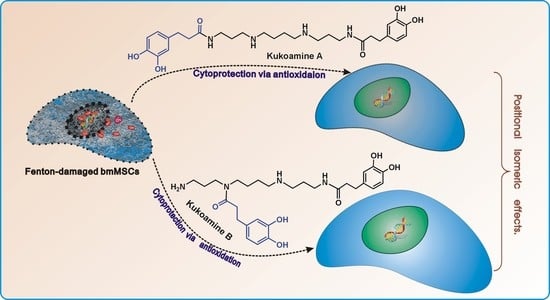
Antioxidant and Cytoprotective Effects of Kukoamines A and B: Comparison and Positional Isomeric Effect
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Chemicals
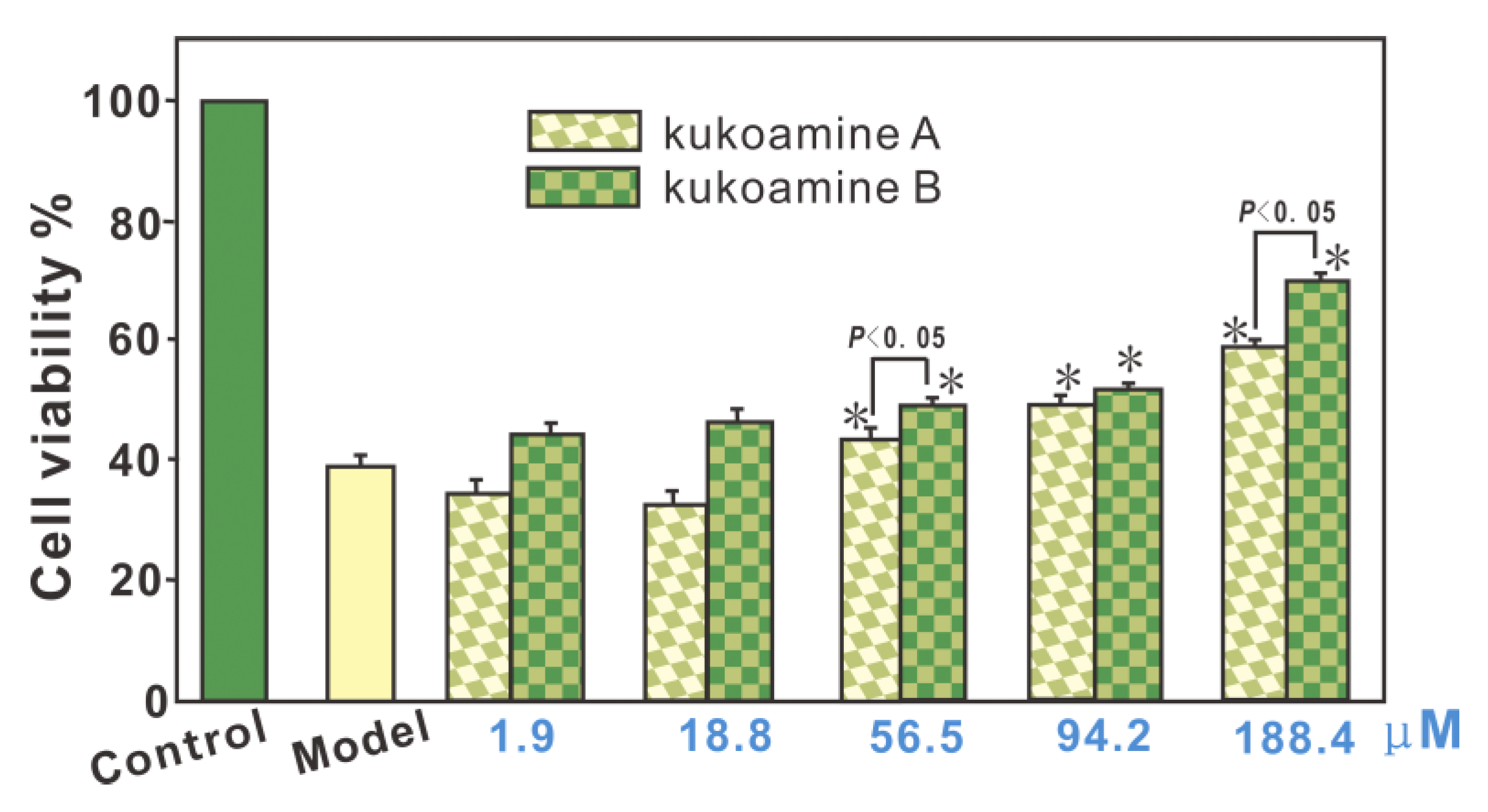
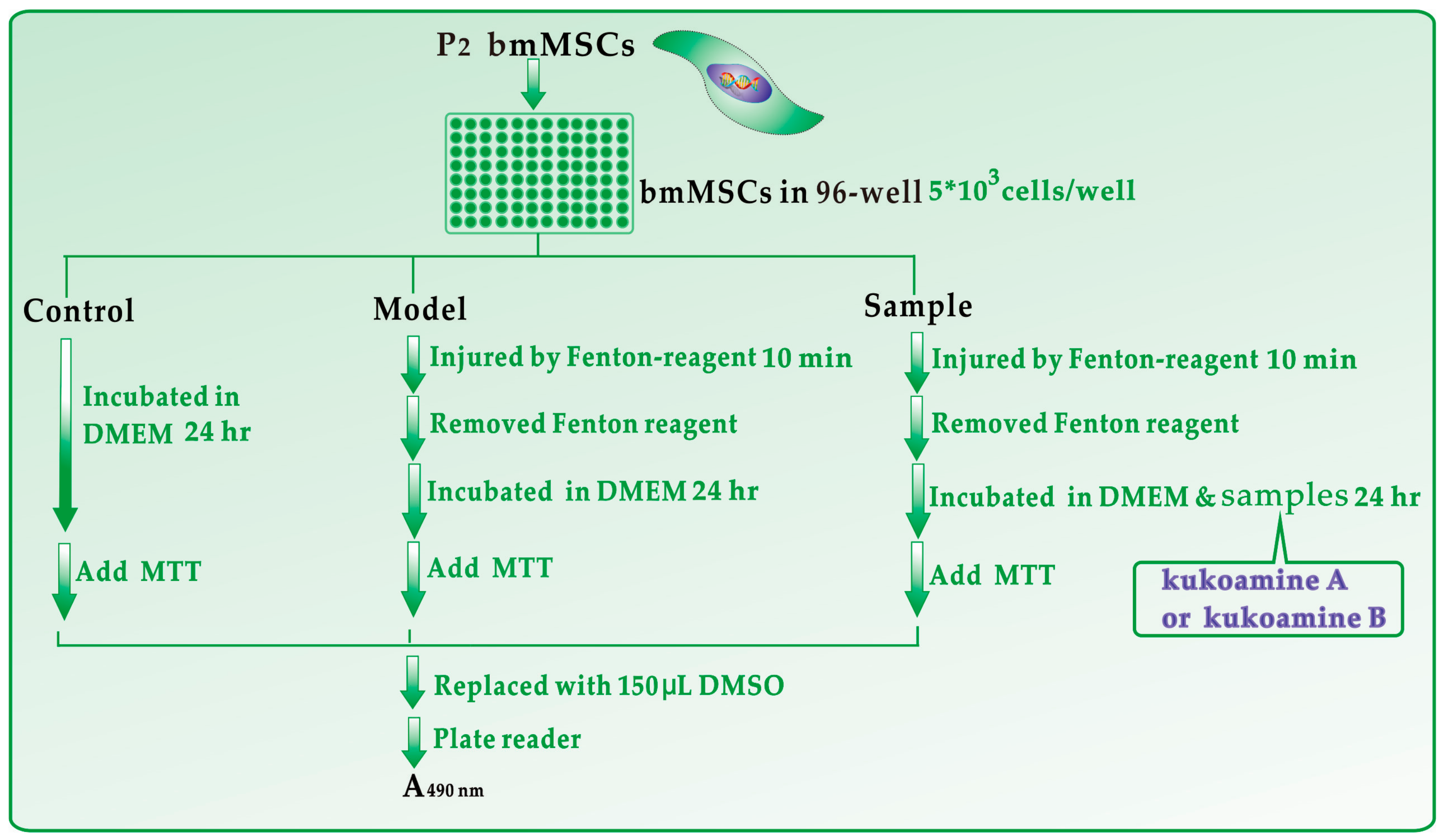
3.2. Protective Effect against Fenton-Induced Damage to bmMSCs (MTT Assay)
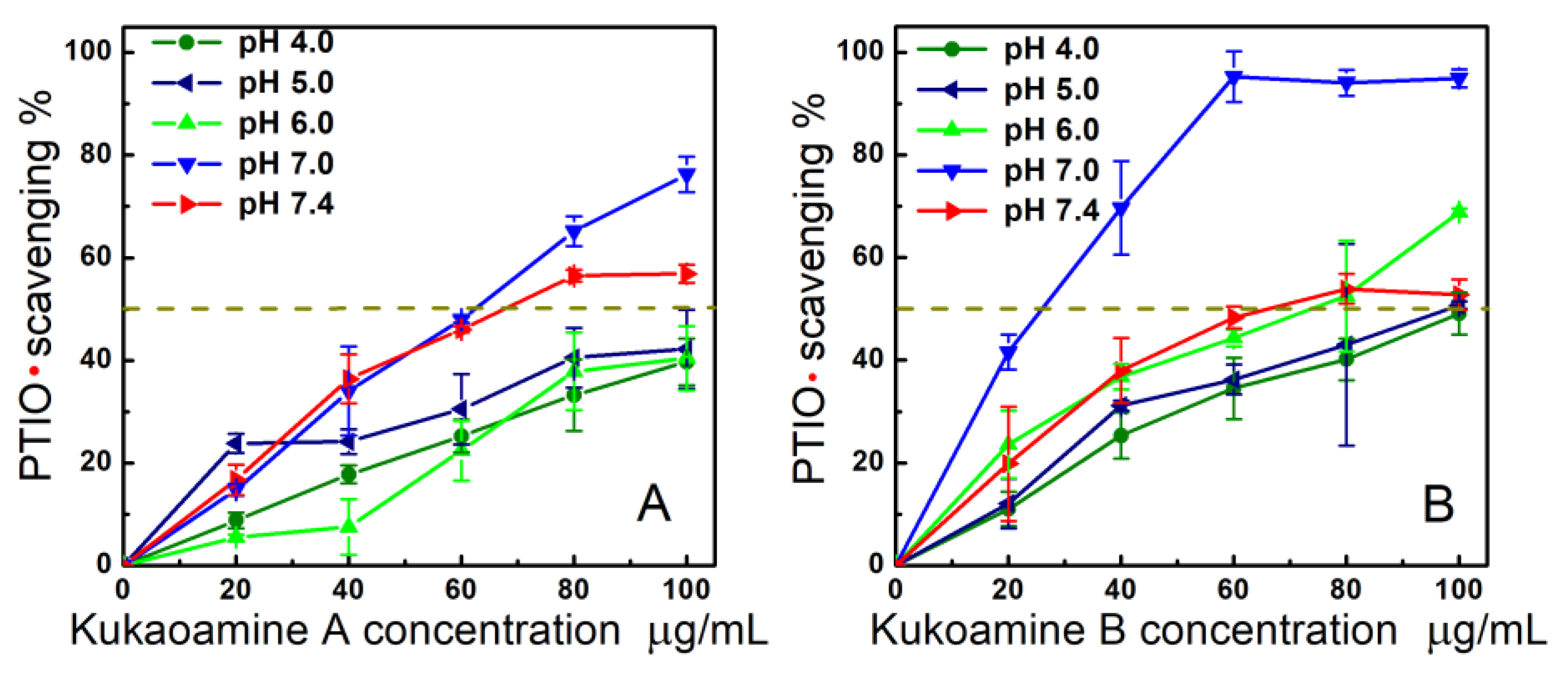
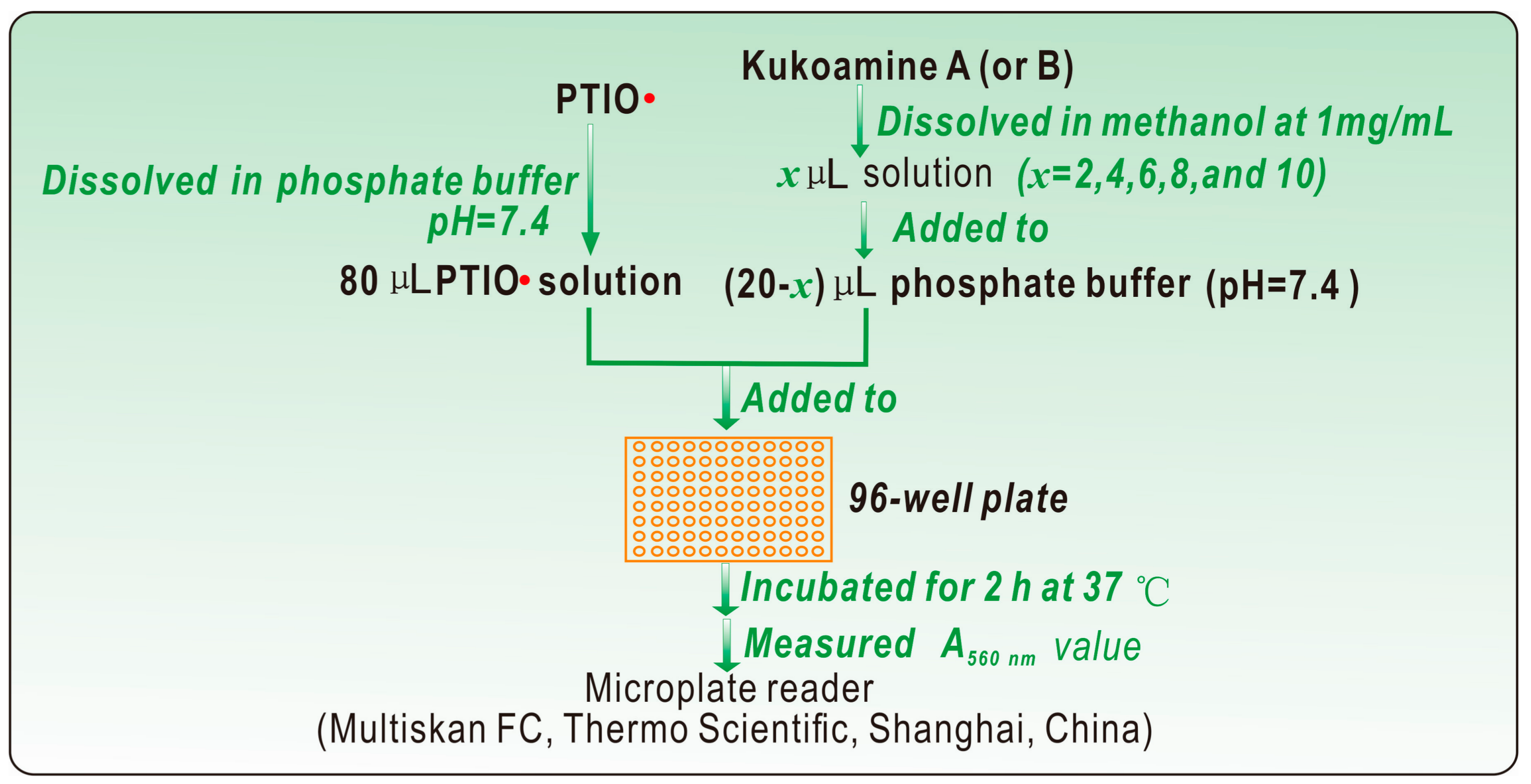
3.3. PTIO•-Scavenging Assay
3.4. Cupric Ions (Cu2+) Reducing Antioxidant Power (CUPRAC) Assay
3.5. DPPH•-Scavenging Assay
3.6. HPLC–ESI–MS/MS Analysis of the Reaction Products of Kukoamine A with DPPH•
3.7. Fe2+-Chelating Assay by Ultraviolet-Visible (UV-Vis) Spectra Analysis
3.8. Deoxyribose Degradation Assay for •OH-Scavenging
3.9. Superoxide Anion Radical (•O2−)-Scavenging Assay
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ABTS | 2,2′-azino-bis(3-ethylbenzo-thiazoline-6-sulfonic acid diammonium salt) |
| bmMSCs | bone marrow-derived mesenchymal stem cells |
| DMEM | Dulbecco’s modified Eagle’s medium |
| DPPH• | 1,1-diphenyl-2-picryl-hydrazl radical |
| FBS | fetal bovine serum |
| FRAP | ferric-reducing antioxidant power |
| HAT | hydrogen atom transfer |
| PTIO• | 2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl 3-oxide radical |
| ROS | reactive oxygen species |
| RAF | radical adduct formation |
| SD | standard deviation |
| TPTZ | 2,4,6-tris(2-pyridyl-s-triazine) |
| Trolox | (±)-6-hydroxyl-2,5,7,8-tetramethlychromane-2-carboxylic acid |
References
- Cai, Y.; Luo, Q.; Sun, M.; Corke, H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004, 74, 2157–2184. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Li, X.; Han, L.; Li, F.; Lu, W.; Bai, Y.; Chen, D. Folium Sennae protects against hydroxyl radical-induced DNA damage via antioxidant mechanism: An in vitro study. Bot. Stud. 2014, 55, 16. [Google Scholar] [CrossRef] [PubMed]
- Shinji, F.; Kozue, Y.; Chohachi, K.; Hiroshi, H. Structure of kukoamine A, a hypotensive principle of Lycium chinense root barks. Tetrahedron Lett. 1980, 21, 1355–1356. [Google Scholar]
- Shinji, F.; Zhang, G.; Shigeo, N.; Kukoamine, B. A spermine alkaloid from Lycium chinense. Phytochemistry 1995, 38, 1529–1531. [Google Scholar]
- Hadjipavlou-Litina, D.; Garnelis, T.; Athanassopoulos, C.M.; Papaioannou, D. Kukoamine A analogs with lipoxygenase inhibitory activity. J. Enzyme Inhib. Med. Chem. 2009, 24, 1188–1193. [Google Scholar] [CrossRef] [PubMed]
- Pegg, A.E. The function of spermine. IUBMB Life 2014, 66, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.L.; Gao, L.Y.; Niu, Y.X.; Tian, X.; Wang, J.; Meng, W.H.; Zhang, Q.; Cui, C.; Han, L.; Zhao, Q.C. Neuroprotection by Kukoamine A against oxidative stress may involve N-methyl-D-aspartate receptors. Biochim. Biophys. Acta 2015, 1850, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cheng, Z.; Wang, C.; Ma, H.; Meng, W.; Zhao, Q. Neuroprotective effects of Kukoamine A against radiation-induced rat brain injury through inhibition of oxidative stress and neuronal apoptosis. Neurochem. Res. 2016, 41, 2549–2558. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Jiang, X.; Zhang, Q.; Lin, S.; Zhu, J.; Zhang, Y.; Du, J.; Hu, X.; Meng, W.; Zhao, Q. Neuroprotective effects of Kukoamine A against cerebral ischemia via antioxidant and inactivation of apoptosis pathway. Neurochem. Int. 2017, 107, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.L.; Guo, L.P.; Song, Q.; Zhang, Q.; Chen, Y.; Wang, J.; Meng, W.H.; Zhao, Q.C.; Kukoamine, B. An amide alkaloid, protects against NMDA-induced neurotoxicity and potential mechanisms in vitro. Neurochem. Int. 2015, 87, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.L.; Niu, Y.X.; Zhang, Q.; Tian, X.; Gao, L.Y.; Guo, L.P.; Meng, W.H.; Zhao, Q.C. Neuroprotective effects of Kukoamine B against hydrogen peroxide-induced apoptosis and potential mechanisms in SH-SY5Y cells. Environ. Toxicol. Pharmacol. 2015, 40, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, X.; Chen, D. Evaluation of antioxidant activity of isoferulic acid in vitro. Nat. Prod. Commun. 2011, 6, 1285–1288. [Google Scholar] [PubMed]
- Koca, S.K.; Sevincek, R.; Akgul, O.; Aygun, M. Positional isomeric effect on the crystallization of chlorine-substituted N-phenyl-2-phthalimidoethanesulfonamide derivatives. Acta Crystallogr. C 2015, 71, 839–843. [Google Scholar] [CrossRef] [PubMed]
- Duan, P.F.; Zhu, X.F.; Liu, M.H. Isomeric effect in the self-assembly of pyridine-containing l-glutamic lipid: Substituent position controlled morphology and supramolecular chirality. Chem. Commun. 2011, 47, 5569–5571. [Google Scholar] [CrossRef] [PubMed]
- Tasso, T.T.; Tsubone, T.M.; Baptista, M.S.; Mattiazzi, L.M.; Acunha, T.V.; Iglesias, B.A. Isomeric effect on the properties of tetraplatinated porphyrins showing optimized phototoxicity for photodynamic therapy. Dalton. Trans. 2017, 46, 11037–11045. [Google Scholar] [CrossRef] [PubMed]
- Denu, R.A.; Hematti, P. Effects of oxidative stress on mesenchymal stem cell biology. Oxid. Med. Cell. Longev. 2016, 1, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Li, X.; Zeng, H. Synthesis, antioxidation activity of (E)-9-p-Tolyl-3-[2-(8-hydroxy-quinol-2-yl)vinyl]-carbazole and (E)-9-(p-Anisyl)-3-[2-(8-hydroxy-quinol-2-yl)vinyl]-carbazole and their induction proliferation of mesenchymal stem cells. Act Chim. Sin. 2009, 67, 9. [Google Scholar]
- Perron, N.R.; Brumaghim, J.L. A review of the antioxidant mechanisms of polyphenol compounds related to iron binding. Cell Biochem. Biophys. 2009, 53, 75–100. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Zhang, S.J.; Zhang, J.L.; Wang, N.; Liu, W.G.; Wang, W. Fenton reaction-initiated formation of biocompatible injectable hydrogels for cell encapsulation. J. Mater. Chem. B 2013, 1, 3932–3939. [Google Scholar] [CrossRef]
- Wang, T.T.; Zeng, G.C.; Li, X.C.; Zeng, H.P. In vitro studies on the antioxidant and protective effect of 2-substituted -8-hydroxyquinoline derivatives against H2O2-induced oxidative stress in BMSCs. Chem. Biol. Drug Des. 2010, 75, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Li, X. 2-Phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl 3-Oxide (PTIO•) radical scavenging: A new and simple antioxidant assay in vitro. J. Agric. Food Chem. 2017, 65, 6288–6297. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.X.; Jin, X.L.; Teng, Q.F.; Chang, J.; Yao, X.J.; Dai, F.; Qian, Y.P.; Tang, J.J.; Li, X.Z.; Zhou, B. Antioxidant activity of α-pyridoin and its derivatives: Possible mechanism. Org. Biomol. Chem. 2010, 8, 1058–1063. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, S.; Russo, A.; Samuni, A. Reactions of PTIO and carboxy-PTIO with •NO, •NO2, and •O2−. J. Biol. Chem. 2003, 278, 50949–50955. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Y.; Wu, W.; Mo, Y.R. Study of proton-coupled electron transfer (PCET) with four explicit diabatic states at the ab initio level. Comput. Theor. Chem. 2017, 1116, 50–58. [Google Scholar] [CrossRef]
- Li, X.; Liu, J.; Lin, J.; Wang, T.; Huang, J.; Lin, Y.; Chen, D. Protective effects of dihydromyricetin against •OH-Induced mesenchymal stem cells damage and mechanistic chemistry. Molecules 2016, 21, 604. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Han, L.; Li, Y.; Zhang, J.; Chen, J.; Lu, W.; Zhao, X.; Lai, Y.; Chen, D.; Wei, G. Protective effect of sinapine against hydroxyl radical-induced damage to mesenchymal stem cells and possible mechanisms. Chem. Pharm. Bull. 2016, 64, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Musialik, M.; Kuzmicz, R.; Pawlowski, T.S.; Litwinienko, G. Acidity of hydroxyl groups: An overlooked influence on antiradical properties of flavonoids. J. Org. Chem. 2009, 74, 2699–2709. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Jiang, Q.; Wang, T.; Liu, J.; Chen, D. Comparison of the antioxidant effects of quercitrin and isoquercitrin: Understanding the role of the 6′’-OH Group. Molecules 2016, 21, 1246. [Google Scholar] [CrossRef] [PubMed]
- Dimic, D.; Milenkovic, D.; Markovic, J.D.; Markovic, Z. Antiradical activity of catecholamines and metabolites of dopamine: Theoretical and experimental study. Phys. Chem. Chem. Phys. 2017, 19, 12970–12980. [Google Scholar] [CrossRef] [PubMed]
- Li, X.C.; Gao, Y.X.; Li, F.; Liang, A.F.; Xu, Z.M.; Bai, Y.; Mai, W.Q.; Han, L.; Chen, D.F. Maclurin protects against hydroxyl radical-induced damages to mesenchymal stem cells: Antioxidant evaluation and mechanistic insight. Chem. Biol. Interact. 2014, 219, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Hung, T.M.; Na, M.; Thuong, P.T.; Su, N.D.; Sok, D.; Song, K.S.; Seong, Y.H.; Bae, K. Antioxidant activity of caffeoyl quinic acid derivatives from the roots of Dipsacus asper Wall. J. Ethnopharmacol. 2006, 108, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic, S.V.; Hara, Y.; Steenken, S.; Simic, M.G. Antioxidant potential of gallocatechins—A pulse-radiolysis and laser photolysis study. J. Am. Chem. Soc. 1995, 117, 9881–9888. [Google Scholar] [CrossRef]
- Nakayama, T.; Uno, B. Importance of proton-coupled electron transfer from natural phenolic compounds in superoxide scavenging. Chem. Pharm. Bull. 2015, 63, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Iuga, C.; Alvarez-Idaboy, J.R.; Russo, N. Antioxidant activity of trans-resveratrol toward hydroxyl and hydroperoxyl radicals: A quantum chemical and computational kinetics study. J. Org. Chem. 2012, 77, 3868–3877. [Google Scholar] [CrossRef] [PubMed]
- Poutsma, M.L. Extension of structure-reactivity correlations for the hydrogen abstraction reaction by bromine atom and comparison to chlorine atom and hydroxyl radical. J. Phys. Chem. A 2016, 120, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.B.; Porter, N.A. Reactivities and products of free radical oxidation of cholestadienols. J. Am. Chem. Soc. 2014, 136, 5443–5450. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.Z.; Deng, G.; Liang, Q.; Chen, D.F.; Guo, R.; Lai, R.C. Antioxidant activity of quercetin and its glucosides from propolis: A theoretical study. Sci. Rep. 2017, 7, 7543. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Y.; Sun, Y.M.; Wang, X.L. Electronic effects on O-H proton dissociation energies of phenolic cation radicals: A DFT study. J. Org. Chem. 2002, 67, 2709–2712. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.F.; Zeng, H.P.; Du, S.H.; Li, H.; Zhou, J.H.; Li, Y.W.; Wang, T.T.; Hua, Z.C. Extracts from Plastrum testudinis promote proliferation of rat bone-marrow-derived mesenchymal stem cells. Cell Prolif. 2007, 40, 196–212. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.T.; Li, X.C.; Li, Y.R.; Chen, D.F. Mechanistic chemistry of extraordinary capacity of salvianolic acid B on oxidatively damaged mesenchymal stem cells. J. Chin. Chem. Soc. 2016, 63, 924–929. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Ozyürek, M.; Esin, K.S.; Erçağ, E. The cupric ion reducing antioxidant capacity and polyphenolic content of some herbal teas. Int. J. Food Sci. Nutr. 2006, 57, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lin, J.; Han, W.; Mai, W.; Wang, L.; Li, Q.; Lin, M.; Bai, M.; Zhang, L.; Chen, D. Antioxidant ability and mechanism of rhizoma Atractylodes macrocephala. Molecules 2012, 17, 13457–13472. [Google Scholar] [CrossRef] [PubMed]
- Li, X. Solvent effects and improvements in the deoxyribose degradation assay for hydroxyl radical-scavenging. Food Chem. 2013, 141, 2083–2088. [Google Scholar] [CrossRef] [PubMed]
- Li, X. Improved pyrogallol autoxidation method: A reliable and cheap superoxide-scavenging assay suitable for all antioxidants. J. Agric. Food Chem. 2012, 60, 6418–6424. [Google Scholar] [CrossRef] [PubMed]
- Li, X.C.; Mai, W.Q.; Chen, D.F. Chemical study on protective effect against hydroxyl-induced DNA damage and antioxidant mechanism of myricitrin. J. Chin. Chem. Soc. 2014, 61, 383–391. [Google Scholar] [CrossRef]
Sample Availability: Sample of the compound kukoamines A is available from the authors. |






| Assays | kukoamine A μg/mL (μM) | kukoamine B μg/mL (μM) | Trolox |
|---|---|---|---|
| PTIO•-scavenging (pH 7.4) | 74.9 ± 2.3 (140.1 ± 4.3 a,B) | 78.1 ± 2.6 (148.6 ± 5.0 a,B) | 83.8 ± 5.4 (333.3 ± 21.5 b) |
| PTIO•-scavenging (pH 7.0) | 63.1 ± 1.4 (118.9 ± 2.6 b,A) | 15.6 ± 3.2 (29.4 ± 6.1 a,A) | ND |
| PTIO•-scavenging (pH 6.0) | 163.7 ± 14.5 (308.4 ± 27.2 b,D) | 69.0 ± 4.1 (130.0 ± 7.6 a,B) | ND |
| PTIO•-scavenging (pH 5.0) | 147.3 ± 27.0 (277.6 ± 51.0 b,C) | 94.4 ± 13.4 (177.9 ± 25.3 a,C) | ND |
| PTIO•-scavenging (pH 4.0) | 162.7 ± 3.3 (306.5 ± 6.1 b,D) | 110.8 ± 9.4 (208.8 ± 17.7 a,D) | ND |
| Cu2+-reducing | 5.5 ± 0.2 (10.3 ± 0.3 b) | 4.7 ± 0.2 (8.9 ± 0.3 a) | 7.04 ± 0.1 (28.1 ± 0.5 d) |
| DPPH•-scavenging | 46.0 ± 0.4 (86.6 ± 0.7 c) | 39.1 ± 0.4 (73.7 ± 0.7 b) | 14.5 ± 0.8 (58.1 ± 3.0 a) |
| •O2--scavenging | 213.0± 2.7 (401.4 ± 5.0 b) | 147.6 ± 1.8 (278.2 ± 3.4 a) | 111.9 ± 0.6 (447.2 ± 2.3 c) |
| •OH-scavenging | 89.0 ± 1.7 (167.7 ± 3.2 b) | 78.6 ± 4.7 (146.9 ± 8.8 a) | 101.6 ± 4.0 (405.8 ± 16.0 c) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Lin, J.; Chen, B.; Xie, H.; Chen, D. Antioxidant and Cytoprotective Effects of Kukoamines A and B: Comparison and Positional Isomeric Effect. Molecules 2018, 23, 973. https://doi.org/10.3390/molecules23040973
Li X, Lin J, Chen B, Xie H, Chen D. Antioxidant and Cytoprotective Effects of Kukoamines A and B: Comparison and Positional Isomeric Effect. Molecules. 2018; 23(4):973. https://doi.org/10.3390/molecules23040973
Chicago/Turabian StyleLi, Xican, Jian Lin, Ban Chen, Hong Xie, and Dongfeng Chen. 2018. "Antioxidant and Cytoprotective Effects of Kukoamines A and B: Comparison and Positional Isomeric Effect" Molecules 23, no. 4: 973. https://doi.org/10.3390/molecules23040973