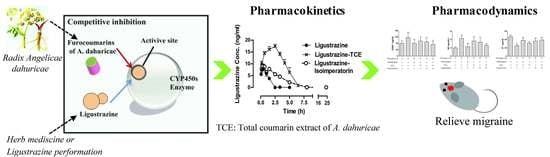
A Metabolism-Based Synergy for Total Coumarin Extract of Radix Angelicae Dahuricae and Ligustrazine on Migraine Treatment in Rats
Abstract
:1. Introduction
2. Results
2.1. TCE Enhanced the Anti-Migraine Effects of Ligustrazine
2.2. TCE (or Isoimperatorin) Increased the Ligustrazine Plasma Concentration in Rats and Inhibited Its Metabolism In Vitro
2.3. Isoimperatorin (or Imperatorin) Inhibited CYP450s Activity and Their Metabolic Information
3. Materials and Methods
3.1. Materials
3.2. Total Coumarin Extract (TCE) of Radix Angelicae Dahuricae Preparation
3.3. Animals, Treatment, and Sampling
3.4. Ligustrazine Metabolism Interference Study in Liver Microsomes
3.5. IC50 of Imperatorin or Isoimperatorin on CYP450s Enzymes
3.6. Metabolism of Imperatorin or Isoimperatorin in Human Liver Microsomes and Recombinant CYP450s
3.7. Analysis of Samples
3.7.1. Determination of Ligustrazine Concentration
3.7.2. Determination of Hydroxy-Ligustrazine Concentration
3.7.3. Determination of Imperatorin or Isoimperatorin Concentration
3.7.4. Analysis of Imperatorin and Isoimperatorin Metabolite Using Ultra-Performance Liquid Chromatography–Mass Spectrometry (UPLC–MS/MS)
3.8. Data Analysis
3.8.1. IC50 Value Calculation
3.8.2. Metabolic Kinetic Constant
3.8.3. Pharmacokinetic Parameters
3.9. Statistical Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| CGRP | calcitonin gene–related peptide |
| ET | endothelin |
| NO | nitric oxide |
| NADPH | β-nicotinamide adenine dinucleotide phosphate |
| TCE | total coumarin extract of Radix Angelicae dahuricae |
References
- Thomford, N.E.; Dzobo, K.; Chopera, D.; Wonkam, A.; Maroyi, A.; Blackhurst, D.; Dandara, C. In Vitro Reversible and Time-Dependent CYP450 Inhibition Profiles of Medicinal Herbal Plant Extracts Newbouldia laevis and Cassia abbreviata: Implications for Herb-Drug Interactions. Molecules 2016, 21. [Google Scholar] [CrossRef] [PubMed]
- Guengerich, F.P. Cytochrome P450s and other enzymes in drug metabolism and toxicity. APS J. 2006, 8. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.; Barbery, G.; Lui, C.W. Complementary and alternative medicine use for headache and migraine: A critical review of the literature. Headache 2013, 53, 459–473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Owen, J.M.; Green, B.N. Homeopathic treatment of headaches: A systematic review of the literature. J. Chiropr. Med. 2004, 3, 45–52. [Google Scholar] [CrossRef]
- Fox, A.W.; Kori, S.H. Pharmacokinetic opportunities for combination therapy in migraine. Neurology 2005, 64, S21–S25. [Google Scholar] [CrossRef] [PubMed]
- Hou, M.; Tang, Q.; Xue, Q.; Zhang, X.; Liu, Y.; Yang, S.; Chen, L.; Xu, X. Pharmacodynamic action and mechanism of Du Liang soft capsule, a traditional Chinese medicine capsule, on treating nitroglycerin-induced migraine. J. Ethnopharmacol. 2017, 195, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Li, J.C.; Shen, X.F.; Meng, X.L.; Zhang, Y.; Lai, X.R. Analgesic effect and mechanism of the three TCM-herbal drug-combination Tou Feng Yu pill on treatment of migraine. Phytomedicine 2011, 18, 788–794. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Wan, H.T.; Yang, J.H.; Zhang, Y.Y.; Ge, L.J.; Bie, X.D. Effect of ligustrazine on levels of amino acid neurotransmitters in rat striatum after cerebral ischemia-reperfusion injury. J. Asian Nat. Prod. Res. 2014, 16, 1060–1067. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Martins-Oliveira, M.; Akerman, S.; Goadsby, P.J. Comparative effects of traditional Chinese and Western migraine medicines in an animal model of nociceptive trigeminovascular activation. Cephalalgia 2017. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Guo, J.; Li, Z.; Zhang, X.; Liu, X.; Yook, C. Development of Chemical Fingerprints for Quality Control of Xiong Ma Tang and its Related Preparations by High-Performance Liquid Chromatography. J. Chromatogr. Sci. 2016, 54, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.G.; Liang, X.L.; Zhu, J.Y.; Zhao, G.W.; Yang, M.; Wang, G.F.; Jiang, Q.Y.; Chen, X.L. Correlation between synergistic action of Radix Angelica dahurica extracts on analgesic effects of Corydalis alkaloid and plasma concentration of dl-THP. J. Ethnopharmacol. 2010, 129, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, K.; Kushida, H.; Yuzurihara, M.; Wakui, Y.; Yanagisawa, T.; Kamei, H.; Ohmori, S.; Kitada, M. Interaction of drugs and Chinese herbs: Pharmacokinetic changes of tolbutamide and diazepam caused by extract of Angelica dahurica. J. Pharm. Pharmacol. 2000, 52, 1023–1029. [Google Scholar] [CrossRef] [PubMed]
- Luszczki, J.J.; Glowniak, K.; Czuczwar, S.J. Imperatorin enhances the protective activity of conventional antiepileptic drugs against maximal electroshock-induced seizures in mice. Eur. J. Pharmacol. 2007, 574, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yang, H.; Yu, C.; Yuan, M.; Li, H. High hepatic exposure of furanocoumarins in Radix Angelica dahuricae is associated with transporter mediated active uptake. J. Ethnopharmacol. 2018, 212, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Mussa, A.R.; An, L.; Liang, R.; Shi, X.; Kabera, J.N.; He, X. Potential Mechanism of Herb-Drug Interaction Mediated by Angelica dahurica: Inhibition on CYP3A Enzymes in Rats. Pharmacol. Pharm. 2016, 7, 153–161. [Google Scholar] [CrossRef]
- Yi, S.; Cho, J.Y.; Lim, K.S.; Kim, K.P.; Kim, J.; Kim, B.H.; Hong, J.H.; Jang, I.J.; Shin, S.G.; Yu, K.S. Effects of Angelicae tenuissima radix, Angelicae dahuricae radix and Scutellariae radix extracts on cytochrome P450 activities in healthy volunteers. Basic Clin. Pharmacol. Toxicol. 2009, 105, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Tong, S.N. Study on metabolism of ligustrazine in rabbit. Acta Pharm. Sin. 1996, 31, 617–621. [Google Scholar]
- Kuang, X.D.; Li, X.H.; Xiong, Y.Q. Study on metabolism of tetramethylpyrazine in system of rat liver microsomes. China J. Chin. Mater. Med. 2005, 31, 1971–1975. [Google Scholar]
- Lai, G.Z.; Huang, H.Z.; Luo, X.N. 82 Cases report for ligustrazine treatment of magrine. Hebei J. Integr. Tradit. Chin. West. Med. 1999, 8, 400–401. [Google Scholar]
- Tassorelli, C.; Joseph, S.A. Systemic nitroglycerin induces Fos immunoreactivity in brainstem and forebrain structures of the rat. Brain Res. 1995, 682, 167–181. [Google Scholar] [CrossRef]
- Zhang, X.; Yao, Y.; Lou, Y.; Jiang, H.; Wang, X.; Chai, X.; Zeng, S. Metabolism of ebracteolata compound B studied in vitro with human liver microsomes, HepG2 cells, and recombinant human enzymes. Drug Metab. Dispos. 2010, 38, 2157–2165. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Yang, A.; Cui, T.; Zhai, Y.; Zhang, F.; Chen, J.; Jin, C.; Fan, Y.; Wu, Z.; Wang, L.; He, X. Reactive metabolite activation by CYP2C19-mediated rhein hepatotoxicity. Xenobiotica 2014, 45, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Pascual, J. Combination therapy for chronic migraine: Bad news but not the last word. Neurology 2012, 78, 940–941. [Google Scholar] [CrossRef] [PubMed]
- Silberstein, S.D.; Ruoff, G. Combination therapy in acute migraine treatment: The rationale behind the current treatment options. Postgrad. Med. 2006, 20–26. [Google Scholar]
- Leung, S.; Bulloch, B.; Young, C.; Yonker, M.; Hostetler, M. Effectiveness of standardized combination therapy for migraine treatment in the pediatric emergency department. Headache 2013, 53, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Schulman, E.A.; Dermott, K.F. Sumatriptan plus metoclopramide in triptan-nonresponsive migraineurs. Headache 2003, 43, 729–733. [Google Scholar] [CrossRef] [PubMed]
- Marumoto, S.; Oda, Y.; Miyazawa, M. Antigenotoxic activity of naturally occurring furanocoumarins. Environ. Mol. Mutagen. 2011, 52, 646–657. [Google Scholar] [CrossRef] [PubMed]
- Qiao, S.; Shi, X.; Shi, R.; Liu, M.; Liu, T.; Zhang, K.; Wang, Q.; Yao, M.; Zhang, L. Identification of urinary metabolites of imperatorin with a single run on an LC/Triple TOF system based on multiple mass defect filter data acquisition and multiple data mining techniques. Anal. Bioanal. Chem. 2013, 405, 6721–6738. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Jin, M.; Du, Y.; Cao, L.; Xu, H. UPLC-QTOF-MS/MS based screening and identification of the metabolites in rat bile after oral administration of imperatorin. J. Chromatogr. B 2016, 1022, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Zhang, Y.; Xu, W.; Kang, T.; Yang, X. Biotransformation of isoimperatorin by rat liver microsomes and its quantification by LC-MS/MS method. Fitoterapia 2014, 93, 88–97. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |





| Group | Dose (mg/kg) | Time after Injection of Nitroglycerin | |||
|---|---|---|---|---|---|
| 0–30 min | 30–60 min | 60–120 min | 120–180 min | ||
| Control | 0.80 ± 0.34 | 1.21 ± 0.67 | 0.92 ± 0.45 | 1.01 ± 0.42 | |
| Model (nitroglycerin) | 10 | 43.07 ± 5.87 ## | 47.90 ± 3.87 ## | 73.96 ± 3.24 ## | 51.61 ± 2.80 ## |
| Sumatriptan | 0.005 | 39.17 ± 3.92 | 41.02 ± 5.67 | 45.30 ± 4.57 ** | 23.57 ± 4.43 ** |
| TCE | 50 | 46.06 ± 8.23 | 46.78 ± 4.45 | 53.24 ± 4.06 ** | 47.93 ± 4.26 |
| Ligustrazine | 15 | 42.90 ± 8.70 | 45 36 ± 2.45 | 50.50 ± 5.47 ** | 38.40 ± 2.95 ** |
| TCE + ligustrazine | 50 + 15 | 38.20 ± 4.43 | 44.81 ± 3.72 | 40.20 ± 4.48 **& | 28.20 ± 3.05 **&& |
| Ligustrazine | Ligustrazine–Isoimperatorin | Ligustrazine–TCE | |
|---|---|---|---|
| Tmax (min) | 30 | 40 | 130 |
| Cmax (µg/mL) | 8.52 ± 0.87 | 8.83 ± 0.38 | 17.68 ± 2.62 ** |
| t1/2 (min) | 35.59 ± 2.54 | 95.59 ± 5.88 ** | 90.43 ± 7.96 ** |
| ke (min−1) | 0.01947 ± 0.0019 | 0.00725 ± 0.001 * | 0.007 ± 0.0007 ** |
| CL (ml/min) | 244.19 ± 88.55 | 15.67 ± 2.33 ** | 2.38 ± 0.67 ** |
| MRT (min) | 58.41 ± 0.637 | 103.6 ± 6.27 * | 152.8 ± 16.6 ** |
| AUC 0-∞ (µg/mL*min) | 43.000 ± 20.000 | 670.000 ± 180.000 * | 4411.2 ± 988.9 ** |
| CYP450 Isoform | IC50 (µM) (95% Confidence Interval) | ||
|---|---|---|---|
| Imperatorin | Isoimperatorin | Positive Control a | |
| CYP1A2 | 0.08 (0.052–0.13) | 8.26 (5.75–11.88) | 0.09 (0.06–0.12) |
| CYP2C9 | 4.81 (3.05–7.71) | 5.69 (3.21–10.09) | 0.14 (0.10–0.18) |
| CYP2C19 | 83.34 (0.66–104.85) | 19.58 (12.72–30.15) | 6.00 (2.76–13.00) |
| CYP2D6 | 9.10 (5.328–15.56) | 1.56 (0.54–4.5) | 0.05 (0.02–0.11) |
| CYP3A4 | 1.10 (0.71–1.71) | 1.47 (0.99–2.17) | 1.38 (0.74–2.56) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, S.; He, X.; Zhong, P.; Zhao, J.; Huang, C.; Hu, Z. A Metabolism-Based Synergy for Total Coumarin Extract of Radix Angelicae Dahuricae and Ligustrazine on Migraine Treatment in Rats. Molecules 2018, 23, 1004. https://doi.org/10.3390/molecules23051004
Feng S, He X, Zhong P, Zhao J, Huang C, Hu Z. A Metabolism-Based Synergy for Total Coumarin Extract of Radix Angelicae Dahuricae and Ligustrazine on Migraine Treatment in Rats. Molecules. 2018; 23(5):1004. https://doi.org/10.3390/molecules23051004
Chicago/Turabian StyleFeng, Shan, Xin He, Peiru Zhong, Jinyi Zhao, Cong Huang, and Zhuohan Hu. 2018. "A Metabolism-Based Synergy for Total Coumarin Extract of Radix Angelicae Dahuricae and Ligustrazine on Migraine Treatment in Rats" Molecules 23, no. 5: 1004. https://doi.org/10.3390/molecules23051004