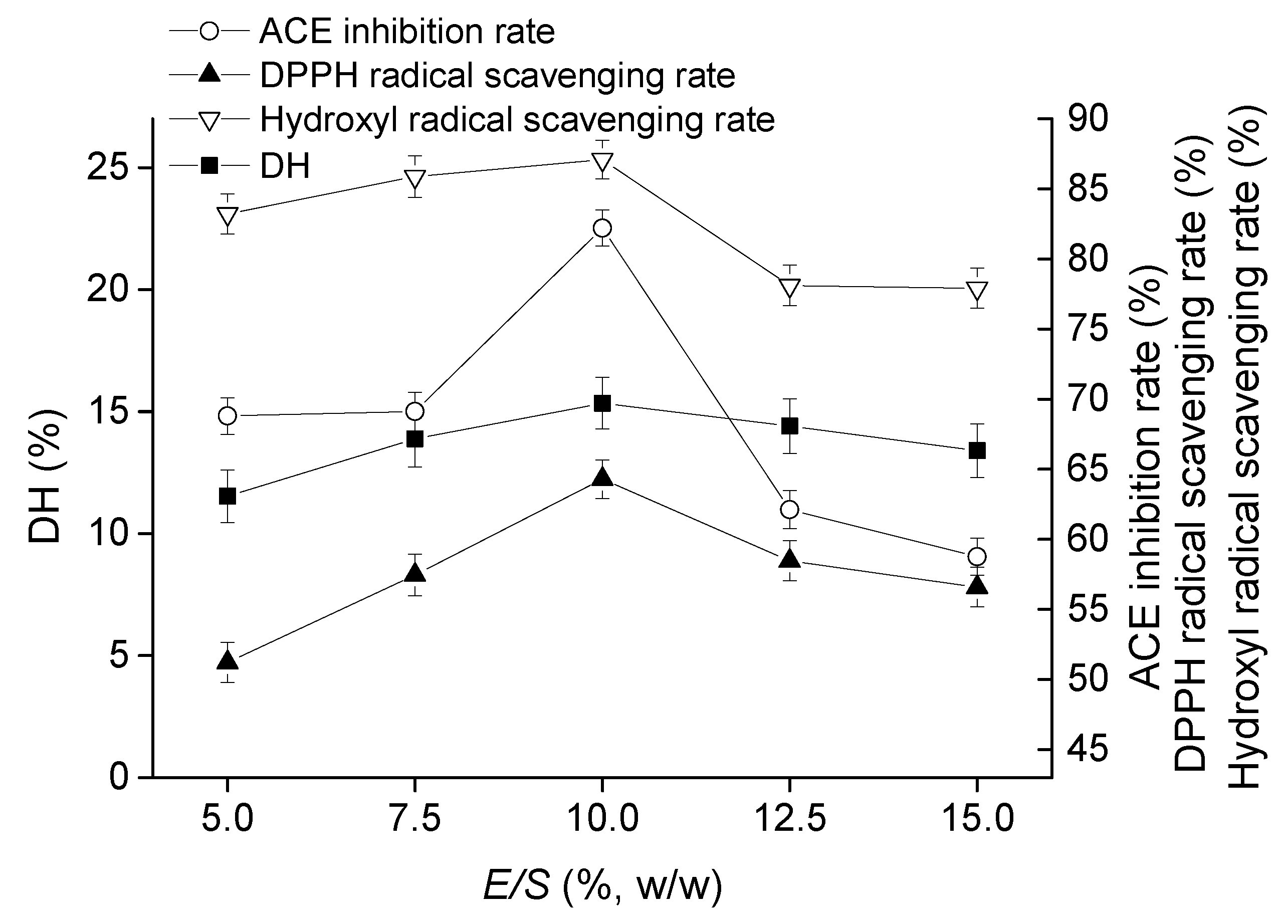
2.1.3. Effect of E/S on Goat Milk Catalyzed by CEP
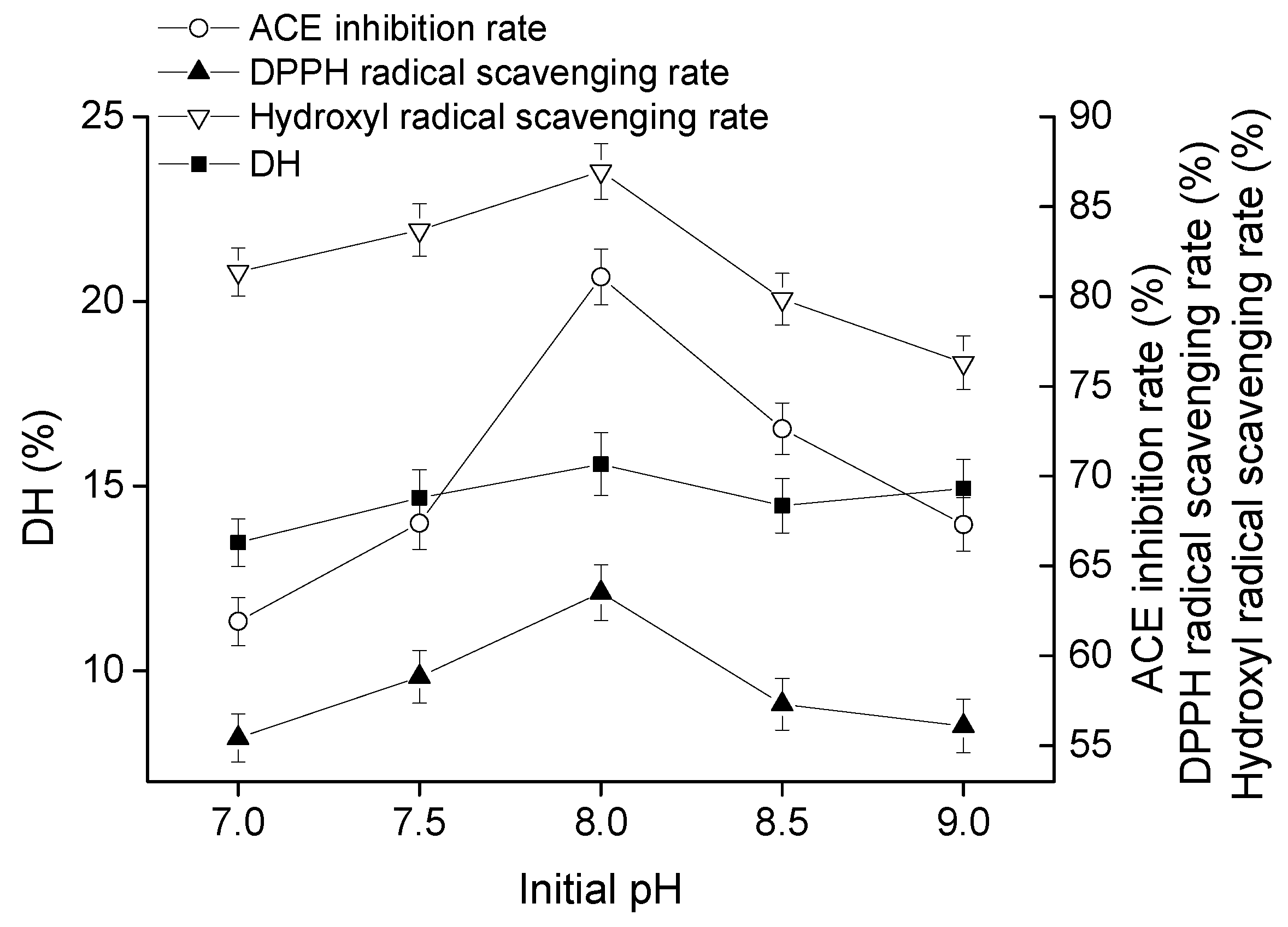
2.1.4. Effect of Initial pH on Goat Milk Catalyzed by CEP
2.1.5. Optimization of the Proteolytic Conditions by Orthogonal Test
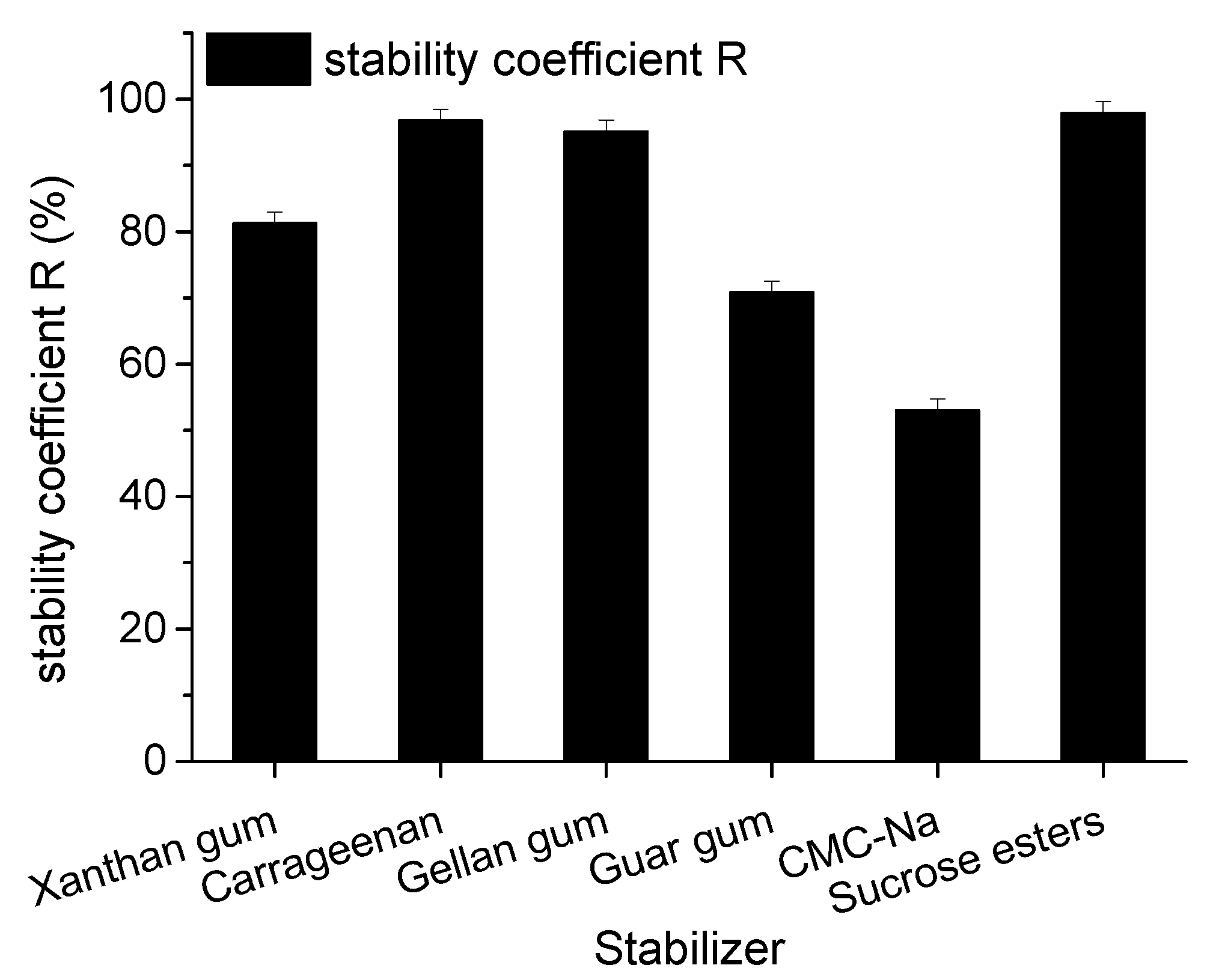
2.2. Optimization of Stabilizer of Goat Milk Catalyzed by CEPs
2.3. Storage Stability of Goat Milk Catalyzed by CEP at 4 °C and Room Temperature (RT)
3. Discussion
4. Materials and Methods
4.1. Materials and Chemicals
4.2. Bacterial Strain and Media
4.3. Preparation of Cell Suspension
4.4. Proteinase Activity Assay
4.5. Optimization of Proteolytic Conditions of Goat Milk Catalyzed by CEP
4.6. Optimization of Stabilizers of Goat Milk Catalyzed by CEP
4.7. The Storage Stability of Goat Milk Catalyzed by CEP
4.8. Analytical Testing Methods
4.8.1. Assay for ACE-Inhibitory Activity
4.8.2. Determination of DPPH Radical Scavenging Rate
4.8.3. Determination of Hydroxyl Radical Scavenging Rate
4.8.4. Determination of DH
4.8.5. Determination of Stability of Goat Milk
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Agyei, D.; Danquah, M.K. In-depth characterisation of Lactobacillus delbrueckii subsp. lactis 313 for growth and cell-envelope-associated proteinase production. Biochem. Eng. J. 2012, 64, 61–68. [Google Scholar] [CrossRef]
- Espeche Turbay, M.B.; Savoy de Giori, G.; Hebert, E.M. Release of the cell-envelope-associated proteinase of Lactobacillus delbrueckii subspecies lactis CRL 581 is dependent upon pH and temperature. J. Agric. Food Chem. 2009, 57, 8607–8611. [Google Scholar] [CrossRef] [PubMed]
- Agyei, D.; Danquah, M.K. Industrial-scale manufacturing of pharmaceuticalgrade bioactive peptides. Biotechnol. Adv. 2011, 29, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, R.; Meisel, H. Food-derived peptides with biological activity: From research to food application. Current Opin. Biotechnol. 2007, 18, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Korhonen, H.; Pihlanto, A. Bioactive peptides: Production and functionally. Int. Dairy J. 2006, 16, 945–960. [Google Scholar] [CrossRef]
- Agyei, D.; Lim, W.; Zass, M.; Tan, D.; Danquah, M.K. Bioanalytical evaluation of Lactobacillus delbrueckii subsp. lactis 313 cell-envelope proteinase extraction. Chem. Eng. Sci. 2013, 95, 323–330. [Google Scholar] [CrossRef]
- Hébert, E.M.; Raya, R.R.; Giori, G.S.D. Characterisation of a cell-envelope proteinase from Lactobacillus helveticus. Biotechnol. Lett. 1999, 21, 831–834. [Google Scholar] [CrossRef]
- Capela, P.; Hay, T.K.C.; Shah, N.P. Effect of cryoprotectants, prebiotics and microencapsulation on survival of probiotic organisms in yoghurt and freeze-dried yoghurt. Food Res. Int. 2006, 39, 203–211. [Google Scholar] [CrossRef]
- Kunji, E.R.S.; Mierau, I.; Hagting, A.; Poolman, B.; Konings, W.N. The proteolytic systems of lactic acid bacteria. Antonie van Leeuwenhoek 1996, 70, 187–221. [Google Scholar] [CrossRef] [PubMed]
- Jensen, S.; Rolin, C.; Ipsen, R. Stabilisation of acidified skimmed milk with HM pectin. Food Hydrocoll. 2010, 24, 291–299. [Google Scholar] [CrossRef]
- Agoda-Tandjawa, G.; Garnec, C.L.; Boulenguer, P.; Gilles, M.; Langendorff, V. Rheological behavior of starch/carrageenan/milk proteins mixed systems: Role of each biopolymer type and chemical characteristics. Food Hydrocoll. 2017, 73, 300–312. [Google Scholar] [CrossRef]
- Foerster, M.; Chang, L.; Gengenbach, T.; Meng, W.W.; Selomulya, C. Reduction of surface fat formation on spray-dried milk powders through emulsion stabilization with λ-carrageenan. Food Hydrocoll. 2017, 70, 163–180. [Google Scholar] [CrossRef]
- Picone, C.S.F.; Cunha, R.L.D. Interactions between milk proteins and gellan gum in acidified gels. Food Hydrocoll. 2010, 24, 502–511. [Google Scholar] [CrossRef]
- Xu, J.; He, Z.; Zeng, M.; Li, B.; Qin, F.; Wang, L.; Wu, S.; Chen, J. Effect of xanthan gum on the release of strawberry flavor in formulated soy beverage. Food Hydrocoll. 2017, 228, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Rohart, A.; Michon, C. Effect of thermomechanical treatment on microstructure of guar gum/acid milk gels. J. Food Eng. 2016, 171, 14–21. [Google Scholar] [CrossRef]
- Nakamura, A.; Yoshida, R.; Maeda, H.; Corredig, M. The stabilizing behaviour of soybean soluble polysaccharide and pectin in acidified milk beverages. Int. Dairy J. 2006, 16, 361–369. [Google Scholar] [CrossRef]
- Cai, Z.; Wu, J.; Du, B.; Zhang, H. Impact of distribution of carboxymethyl substituents in the stabilizer of carboxymethyl cellulose on the stability of acidified milk drinks. Food Hydrocoll. 2018, 76, 150–157. [Google Scholar] [CrossRef]
- Li, M.; Xia, J.; Ding, H.; Ding, C.; Wang, M.; Li, S.H. Optimal design, characterization, and thermal stability of bio-based Ca/Na/Zn composite stabilizer derived from myrcene for poly(vinyl chloride). Food Hydrocoll. 2017, 139, 117–129. [Google Scholar] [CrossRef]
- Tsakalidou, E.; Anastasiou, R.; Vandenberghe, I.; Van, J.B.; Kalantzopoulos, G. Cell-Wall-Bound Proteinase of Lactobacillus delbrueckii subsp. Lactis ACA-DC 178: Characterization and specificity for β-Casein. Appl. Environ. Microbiol. 1999, 65, 2035–2040. [Google Scholar] [PubMed]
- Pan, D.D.; Luo, Y.K.; Tanokura, M. Antihypertensive peptides from skimmed milk hydrolysate digested by cell-free extract of Lactobacillus helveticus JCM1004. Food Chem. 2005, 91, 123–129. [Google Scholar] [CrossRef]
- Tian, J.Y.; Pan, D.D.; Guo, Y.X. Optimization of Enzymatic Hydrolysis Technology of Casein by Proteinases from Lactobacillus helveticus for ACE Inhibitory Peptide Production. Food Sci. 2009, 30, 170–174. [Google Scholar] [CrossRef]
- Tuinier, R.; Kruif, C.G.D. Stability of casein micelles in milk. J. Chem. Phys. 2002, 117, 1290–1295. [Google Scholar] [CrossRef] [Green Version]
- Natalia, M.D.; Darío, S.; Natael, W.; Valeria, B.; Fernando, L.B.S. A combined experimental and molecular simulation study of factors influencing interaction of quinoa proteins-carrageenan. Int. J. Biol. Macromol. 2018, 107, 949–956. [Google Scholar]
- Javier, F.; Harold, E.S. Interaction of Sucrose Esters with Skim Milk Proteins as Characterized by Affinity Chromatography. J. Dairy Sci. 1994, 77, 3545–3551. [Google Scholar]
- Amine, K.M.; Champagne, C.P.; Salmieri, S.; Britten, M.; St-Gelais, D.; Fustier, P. Effect of palmitoylated alginate microencapsulation on viability of Bifidobacterium longum, during freeze-drying. LWT Food Sci. Technol. 2014, 56, 111–117. [Google Scholar] [CrossRef]
- Shu, G.W.; Wang, Z.; Chen, L.; Wan, H.C.; Chen, H. Characterization of freeze-dried Lactobacillus acidophilus in goat milk powder and tablet: Optimization of the composite cryoprotectants and evaluation of storage stability at different temperature. LWT Food Sci. Technol. 2018, 90, 70–76. [Google Scholar] [CrossRef]
- Rasolofo, E.A.; Stgelais, D.; Lapointe, G.; Roy, D. Molecular analysis of bacterial population structure and dynamics during cold storage of untreated and treated milk. Int. J. Food Microbiol. 2010, 138, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Cid, F.J.; Centeno, J.A.; Martínez, S.; Carballo, J. Changes in the chemical and physical characteristics of cow’s milk butter during storage: Effects of temperature and addition of salt. J. Food Compos. Anal. 2017, 63, 121–132. [Google Scholar] [CrossRef]
- Agyei, D.; He, L.Z. Evaluation of cross-linked enzyme aggregates of lactobacillus cell-envelope proteinases, for protein degradation. Food Bioprod. Process. 2015, 94, 59–69. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |


| Trial No. | Factors | Y1/% | Y2/% | Y3/% | Y4/% | ||||
|---|---|---|---|---|---|---|---|---|---|
| A/h | B/°C | C/% | D | ||||||
| 1 | 1(3.5) | 1(40) | 1(8) | 1(7.5) | 13.73 ± 0.39 | 63.85 ± 0.49 | 56.37 ± 1.80 | 85.71 ± 1.07 | |
| 2 | 1 | 2(41) | 2(10) | 2(8.0) | 12.89 ± 1.31 | 68.57 ± 0.68 | 61.92 ± 0.51 | 80.46 ± 0.72 | |
| 3 | 1 | 3(42) | 3(12) | 3(8.5) | 13.50 ± 0.77 | 74.09 ± 1.39 | 60.48 ± 1.50 | 87.04 ± 0.36 | |
| 4 | 2(4.0) | 1 | 2 | 3 | 14.29 ± 1.15 | 78.46 ± 1.07 | 58.57 ± 1.71 | 79.53 ± 0.41 | |
| 5 | 2 | 2 | 3 | 1 | 15.61 ± 0.46 | 61.57 ± 0.99 | 62.39 ± 2.07 | 82.16 ± 1.14 | |
| 6 | 2 | 3 | 1 | 2 | 14.03 ± 0.72 | 80.51 ± 1.03 | 59.02 ± 0.73 | 78.47 ± 1.03 | |
| 7 | 3(4.5) | 1 | 3 | 2 | 12.74 ± 0.22 | 76.06 ± 0.21 | 60.64 ± 0.93 | 86.39 ± 0.58 | |
| 8 | 3 | 2 | 1 | 3 | 15.04 ± 0.34 | 71.43 ± 0.65 | 63.42 ± 1.39 | 90.62 ± 0.29 | |
| 9 | 3 | 3 | 2 | 1 | 14.75 ± 1.55 | 75.02 ± 1.22 | 58.45 ± 1.21 | 84.80 ± 1.17 | |
| Y1/% | 13.37 | 13.59 | 14.27 | 14.70 | |||||
| 14.64 | 14.51 | 13.98 | 13.22 | ||||||
| 14.18 | 14.09 | 13.95 | 14.28 | ||||||
| G | 1.27 | 0.92 | 0.32 | 1.48 | |||||
| Y2/% | 63.84 | 72.79 | 71.93 | 66.81 | |||||
| 73.51 | 67.19 | 74.02 | 75.05 | ||||||
| 74.17 | 76.54 | 70.57 | 74.66 | ||||||
| G | 10.33 | 9.35 | 3.45 | 8.24 | |||||
| Y3/% | 59.59 | 58.53 | 59.6 | 59.07 | |||||
| 60.00 | 62.58 | 59.64 | 60.53 | ||||||
| 60.84 | 59.32 | 61.17 | 60.82 | ||||||
| G | 1.25 | 4.05 | 1.57 | 1.75 | |||||
| Y4/% | 84.40 | 83.88 | 84.94 | 84.22 | |||||
| 80.05 | 84.41 | 81.60 | 81.77 | ||||||
| 87.27 | 83.44 | 85.20 | 85.73 | ||||||
| G | 7.22 | 0.97 | 3.60 | 3.96 | |||||
| Trial No. | Factors | Stability Coefficient R/% | ||
|---|---|---|---|---|
| Carrageenan (w/v)/% | Gellan Gum (w/v)/% | Sucrose Esters (w/v)/% | ||
| 1 | 1(0.05) | 1(0.05) | 1(0.05) | 96.75 ± 0.24 |
| 2 | 1 | 2(0.10) | 2(0.10) | 95.86 ± 0.47 |
| 3 | 1 | 3(0.15) | 3(0.15) | 98.57 ± 0.22 |
| 4 | 2(0.10) | 1 | 2 | 91.50 ± 0.26 |
| 5 | 2 | 2 | 3 | 91.65 ± 0.30 |
| 6 | 2 | 3 | 1 | 94.03 ± 0.21 |
| 7 | 3(0.15) | 1 | 3 | 97.52 ± 0.48 |
| 8 | 3 | 2 | 1 | 92.45 ± 0.23 |
| 9 | 3 | 3 | 2 | 96.85 ± 0.16 |
| 97.06 | 93.57 | 94.41 | ||
| 92.39 | 93.32 | 94.74 | ||
| 95.60 | 96.48 | 95.91 | ||
| G | 4.67 | 3.16 | 1.50 | |
| Level | Factors | |||
|---|---|---|---|---|
| Time/h, (A) | Temperature/°C, (B) | E/S (w/w)/%, (C) | Initial pH, (D) | |
| 1 | 3.5 | 40 | 8 | 7.5 |
| 2 | 4.0 | 41 | 10 | 8.0 |
| 3 | 4.5 | 42 | 12 | 8.5 |
| Level | Factors | ||
|---|---|---|---|
| Carrageenan (w/v)/% | Gellan Gum (w/v)/% | Sucrose Esters (w/v)/% | |
| 1 | 0.05 | 0.05 | 0.05 |
| 2 | 0.10 | 0.10 | 0.10 |
| 3 | 0.15 | 0.15 | 0.15 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shu, G.; Huang, J.; Chen, L.; Lei, N.; Chen, H. Characterization of Goat Milk Hydrolyzed by Cell Envelope Proteinases from Lactobacillus plantarum LP69: Proteolytic System Optimization, Bioactivity, and Storage Stability Evaluation. Molecules 2018, 23, 1317. https://doi.org/10.3390/molecules23061317
Shu G, Huang J, Chen L, Lei N, Chen H. Characterization of Goat Milk Hydrolyzed by Cell Envelope Proteinases from Lactobacillus plantarum LP69: Proteolytic System Optimization, Bioactivity, and Storage Stability Evaluation. Molecules. 2018; 23(6):1317. https://doi.org/10.3390/molecules23061317
Chicago/Turabian StyleShu, Guowei, Jie Huang, Li Chen, Ni Lei, and He Chen. 2018. "Characterization of Goat Milk Hydrolyzed by Cell Envelope Proteinases from Lactobacillus plantarum LP69: Proteolytic System Optimization, Bioactivity, and Storage Stability Evaluation" Molecules 23, no. 6: 1317. https://doi.org/10.3390/molecules23061317





