Cultivar and Harvest Month Influence the Nutrient Content of Opuntia spp. Cactus Pear Cladode Mucilage Extracts
Abstract
:1. Introduction
2. Results and Discussion
2.1. Differences in the Nutrient Content Observed among the Four Cultivars
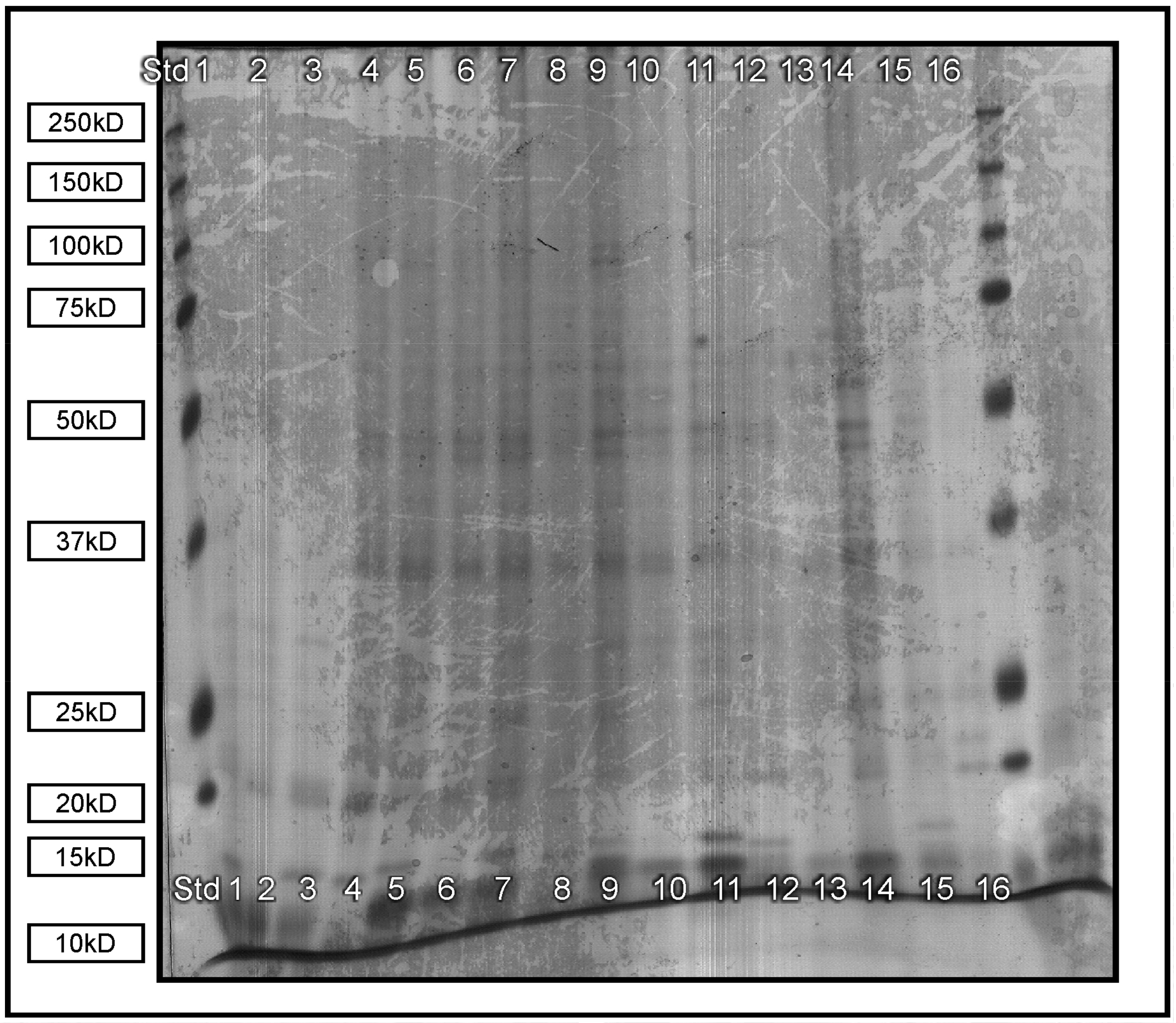
2.1.1. Protein Content
2.1.2. Total Extractable Fat Content (EFC) and Fatty Acid Analysis
2.1.3. Indigestible Carbohydrates
2.1.4. Mineral Content
2.1.5. Calcium Oxalate Crystals
2.2. Differences in Nutrient Content Observed over the Six Months of Harvest
2.2.1. Gross Energy and Digestible Carbohydrates
2.2.2. Ash and Mineral Content
3. Materials and Methods
3.1. Sample Collection
3.2. Methods
3.3. Statistical Methods
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| CAM | Crassulacean acid metabolism |
| EFC | Extractable fat content |
| MUFA | Monounsaturated fatty acids |
| PUFA | Polyunsaturated fatty acids |
| SFA | Saturated fatty acids |
References
- Li, J.M.; Nie, S.P. The functional and nutritional aspects of hydrocolloids in foods. Food Hydrocoll. 2014, 53, 46–61. [Google Scholar] [CrossRef]
- Du Toit, A.; De Wit, M.; Naudé, S.; Taljaard, M.; Fouché, H.J.; Hugo, A.; Venter, S.L. Determination of the functional properties of cactus pear mucilage from cladodes of four South African cultivars. In Proceedings of the 9th International Congress on Cactus Pear and Cochineal, Coquimbo, Chile, 26–30 March 2017. [Google Scholar]
- Sepúlveda, E.; Sáenz, C.; Aliaga, E.; Aceituno, C. Extraction and characterization of mucilage in Opuntia spp. J. Arid Environ. 2007, 68, 534–545. [Google Scholar] [CrossRef]
- Cárdenas, A.; Higuera-Ciapara, I.; Goycoolea, F.M. Rheology and aggregation of cactus (Opuntia ficus-indica) mucilage in solution. J. Prof. Assoc. Cactus Dev. 1997, 2, 152–159. [Google Scholar]
- Du Toit, A.; De Wit, M.; Seroto, K.D.; Fouché, H.J.; Hugo, A.; Venter, S.L. Rheological characterization of cactus pear mucilage for application in nutraceutical food products. In Proceedings of the 9th International Congress on Cactus Pear and Cochineal, Coquimbo, Chile, 26–30 March 2017. [Google Scholar]
- Rocchetti, G.; Pellizzoni, M.; Montesano, D.; Lucini, L. Italian Opuntia ficus-indica cladodes as rich source of bioactive compounds with health-promoting properties. Foods 2018, 7, 24. [Google Scholar] [CrossRef] [PubMed]
- Nazareno, M.A. New insights about medicinal uses and health-beneficial properties of cactus products. In Proceedings of the 7th International Congress on Cactus Pear and Cochineal, Agadir, Morocco, 17–22 October 2010. [Google Scholar]
- Feugang, J.M.; Konarski, P.; Zou, C.; Stintzing, F.C. Nutritional and medicinal use of cactus pear (Opuntia spp.) cladodes and fruits. Front. Biosci. 2006, 11, 2574–2589. [Google Scholar] [CrossRef] [PubMed]
- Salisbury, F.B.; Ross, C.W. Plant Physiology, 4th ed.; Wadsworth Publishing Company: Belmont, CA, USA, 1992; ISBN 100534151620. [Google Scholar]
- Van Sittert, L. ‘Our irrepressible fellow-colonist’: The biological invasion of prickly pear (Opuntia ficus-indica) in the Eastern Cape c.1890–c.1910. J. Hist. Geogr. 2002, 28, 397–419. [Google Scholar] [CrossRef]
- Moreno, F.J.; Clemente, A. 2S Albumin Storage Proteins: What Makes them Food Allergens? Open Biochem. J. 2008, 2, 16–28. [Google Scholar] [CrossRef] [PubMed]
- El-Mostafa, K.; El Kharrassi, Y.; Badreddine, A.; Andreoletti, P.; Vamecq, J.; El Kebbaj, M.S.; Latruffe, N.; Lizard, G.; Nasser, B.; Cherkaoui-Malki, M. Nopal cactus (Opuntia ficus-indica) as a source of bioactive compounds for nutrition, health and disease. Molecules 2014, 19, 14879–14901. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Urbiola, M.I.; Contreras-Padilla, M.; Pérez-Torrero, E.; Hernández-Quevedo, G.; Rojas-Molina, J.I.; Cortes, M.E.; Rodríquez-Garcia, M.E. Study of nutritional composition of nopal (Opuntia ficus-indica cv. Redonda) at different maturity stages. Open Nutr. J. 2010, 4, 11–16. [Google Scholar] [CrossRef]
- Majdoub, H.; Roudesli, S.; Picton, L.; Le Cerf, D.; Muller, G.; Grisel, M. Prickly pear nopals pectin from Opuntia ficus-indica physico-chemical study in dilute and semi-dilute solutions. Carbohydr. Polym. 2001, 46, 69–79. [Google Scholar] [CrossRef]
- Gebresamuel, N.; Gebre-Mariam, T. Comparative physico-chemical characterization of the mucilages of two cactus pears (Opuntia spp.) obtained from Mekelle, Northern Ethiopia. J. Biomater. Nanobiotechnol. 2012, 3, 79–86. [Google Scholar] [CrossRef]
- Fenema, O.R. Food Chemistry, 3rd ed.; Fennema, O.R., Ed.; Marcel Dekker: Madison, WI, USA, 1996; ISBN 0824793463. [Google Scholar]
- Akoh, C.C. Fat replacers. Food Technol. 1998, 52, 47–53. [Google Scholar]
- Charley, H. Food Science, 2nd ed.; John Wiley and Sons: Etobicoke, ON, Canada, 1982. [Google Scholar]
- Whitney, E.; Rolfes, S.R. Understanding Nutrition, 14th ed.; Wadsworth Publishing Company: Belmont, CA, USA, 2015; ISBN 101305396456. [Google Scholar]
- Bennion, M. Introductory Foods, 8th ed.; Collier Macmillan Publishers: New York, NY, USA, 1985. [Google Scholar]
- Sáenz, C. Cladodes: A source of dietary fiber. J. Prof. Assoc. Cactus Dev. 1997, 2, 117–123. [Google Scholar]
- Franceschi, V.R.; Horner, H.J. Calcium oxalate crystals in plants. Bot. Rev. 1980, 46, 361–427. [Google Scholar] [CrossRef]
- McConn, M.M.; Nakata, P.A. Oxalate reduces calcium availability in the pads of the prickly pear cactus through formation of calcium oxalate crystals. J. Agric. Food Chem. 2004, 52, 1371–1374. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Padilla, M.; Rivera-Muñoz, E.M.; Gutiérrez-Cortez, E.; del López, A.R.; Rodríguez-García, M.E. Characterization of crystalline structures in Opuntia ficus-indica. J. Biol. Phys. 2015, 41. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Molina, I.; Gutiérrez-Cortez, E.; Bah, M.; Rojas-Molina, A.; Ibarra-Alvarado, C.; Rivera-Muñoz, E.; Del Real, A.; Aguilera-Barreiro, M. Characterization of calcium compounds in Opuntia ficus-indica as a source of calcium for human diet. J. Chem. 2015, 7, 1–8. [Google Scholar] [CrossRef]
- World Weather and Climate Information Bloemfontein, South Africa. Available online: https://weather-and-climate.com/average-monthly-Rainfall-Temperature-Sunshine,bloemfontein,South-Africa (accessed on 24 June 2016).
- Potgieter, J.P. The Influence of Environmental Factors on Spineless Cactus Pear (Opuntia spp.) Fruit Yield in Limpopo Province, South Africa. Ph.D. Thesis, University of the Free State, Bloemfontein, South Africa, 2007. [Google Scholar]
- Sáenz, C.; Pak, N.; Lecaros, M.; Sepulveda, E. Chemical and physical characterization of cactus cladode (Opuntia ficus-indica) powder. Ital. J. Food Sci. 2010, 22, 416–423. [Google Scholar]
- Ayadi, M.A.; Abdelmaksoud, W.; Ennouri, M.; Attia, H. Cladodes from Opuntia ficus-indica as a source of dietary fiber: Effect on dough characteristics and cake making. Ind. Crops Prod. 2009, 30, 40–47. [Google Scholar] [CrossRef]
- Aguilera-Barreiro, M.; Rivera-Marquez, J.A.; Trujillo-Arriaga, H.M.; Orozco, J.A.T.; Barreira-Mercado, E.; Rodrıquez-Garcia, M.E. Intake of dehydrated nopal (Opuntia ficus-indica) improves bone mineral density and calciuria in adult Mexican woman. Food Nutr. Res. 2013. [Google Scholar] [CrossRef] [PubMed]
- Du Toit, A.; De Wit, M. A Process for Extracting Mucilage from Opuntia ficus-indica and Aloe barbadensis 2011. Patent No. PA153178/P, 12 May 2011. [Google Scholar]
- Aeckerle, E. Process for Improving the Stability of Hygroscopic Substances 1941. U.S. Patent US2238149A, 15 April 1941. [Google Scholar]
- Du Toit, A. Selection, Extraction, Characterization and Application of Mucilage from Cactus Pear (Opuntia ficus-indica and Opuntia robusta) Cladodes. Ph.D. Thesis, University of the Free State, Bloemfontein, South Africa, 2016. [Google Scholar]
- Leco FP-528 Protein/Nitrogen Determinator. FP-528 Instruction Manual, version 1.2.; Leco Corporation: Saint Joseph, MI, USA, 2001.
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis of AOAC International, 17th ed.; Horwitz, Ed.; AOAC: Washington, DC, USA, 2000; Volume 1. [Google Scholar]
- Laemmli, U.K. Most commonly used discontinious buffer systems for SDS electrophoresis. Nature 1970, 277, 680–685. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Slaone-Stanley, G.H. A simple method for the isolation and purification of total lipids from animal tissue. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [PubMed]
- Park, P.W.; Goins, R.E. In situ preparation of fatty acid methyl esters for analysis of fatty acid composition in foods. J. Food Sci. 1994, 59, 1262–1266. [Google Scholar] [CrossRef]
- Food and Agriculture Organization (FAO). Food and Agriculture Organization (FAO). Food energy—Methods of analysis and convertion factors. In Food and Agriculture Organization of the United Nations Technical Workshop Report; FAO: Rome, Italy, 2003; pp. 1–7. ISBN 92-5-105014-7. [Google Scholar]
- Goering, H.K.; Van Soest, P.J. Forage fiber analyses (apparatus, reagents, procedures, and some applications). In Agriculture Handbook; U.S. Agricultural Research Service: New Orleans, LA, USA, 1970; p. 20. [Google Scholar]
- Roberson, J.B.; Van Soest, P.J. The Detergent System of Analysis and its application to human foods. In The Analysis of Dietary Fiber in Food; James, W.P.T., Theader, O.D., Eds.; Marcel Dekker: New York, NY, USA, 1981; pp. 123–158. [Google Scholar]
- Anonymous. Methods of Biochemical Analysis and Food Analysis Using Test-Combinations; Boehringer Mannheim GmbH: Mannheim, Germany, 1986. [Google Scholar]
- Hesse, P.R. A Textbook of Soil Chemical Analysis; John Murray Ltd.: London, UK, 1971. [Google Scholar]
- The Non-Affiliated Soil Analysis Work Committee. Handbook of Standard Soil Testing Methods for Advisory Purposes; Soil Science Society of South Africa: Pretoria, South Africa, 1990. [Google Scholar]
- Glauert, A.M. (Ed.) Practical Methods in Electron Microscopy, 3rd ed.; North Holland Publishing Company: Amsterdam, The Netherlands, 1974. [Google Scholar]
- NCSS Statistical Software. Statistical Systems for Windows: Number Cruncher Statistical System; NCSS: Kaysville, UT, USA, 2007. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |
| Extractable Fat Content | Gross Energy | Total Carbohydrates | Starch | Acid-Detergent Fibre | Neutral-Detergent Fibre | ||
|---|---|---|---|---|---|---|---|
| g/100 g | kJ/g | g/100 g | g/100 g | g/kg | g/kg | ||
| Cultivar | Algerian | 0.5 ± 0.2 a | 9.8 ± 0.9 | 62.4 ± 3.5 | 5.7 ± 1.1 | 0.9 ± 0.8 | 0.7 ± 0.6 a |
| Morado | 0.6 ± 0.2 ab | 10.3 ± 1.1 | 62.8 ± 3.5 | 5.5 ± 0.1 | 1.1 ± 0.1 | 1.4 ± 0.1 a | |
| Gymno-Carpo | 0.4 ± 0.2 a | 10.1 ± 0.1 | 62.6 ± 3.4 | 6.1 ± 0.9 | 1.5 ± 1.5 | 1.7 ± 2.1 ab | |
| Robusta | 0.9 ± 0.3 b | 10.1 ± 0.1 | 62.1 ± 5.2 | 6.5 ± 1.9 | 2.3 ± 1.9 | 4.5 ± 3.1 b | |
| Significance level | (p = 0.012) | (p = 0.332) | (p = 0.989) | (p < 0.586) | (p = 0.321) | (p = 0.014) | |
| Month | February | 0.5 ± 0.2 | 9.1 ± 0.3 a | 59.1 ± 2.7 a | 5.4 ± 0.3 ab | 1.4 ± 1.1 | 1.6 ± 0.4 |
| April | 0.4 ± 0.2 | 9.2 ± 0.6 a | 56.6 ± 0.6 a | 5.1 ± 1.1 a | 0.5 ± 0.4 | 0.6 ± 0.5 | |
| May | 0.7 ± 0.2 | 10.3 ± 0.5 b | 63.6 ± 0.9 b | 5.5 ± 0.9 ab | 1.8 ± 1.6 | 3.3 ± 3.1 | |
| June | 0.7 ± 0.2 | 10.6 ± 0.3 bc | 65.8 ± 1.0 b | 5.7 ± 0.8 ab | 2.4 ± 0.3 | 2.0 ± 1.8 | |
| July | 0.6 ± 0.4 | 10.7 ± 0.6 bc | 64.9 ± 0.6 b | 6.2 ± 0.9 ab | 1.2 ± 2.3 | 2.7 ± 4.5 | |
| August | 0.8 ± 0.4 | 11.6 ± 0.5 c | 64.9 ± 1.9 b | 7.7 ± 1.7 b | 1.4 ± 1.4 | 2.4 ± 1.5 | |
| Significance level | (p = 0.359) | (p < 0.001) | (p < 0.001) | (p = 0.032) | (p = 0.523) | (p = 0.694) | |
| Means | 0.6 ± 0.2 | 10.2 ± 0.1 | 62.5 ± 3.7 | 5.6 ± 0.9 | 1.4 ± 0.7 | 2.1 ± 0.1 | |
| Fatty Acids | Abbreviation | Algerian | Morado | Gymno-Carpo | Robusta | Mean | Significance Level | |
|---|---|---|---|---|---|---|---|---|
| % of Total Fatty Acids | ||||||||
| Individual fatty Acids: | Myristic | C14:0 | 0.1 ± 0.03 | 0.1 ± 0.01 | 0.1 ± 0.02 | 0.1 ± 0.01 | 0.1 ± 0.01 | p = 0.614 |
| Palmitic | C16:0 | 14.1 ± 1.8 | 13.8 ± 1.2 | 12.8 ± 1.5 | 15.2 ± 1.0 | 14.0 ± 0.5 | p = 0.094 | |
| Palmitoleic | C16:1c9 | 0.7 ± 0.04 | 0.7 ± 0.1 | 0.6 ± 0.2 | 0.6 ± 0.1 | 0.7 ± 0.05 | p = 0.142 | |
| Margaric | C17:0 | 0.02 ± 0.001 | 0.02 ± 0.001 | 0.02 ± 0.001 | 0.02 ± 0.001 | 0.02 ± 0.001 | p = 0.103 | |
| Heptadecenoic | C17:1c10 | 0.04 ± 0.01 | 0.1 ± 0.04 | 0.1 ± 0.1 | 0.1 ± 0.02 | 0.1 ± 0.02 | p = 0.59 | |
| Stearic acid | C18:0 | 3.1 ± 0.1 | 2.9 ± 0.4 | 2.9 ± 0.3 | 2.9 ± 0.4 | 3.0 ± 0.4 | p = 0.823 | |
| Oleic | C18:1c9 | 15.6 ± 4.5 a | 20.1 ± 4.1 ab | 16.1 ± 5.4 ab | 23.5 ± 4.2 b | 19.1 ± 1.8 | p = 0.023 | |
| Linoleic | C18:2c9,12 (n−6) | 65.3 ± 5.2 ab | 60.6 ± 5.0 ab | 66.5 ± 6.8 b | 56.2 ± 5.2 a | 62.1 ± 2.0 | p = 0.018 | |
| Arachidic | C20:0 | 0.1 ± 0.04 a | 0.2 ± 0.1 a | 0.1 ± 0.04 a | 0.5 ± 0.12 b | 0.2 ± 0.1 | p < 0.001 | |
| Eicosenoic | C20:1c11 | 0.2 ± 0.02 | 0.2 ± 0.02 | 0.2 ± 0.02 | 0.2 ± 0.03 | 0.2 ± 0.01 | p = 0.335 | |
| α-Linolenic | C18:3c9,12,15 (n-3) | 0.2 ± 0.1 a | 0.2 ± 0.1 a | 0.1 ± 0.1 a | 0.3 ± 0.04 b | 0.2 ± 0.1 | p = 0.018 | |
| Behenic | C22:0 | 0.1 ± 0.03 a | 0.2 ± 0.1 ab | 0.2 ± 0.1 ab | 0.2 ± 0.04 b | 0.2 ± 0.02 | p = 0.041 | |
| Eicosatrienoic | C20:3c8,11,14 (n-6) | 0.1 ± 0.1 a | 0.1 ± 0.1 a | 0.1 ± 0.1 a | 0.4 ± 0.2 b | 0.2 ± 0.1 | p < 0.001 | |
| Lignoceric | C24:0 | 0.03 ± 0.02 | 0.04 ± 0.02 | 0.03 ± 0.02 | 0.1 ± 0.03 | 0.04 ± 0.02 | p = 0.163 | |
| Fatty Acid Ratios: | Saturated Fatty Acids | 17.7 ± 2.7 | 17.3 ± 1.3 | 16.3 ± 1.5 | 18.8 ± 1.2 | 17.5 ± 0.5 | p = 0.117 | |
| Monounsaturated | 16.8 ± 4.5 a | 21.9 ± 4.1 ab | 17.0 ± 5.4 a | 24.3 ± 4.1 b | 20.0 ± 1.2 | p = 0.023 | ||
| Polyunsaturated | 65.5 ± 5.1 ab | 60.9 ± 4.5 ab | 66.8 ± 6.7 b | 56.9 ± 5.2 a | 64.4 ± 1.9 | p = 0.022 | ||
| PUFA:SFA Ratio | 3.8 ± 0.8 ab | 3.6 ± 0.6 ab | 4.2 ± 0.7 b | 3.0 ± 0.4 a | 3.6 ± 0.2 | p = 0.044 | ||
| Ash | Ca | K | Mg | P | Na | Mn | Fe | Cu | Zn | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| g/100 g | g/100 g | g/100 g | g/100 g | mg/kg | mg/kg | mg/kg | mg/kg | mg/kg | mg/kg | ||
| Cultivar | Algerian | 18.8 ± 2.5 | 3.3 ± 0.9 | 2.9 ± 0.7 ab | 2.6 ± 0.3 b | 113.8 ± 24.1 | 86.3 ± 34.6 | 190.9 ± 30.1 | 32.3 ± 25.3 | 6.2 ± 1.0 | 23.7 ± 4.6 |
| Morado | 17.1 ± 2.0 | 3.0 ± 0.9 | 2.3 ± 0.5 a | 2.6 ± 0.2 b | 93.9 ± 11.3 | 129.3 ± 50.1 | 185.8 ± 118.7 | 18.3 ± 18.0 | 5.7 ± 0.7 | 25.7 ± 4.2 | |
| Gymno-Carpo | 18.1 ± 2.5 | 3.0 ± 0.8 | 2.6 ± 1.0 ab | 2.7 ± 0.3 b | 100.2 ± 26.7 | 114.3 ± 46.4 | 220.7 ± 88.1 | 15.7 ± 18.8 | 5.4 ± 1.1 | 24.3 ± 3.0 | |
| Robusta | 16.8 ± 3.4 | 2.3 ± 1.2 | 3.3 ± 0.1 b | 2.0 ± 0.1 a | 129.9 ± 132.0 | 143.2 ± 72.6 | 156.2 ± 84.1 | 22.2 ± 23.7 | 4.9 ± 1.2 | 24.5 ± 6.4 | |
| Significance level | (p = 0.545) | (p = 0.854) | (p = 0.043) | (p < 0.001) | (p = 0.808) | (p = 0.311) | (p = 0.647) | (p = 0.572) | (p = 0.235) | (p = 0.904) | |
| Month | February | 21.0 ± 1.1 b | 3.5 ± 0.9 bc | 3.7 ± 0.8 | 2.8 ± 0.4 | 125.2 ± 26.3 | 170.0 ± 18.7 b | 302.5 ± 82.1 b | 31.6 ± 4.8 ab | 7.1 ± 0.8 b | 27.8 ± 3.3 b |
| April | 20.1 ± 1.1 b | 4.6 ± 0.4 c | 2.6 ± 1.0 | 2.4 ± 0.2 | 103.0 ± 18.1 | 133.1 ± 92.9 ab | 136.9 ± 16.4 a | 3.9 ± 1.3 a | 5.6 ± 0.5 ab | 26.5 ± 5.1 ab | |
| May | 17.0 ± 0.7 a | 2.1 ± 0.4 a | 2.8 ± 0.5 | 2.5 ± 0.4 | 85.9 ± 14.5 | 79.8 ± 14.5 ab | 136.4 ± 50.4 a | 2.6 ± 1.3 a | 5.0 ± 0.4 a | 28.3 ± 4.3 b | |
| June | 16.1 ± 1.5 a | 2.5 ± 0.5 ab | 2.4 ± 0.5 | 2.5 ± 0.4 | 166.2 ± 156.1 | 70.5 ± 20.5 a | 129.9 ± 53.2 a | 16.9 ± 28.1 ab | 4.9 ± 1.4 a | 22.3 ± 1.7 ab | |
| July | 15.6 ± 1.2 a | 2.6 ± 0.4 ab | 2.6 ± 0.4 | 2.3 ± 0.3 | 88.7 ± 20.6 | 113.0 ± 37.9 ab | 222.3 ± 65.0 ab | 28.8 ± 20.9 ab | 5.3 ± 0.7 a | 20.5 ± 3.3 a | |
| August | 15.6 ± 1.4 a | 2.75 ± 0.5 ab | 2.5 ± 0.2 | 2.3 ± 0.4 | 87.8 ± 16.8 | 143.3 ± 38.4 ab | 202.4 ± 80.2 ab | 48.9 ± 9.0 b | 5.4 ± 0.9 ab | 22.0 ± 3.0 ab | |
| Significance level | (p < 0.001) | (p < 0.001) | (p = 0.090) | (p = 0.463) | (p = 0.498) | (p = 0.034) | (p = 0.005) | (p = 0.002) | (p = 0.015) | (p = 0.023) | |
| Means | 17.7 ± 2.3 | 3.0 ± 0.9 | 2.8 ± 0.5 | 2.5 ± 0.2 | 109.5 ± 31.5 | 118.3 ± 38.3 | 188.4 ± 68.0 | 22.1 ± 17.8 | 5.5 ± 0.8 | 24.5 ± 3.34 | |
| % Ash | − | − | − | − | − | 0.01 | 0.002 | N/A | 0.002 | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du Toit, A.; De Wit, M.; Hugo, A. Cultivar and Harvest Month Influence the Nutrient Content of Opuntia spp. Cactus Pear Cladode Mucilage Extracts. Molecules 2018, 23, 916. https://doi.org/10.3390/molecules23040916
Du Toit A, De Wit M, Hugo A. Cultivar and Harvest Month Influence the Nutrient Content of Opuntia spp. Cactus Pear Cladode Mucilage Extracts. Molecules. 2018; 23(4):916. https://doi.org/10.3390/molecules23040916
Chicago/Turabian StyleDu Toit, Alba, Maryna De Wit, and Arno Hugo. 2018. "Cultivar and Harvest Month Influence the Nutrient Content of Opuntia spp. Cactus Pear Cladode Mucilage Extracts" Molecules 23, no. 4: 916. https://doi.org/10.3390/molecules23040916
APA StyleDu Toit, A., De Wit, M., & Hugo, A. (2018). Cultivar and Harvest Month Influence the Nutrient Content of Opuntia spp. Cactus Pear Cladode Mucilage Extracts. Molecules, 23(4), 916. https://doi.org/10.3390/molecules23040916







