Synthesis and Antibacterial Activity of New Thiazolidine-2,4-dione-Based Chlorophenylthiosemicarbazone Hybrids
Abstract
:1. Introduction
2. Results and Discussion
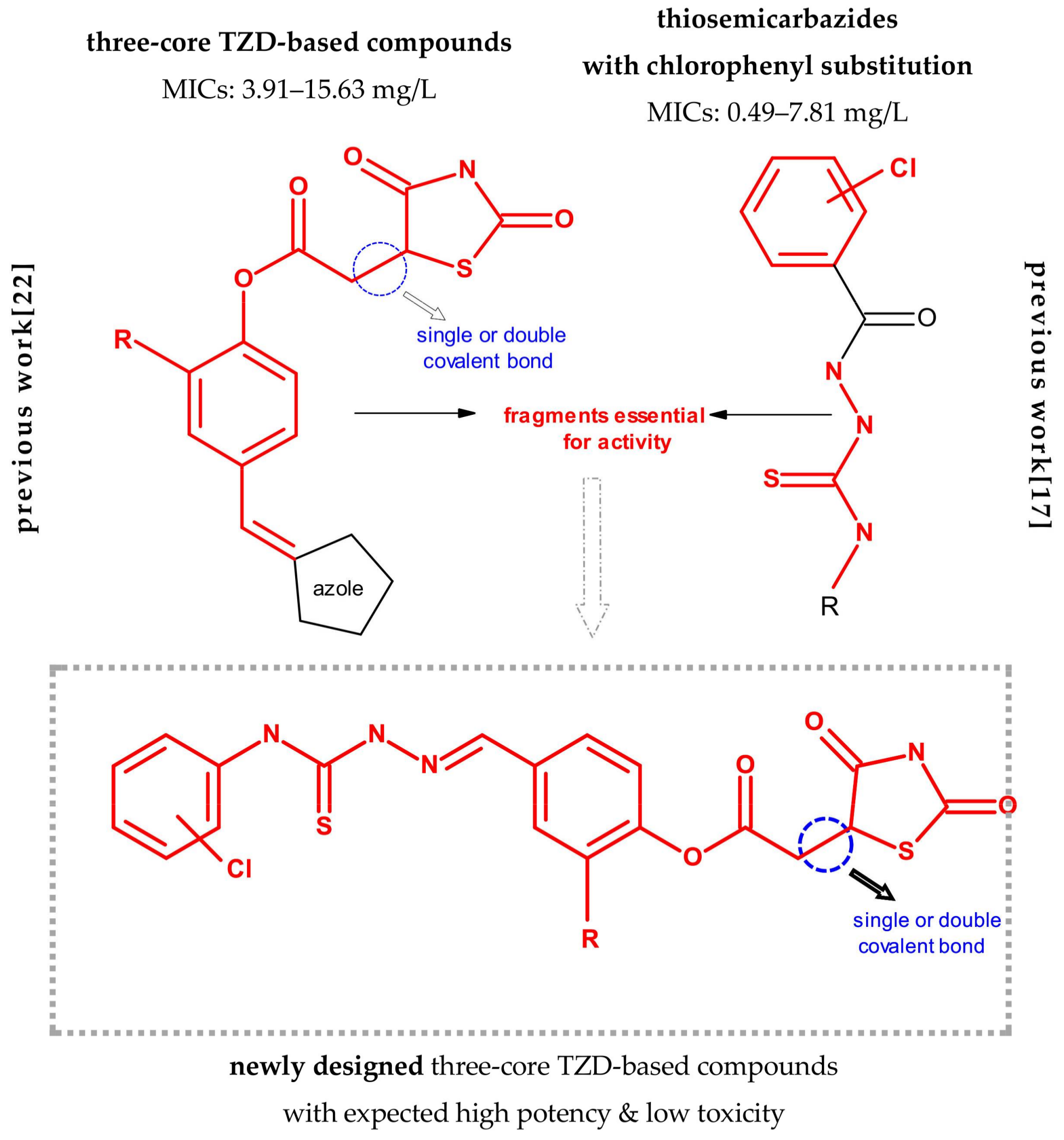
2.1. Rationale
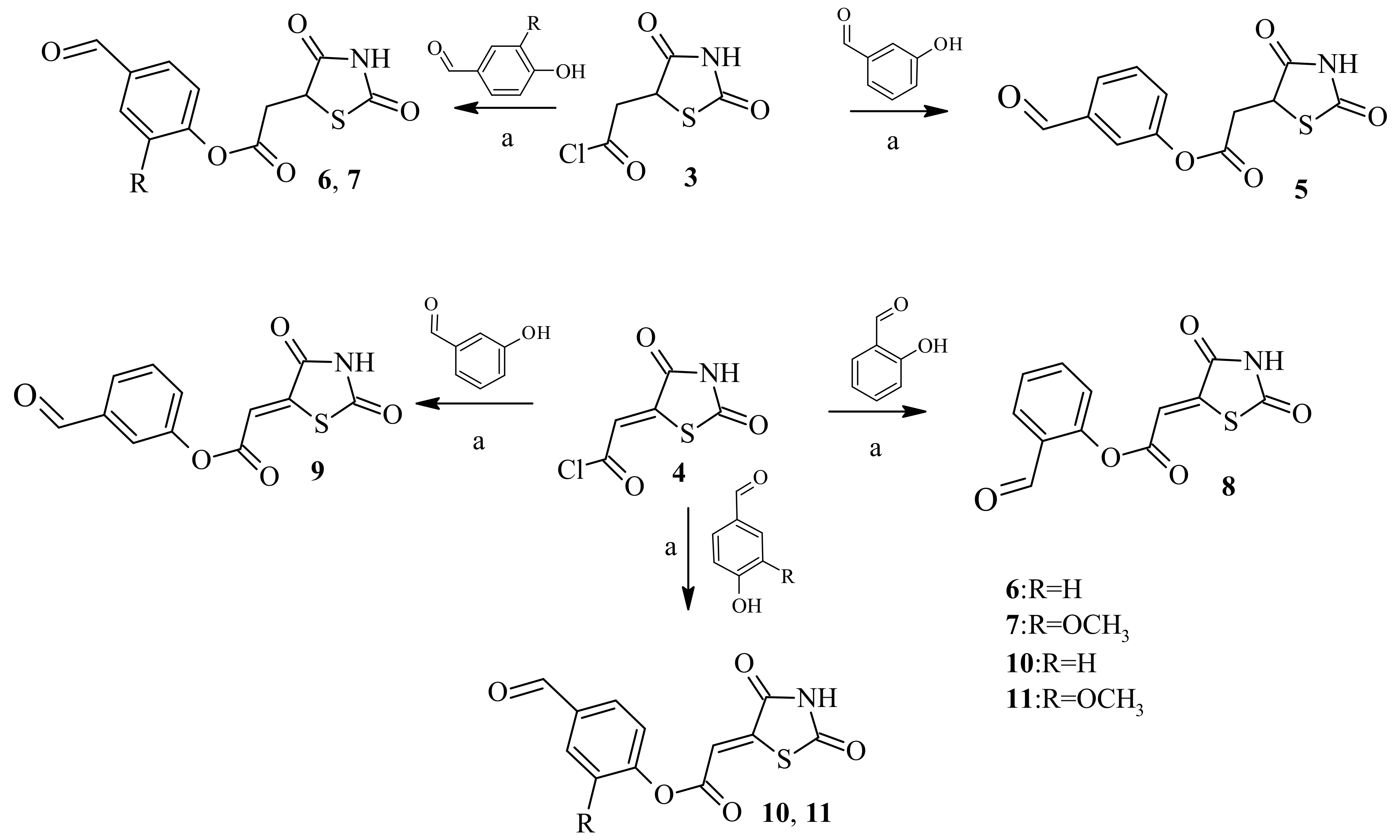
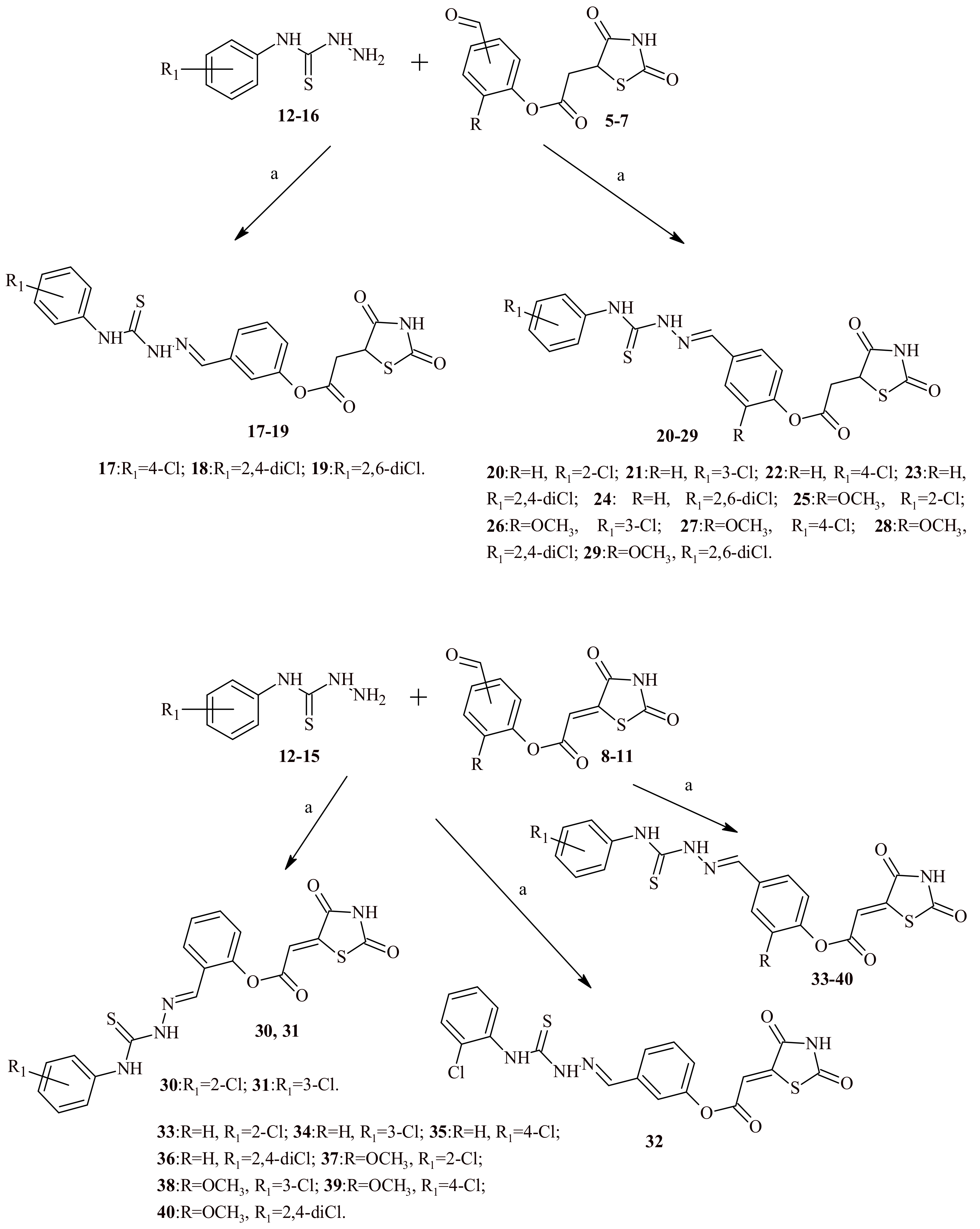
2.2. Chemistry
2.3. Antibacterial Screening
2.4. Bactericidal and Bacteriostatic Activity
2.5. Computational Studies
2.6. Cytotoxicity Studies
3. Materials and Methods
3.1. Chemistry
3.1.1. Synthesis of Chlorophenylthiosemicarbazones (17–40)
3.1.2. Synthesis of 4-[(2,5-dioxoimidazolidin-4-ylidene)methyl]-2-methoxyphenyl (2,4-dioxo-1,3-thiazolidin-5-yl/ylidene)acetates (41, 42)
3.2. Microbiology Tests
3.3. Computational Details
3.4. Cytotoxicity Studies
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Schillaci, D.; Spano, V.; Parrino, B.; Carbone, A.; Montalbano, A.; Barraja, P.; Diana, P.; Cirrincione, G.; Cascioferro, S. Pharmaceutical approaches to target antibiotic resistance mechanisms. J. Med. Chem. 2017, 60, 8268–8297. [Google Scholar] [CrossRef] [PubMed]
- Lesyk, R.B.; Zimenkovsky, B.S. 4-Thiazolidones: Centenarian history, current status and perspectives for modern organic and medicinal chemistry. Curr. Org. Chem. 2004, 8, 1547–1577. [Google Scholar] [CrossRef]
- Jain, V.S.; Vora, D.K.; Ramaa, C.S. Thiazolidine-2,4-diones: Progress towards multifarious applications. Bioorg. Med. Chem. 2013, 21, 1599–1620. [Google Scholar] [CrossRef] [PubMed]
- Kaminskyy, D.; Kryshchyshyn, A.; Lesyk, R. 5-Ene-4-thiazolidinones—An efficient tool in medicinal chemistry. Eur. J. Med. Chem. 2017, 140, 542–594. [Google Scholar] [CrossRef] [PubMed]
- Naim, M.J.; Alam, M.J.; Ahmad, S.; Nawaz, F.; Shrivastava, N.; Sahu, M.; Alam, O. Therapeutic journey of 2,4-thiazolidinediones as a versatile scaffold: An insight into structure activity relationship. Eur. J. Med. Chem. 2017, 129, 218–250. [Google Scholar] [CrossRef] [PubMed]
- Sucheta, S.T.; Prabhakar, K.V. Biological potential of thiazolidinedione derivatives of synthetic origin. Chem. Cent. J. 2017, 11, 130. [Google Scholar] [CrossRef] [PubMed]
- Barros, C.D.; Amato, A.A.; DeOliveira, T.B.; Iannini, K.B.R.; DaSilva, A.L.; DaSilva, T.G.; Leite, E.S.; Hernandes, M.Z.; DeLima, M.C.A.; Galdino, S.L.; et al. Synthesis and anti-inflammatory activity of new arylidene-thiazolidine-2,4-diones as PPARg ligands. Bioorg. Med. Chem. 2010, 18, 3805–3811. [Google Scholar] [CrossRef] [PubMed]
- Youssef, A.M.; White, M.S.; Villanueva, E.B.; Ashmawy, I.M.; Klegeris, A. Synthesis and evaluation of novel pyrazolyl-2,4-thiazolidinediones a anti-inflammatory and neuroprotective agents. Bioorg. Med. Chem. 2010, 18, 2019–2028. [Google Scholar] [CrossRef] [PubMed]
- Alegaon, S.G.; Alagawadi, K.R. New thiazolidinedione-5-acetic acid amide derivatives: Synthesis, characterization and investigation of antimicrobial and cytotoxic properties. Med. Chem. Res. 2012, 21, 816–824. [Google Scholar] [CrossRef]
- Moorthy, P.; Ekambaram, S.P.; Perumal, S.S. Synthesis, characterization and antimicrobial evaluation of imidazolyl thiazolidinedione derivatives. Arab. J. Chem. 2014, 8, 1–7. [Google Scholar] [CrossRef]
- Liu, X.F.; Zheng, C.J.; Sun, L.P.; Liu, X.K.; Piao, H.R. Synthesis of new chalcone derivatives bearing 2,4-thiazolidinedione and benzoic acid moieties as potential antibacterial agents. Eur. J. Med. Chem. 2011, 46, 3469–3473. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Chawla, A.; Jain, S.; Kumar, P.; Kumar, S. 3-Aryl-2-{4-[4-(2,4-dioxothiazolidin-5-ylmethyl)phenoxy]-phenyl}-acrylic acid alkyl ester: Synthesis and antihyperglycemic evaluation. Med. Chem. Res. 2011, 20, 678–686. [Google Scholar] [CrossRef]
- Kar, K.; Krithika, U.; Basu, P.; Kumar, S.S.; Reji, A.; Kumar, B.P. Design, synthesis and glucose uptake activity of some novel glitazones. Bioorg. Chem. 2014, 56, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Asati, V.; Bhati, S.K. Design, synthesis and molecular modelling studies of novel thiazolidine-2,4-dione derivatives as potential anti-cancer agents. J. Mol. Struct. 2018, 1154, 406–417. [Google Scholar] [CrossRef]
- Sharma, P.; Reddy, T.S.; Thummuri, D.; Senwar, K.R.; Kumar, N.P.; Naidu, V.G.; Bhargava, S.K.; Shankaraiah, N. Synthesis and biological evaluation of new benzimidazole-thiazolidinedione hybrids as potential cytotoxic and apoptosis inducing agents. Eur. J. Med. Chem. 2016, 124, 608–621. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Rao, W.; Parikh, H.; Li, Q.; Guo, T.L.; Grant, S.; Kellogg, G.E.; Zhang, S. 3,5-Disubstituted-thiazolidine-2,4-dione analogs as anticancer agents: Design, synthesis and biological characterization. Eur. J. Med. Chem. 2012, 47, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Paneth, A.; Plech, T.; Kaproń, B.; Hagel, D.; Kosikowska, U.; Kuśmierz, E.; Dzitko, K.; Paneth, P. Design, synthesis and biological evaluation of 4-benzoyl-1-dichlorobenzoylthiosemicarbazides as potent Gram-positive antibacterial agents. J. Enzyme Inhib. Med. Chem. 2016, 31, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Plech, T.; Paneth, A.; Kaproń, B.; Kosikowska, U.; Malm, A.; Strzelczyk, A.; Stączek, P. Structure–activity Relationship studies of microbiologically active thiosemicarbazides derived from hydroxybenzoic acid hydrazides. Chem. Biol. Drug Des. 2015, 85, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Paneth, A.; Stączek, P.; Plech, T.; Strzelczyk, A.; Janowska, D.; Stefańska, J.; Dzitko, K.; Wujec, M.; Kosiek, S.; Paneth, P. Synthesis and antibacterial activity of 1,4-dibenzoylthiosemicarbazide derivatives. Biomed. Pharmacother. 2017, 88, 1235–1242. [Google Scholar] [CrossRef]
- Plech, T.; Wujec, M.; Siwek, A.; Kosikowska, U.; Malm, A. Synthesis and antimicrobial activity of thiosemicarbazides, s-triazoles and their Mannich bases bearing 3-chlorophenyl moiety. Eur. J. Med. Chem. 2011, 46, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Siwek, A.; Stączek, P.; Wujec, M.; Stefańska, J.; Kosikowska, U.; Malm, A.; Jankowski, S.; Paneth, P. Biological and docking studies of topoisomerase IV inhibition by thiosemicarbazides. J. Mol. Model. 2011, 17, 2297–2303. [Google Scholar] [CrossRef] [PubMed]
- Trotsko, N.; Kosikowska, U.; Paneth, A.; Wujec, M.; Malm, A. Synthesis and antibacterial activity of new (2,4-dioxothiazolidin-5-yl/ylidene)acetic acid derivatives with thiazolidine-2,4-dione, rhodanine and 2-thiohydantoin moieties. Saudi Pharm. J. 2018. [Google Scholar] [CrossRef]
- Trotsko, N.; Przekora, A.; Zalewska, J.; Ginalska, G.; Paneth, A.; Wujec, M. Synthesis and in vitro antiproliferative and antibacterial activity of new thiazolidine-2,4-dione derivatives. J. Enzyme Inhib. Med. Chem. 2018, 33, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Dzitko, K.; Paneth, A.; Plech, T.; Pawełczyk, J.; Stączek, P.; Stefańska, J.; Paneth, P. 1,4-Disubstituted thiosemicarbazide derivatives are potent inhibitors of Toxoplasma gondii proliferation. Molecules 2014, 19, 9926–9943. [Google Scholar] [CrossRef] [PubMed]
- Siwek, A.; Stączek, P.; Stefańska, J. Synthesis and structure-activity relationship studies of 4-arylthiosemicarbazides as topoisomerase IV inhibitors with Gram-positive antibacterial activity. Search for molecular basis of antibacterial activity of thiosemicarbazides. Eur. J. Med. Chem. 2011, 46, 5717–5726. [Google Scholar] [CrossRef] [PubMed]
- Trotsko, N.; Król, J.; Siwek, A.; Wujec, M.; Kosikowska, U.; Malm, A. Synthesis and antimicrobial evaluation of new 1-{[4-(4-halogenophenyl)-4H-1,2,4-triazol-3-yl]sulfanyl}acetyl-4-substituted thiosemicarbazides and products of their cyclization. Heteroat. Chem. 2012, 23, 117–121. [Google Scholar] [CrossRef]
- Jones, R.N. Microbiological features of vancomycin in the 21st century: Minimum inhibitory concentration creep, bactericidal/static activity, and applied breakpoints to predict clinical outcomes or detect resistant strains. Clin. Infect. Dis. 2006, 42, S13–S24. [Google Scholar] [CrossRef] [PubMed]
- Hyperchem 8.0.3; HyperCube Inc.: Gainsville, FL, USA, 2007.
- Siwek, A.; Świderek, K.; Jankowski, S. Problems with molecular mechanics implementations on the example of 4-benzoyl-1-(4-methyl-imidazol-5-yl)-carbonylthiosemicarbazide. J. Mol. Model. 2012, 18, 843–849. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 17–40 are available from the authors. |
| Compound | S. aureus | S. aureus | S. epidermidis | B. subtilis | B. cereus | M. luteus | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ATCC 6538 | ATCC 25923 | ATCC 12228 | ATCC 6633 | ATCC 10876 | ATCC 10240 | |||||||
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| 19 | 125 | >1000 | 62.5 | >1000 | 62.5 | >1000 | 62.5 | 500 | 62.5 | >1000 | 62.5 | 1000 |
| 251.3 | >2010.6 | 125.7 | >2010.6 | 125.7 | >2010.6 | 125.7 | 1005.3 | 125.7 | >2010.6 | 125.7 | 2010.6 | |
| 20 | 3.91 | 3.91 | 3.91 | 62.5 | 31.25 | 62.5 | 31.25 | 125 | 3.91 | 125 | 7.81 | 125 |
| 8.4 | 8.4 | 8.4 | 135 | 67.5 | 135 | 67.5 | 270 | 8.4 | 270 | 16.9 | 270 | |
| 21 | 31.25 | >1000 | 7.81 | 1000 | 31.25 | >1000 | 62.5 | 250 | 31.25 | 250 | 15.63 | 15.63 |
| 67.5 | >2160.2 | 16.9 | 2160.2 | 67.5 | >2160.2 | 135 | 540 | 67.5 | 540 | 33.8 | 33.8 | |
| 22 | 15.63 | 1000 | 31.25 | 1000 | 15.63 | 1000 | 31.25 | >1000 | 7.81 | >1000 | 3.91 | 500 |
| 33.8 | 2160.2 | 67.5 | 2160.2 | 33.8 | 2160.2 | 67.5 | >2160.2 | 16.9 | >2160.2 | 8.4 | 1080.1 | |
| 23 | 3.91 | >1000 | 3.91 | >1000 | 7.81 | 1000 | 7.81 | 62.5 | 7.81 | >1000 | 7.81 | 7.81 |
| 7.9 | >2010.6 | 7.9 | >2010.6 | 15.7 | 2010.6 | 15.7 | 125.7 | 15.7 | >2010.6 | 15.7 | 15.7 | |
| 24 | 125 | >1000 | 62.5 | >1000 | 31.25 | >1000 | 125 | 250 | 62.5 | >1000 | 62.5 | >1000 |
| 251.3 | >2010.6 | 125.7 | >2010.6 | 62.8 | >2010.6 | 251.3 | 502.6 | 125.7 | >2010.6 | 125.7 | >2010.6 | |
| 26 | 15.63 | >1000 | 15.63 | >1000 | 31.25 | >1000 | 15.63 | 500 | 62.5 | >1000 | 3.91 | 1000 |
| 31.7 | >2028.6 | 31.7 | >2028.6 | 63.4 | >2028.6 | 31.7 | 1014.3 | 126.8 | >2028.6 | 7.9 | 2028.6 | |
| 29 | 125 | >1000 | 62.5 | >1000 | 31.25 | >1000 | 31.25 | 500 | 15.63 | 1000 | 7.81 | 1000 |
| 237 | >1896.1 | 118.5 | >1896.1 | 59.3 | >1896.1 | 59.3 | 948 | 29.6 | 1896.1 | 14.8 | 1896.1 | |
| 30 | 7.81 | 250 | 7.81 | 250 | 7.81 | 250 | 7.81 | 250 | 3.91 | 500 | 7.81 | 250 |
| 16.9 | 542.4 | 16.9 | 542.4 | 16.9 | 542.4 | 16.9 | 542.4 | 8.5 | 1084.8 | 16.9 | 542.4 | |
| 31 | 7.81 | 500 | 7.81 | 500 | 7.81 | 500 | 7.81 | 500 | 3.91 | 500 | 7.81 | 1000 |
| 16.9 | 1084.8 | 16.9 | 1084.8 | 16.9 | 1084.8 | 16.9 | 1084.8 | 8.5 | 1084.8 | 16.9 | 2169.6 | |
| 33 | 15.63 | 1000 | 15.63 | 250 | 31.25 | 500 | 15.63 | 500 | 62.5 | >1000 | 62.5 | >1000 |
| 33.9 | 2169.6 | 33.9 | 542.4 | 67.8 | 1084.8 | 33.9 | 1084.8 | 135.6 | >2169.6 | 135.6 | >2169.6 | |
| 34 | 31.25 | 500 | 7.81 | 500 | 15.63 | 500 | 15.63 | 500 | 7.81 | 1000 | 62.5 | 500 |
| 67.8 | 1084.8 | 16.9 | 1084.8 | 33.9 | 1084.8 | 33.9 | 1084.8 | 16.9 | 2169.6 | 135.6 | 1084.8 | |
| 35 | 500 | 1000 | 1000 | 500 | 500 | 500 | 500 | 500 | 1000 | 1000 | 1000 | 1000 |
| 1084.8 | 2169.6 | 2169.6 | 1084.8 | 1084.8 | 1084.8 | 1084.8 | 1084.8 | 2169.6 | 2169.6 | 2169.6 | 2169.6 | |
| 36 | 125 | 500 | 7.81 | 125 | 7.81 | 62.5 | 250 | 1000 | 7.81 | 1000 | 7.81 | 250 |
| 252.3 | 1009.4 | 15.8 | 252.3 | 15.8 | 126.2 | 504.7 | 2018.7 | 15.8 | 2018.7 | 15.8 | 504.7 | |
| 38 | 31.25 | >1000 | 7.81 | >1000 | 7.81 | 250 | 3.91 | 62.5 | 3.91 | 62.5 | 3.91 | 125 |
| 63.7 | >2036.9 | 15.9 | >2036.9 | 15.9 | 509.2 | 8 | 127.3 | 8 | 127.3 | 8 | 254.6 | |
| 39 | 250 | 1000 | 250 | 1000 | 500 | 1000 | 500 | 500 | 1000 | 1000 | 1000 | 1000 |
| 509.2 | 2036.9 | 509.2 | 2036.9 | 1018.5 | 2036.9 | 1018.5 | 1018.5 | 2036.9 | 2036.9 | 2036.9 | 2036.9 | |
| 40 | 500 | 1000 | 250 | 1000 | 250 | 1000 | 500 | 1000 | 1000 | 1000 | 1000 | 1000 |
| 951.7 | 1903.4 | 475.8 | 1903.4 | 475.8 | 1903.4 | 951.7 | 1903.4 | 1903.4 | 1903.4 | 1903.4 | 1903.4 | |
| Cefuroxime | 0.98 | - | 0.49 | - | 0.24 | - | 15.63 | - | 31.25 | - | 0.98 | - |
| 2.3 | 1.2 | 0.6 | 36.8 | 73.6 | 2.3 | |||||||
| Ciprofloxacin | 0.49 | 0.49 | - | - | 0.49 | 0.49 | 0.015 | 0.12 | 0.12 | 0.12 | 0.98 | 1.95 |
| 1.5 | 1.5 | 1.5 | 1.5 | 0.05 | 0.4 | 0.4 | 0.4 | 3.0 | 5.9 | |||
| Oxacillin | 0.06 | 0.06 | - | - | 0.12 | 0.12 | 0.06 | 0.12 | 62.5 | 62.5 | 0.98 | 0.98 |
| 0.15 | 0.15 | 0.3 | 0.3 | 0.15 | 0.3 | 155.7 | 155.7 | 2.4 | 2.4 | |||
| Compound | EC70 ± SD (mg/L) (Toxicity Threshold) |
|---|---|
| 20 | 30.82 ± 3.73 |
| 23 | 21.28 ± 3.84 |
| 30 | 14.86 ± 1.12 |
| 31 | 10.94 ± 1.35 |
| 38 | 19.01 ± 3.80 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trotsko, N.; Kosikowska, U.; Paneth, A.; Plech, T.; Malm, A.; Wujec, M. Synthesis and Antibacterial Activity of New Thiazolidine-2,4-dione-Based Chlorophenylthiosemicarbazone Hybrids. Molecules 2018, 23, 1023. https://doi.org/10.3390/molecules23051023
Trotsko N, Kosikowska U, Paneth A, Plech T, Malm A, Wujec M. Synthesis and Antibacterial Activity of New Thiazolidine-2,4-dione-Based Chlorophenylthiosemicarbazone Hybrids. Molecules. 2018; 23(5):1023. https://doi.org/10.3390/molecules23051023
Chicago/Turabian StyleTrotsko, Nazar, Urszula Kosikowska, Agata Paneth, Tomasz Plech, Anna Malm, and Monika Wujec. 2018. "Synthesis and Antibacterial Activity of New Thiazolidine-2,4-dione-Based Chlorophenylthiosemicarbazone Hybrids" Molecules 23, no. 5: 1023. https://doi.org/10.3390/molecules23051023





