Synthesis, Structural Studies and Biological Evaluation of Connections of Thiosemicarbazide, 1,2,4-Triazole and 1,3,4-Thiadiazole with Palmitic Acid
Abstract
:1. Introduction
2. Results and Discussion
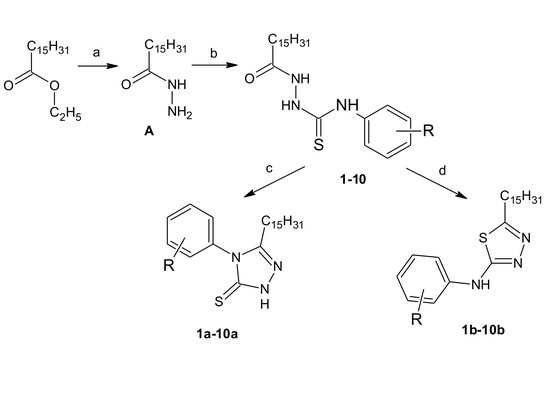
2.1. Chemistry
2.2. Biological Activity
2.2.1. Antimicrobial Study
2.2.2. Antifungal Study
2.2.3. Cytotoxic Studies
2.3. Quantitative Structure-Activity Relationships
3. Materials and Methods
3.1. Chemistry
3.1.1. Palmitohydrazide (A)
3.1.2. General Procedure for the Preparation of Palmitic Acid Thiosemicarbazide (1–10)
3.1.3. General Procedure for Derivatives of 3-Pentadecyl-1H-1,2,4-Triazole-5(4H)-Thione (1a–10a)
3.1.4. General Procedure for Derivatives of 5-Pentadecyl-N-(Substituted Phenyl)-1,3,4-Thiadiazol-2-Amine (1b–10b)
3.2. Biological Assay
3.2.1. In Vitro Evaluation of Antimicrobial Activity
3.2.2. Media, Growth Conditions and Antimicrobial Assay
3.2.3. Cytotoxicity Studies
3.2.4. MTT Cytotoxicity Assay
3.3. QSAR Modeling
3.3.1. Physicochemical Parameters
3.3.2. QSAR Models
- Model linearity,
- coincidence condition,
- uniform dispersion (homoscedasticity) condition,
- normal distribution of model residue.
3.3.3. Model Validation
3.3.4. Cluster Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Armstrong-James, D.; Meintjes, G.; Brown, G.D. A neglected epidemic: Fungal infections in HIV/AIDS. Trends Microbiol. 2014, 22, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Batzlaff, A.H.L.C.M. When to Consider the Possibility of a Fungal Infection: An Overview of Clinical Diagnosis and Laboratory Approaches. Clin. Chest Med. 2017, 38, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Lamb, D.C.; Kelly, D.E.; Venkateswarlu, K.; Manning, N.J.; Bligh, H.F.J.; Schunck, W.; Kelly, S.L. Generation of a complete, soluble, and catalytically active sterol 14α- demethylase - Reductase complex. Biochemistry 1999, 38, 8733–8738. [Google Scholar] [CrossRef] [PubMed]
- Morschhäuser, J. The genetic basis of fluconazole resistance development in Candida albicans. Biochim. Biophys. Acta 2002, 1587, 240–248. [Google Scholar] [CrossRef]
- Sun, W.; Wang, D.; Yu, C.; Huang, X.; Li, X.; Sun, S. Strong synergism of dexamethasone in combination with fluconazole against resistant Candida albicans mediated by inhibiting drug efflux and reducing virulence. Int. J. Antimicrob. Agents 2017, 50, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Salari, S.; Khosravi, A.R.; Mousavi, S.A.A.; Nikbakht-Brojeni, G.H. Mechanisms of resistance to fluconazole in Candida albicans clinical isolates from Iranian HIV-infected patients with oropharyngeal candidiasis. J. Mycol. Med. 2016, 26, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Upadhayaya, R.S.; Jain, S.; Sinha, N.; Kishore, N.; Chandra, R.; Arora, S.K. Synthesis of novel substituted tetrazoles having antifungal activity. Eur. J. Med. Chem. 2004, 39, 579–592. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, S.M.; Badali, H.; Irannejad, H.; Shokrzadeh, M.; Emami, S. Synthesis and biological evaluation of fluconazole analogs with triazole-modified scaffold as potent antifungal agents. Bioorg. Med. Chem. 2015, 23, 1481–1491. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.-Y.; Xu, J.M.; Cao, Y.B.; Zhang, W.N.; Wu, Q.Y.; Zhang, D.Z.; Zhang, J.; Zhao, H.Q.; Jiang, Y.Y. Synthesis of novel triazole derivatives as inhibitors of cytochrome P450 14alpha-demethylase (CYP51). Eur. J. Med. Chem. 2007, 42, 1226–1233. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Cao, Y.; Zhang, J.; Yu, S.; Zou, Y.; Chai, X.; Wu, Q.; Zhang, D.; Jiang, Y.; Sun, Q. Design, synthesis and antifungal activities of novel 1,2,4-triazole derivatives. Eur. J. Med. Chem. 2011, 46, 3142–3148. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.K.; Sharma, S.; Vaidya, A.; Ravichandran, V.; Agrawal, R.K. 1,3,4-thiadiazole and its derivatives: A review on recent progress in biological activities. Chem. Biol. Drug Des. 2013, 81, 557–576. [Google Scholar] [CrossRef] [PubMed]
- Sarigol, D.; Uzgoren-Baran, A.; Tel, B.C.; Somuncuoglu, E.I.; Kazkayasi, I.; Ozadali-Sari, K.; Unsal-Tan, O.; Okay, G.; Ertan, M.; Tozkoparan, B. Novel thiazolo[3,2-b]-1,2,4-triazoles derived from naproxen with analgesic/anti-inflammatory properties: Synthesis, biological evaluation and molecular modeling studies. Bioorg. Med. Chem. 2015, 23, 2518–2528. [Google Scholar] [CrossRef] [PubMed]
- Yehye, W.A.; Abdul Rahman, N.; Saad, O.; Ariffin, A.; Abd Hamid, S.B.; Alhadi, A.A.; Kadir, F.A.; Yaeghoobi, M.; Matlob, A.A. Rational design and synthesis of new, high efficiency, multipotent Schiff base-1,2,4-triazole antioxidants bearing butylated hydroxytoluene moieties. Molecules 2016, 21, 847. [Google Scholar] [CrossRef] [PubMed]
- Yadagiri, B.; Gurrala, S.; Bantu, R.; Nagarapu, L.; Polepalli, S.; Srujana, G.; Jain, N. Synthesis and evaluation of benzosuberone embedded with 1,3,4-oxadiazole, 1,3,4-thiadiazole and 1,2,4-triazole moieties as new potential anti proliferative agents. Bioorg. Med. Chem. Lett. 2015, 25, 2220–2224. [Google Scholar] [CrossRef] [PubMed]
- Demirbas, N.; Demirbas, A.; Sancak, K. Synthesis and antimicrobial activities of some new 1-(5-phenylamino-[1,3,4]thiadiazol-2-yl)methyl-5-oxo-[1,2,4]triazole and 1-(4-phenyl-5-thioxo-[1,2,4]triazol-3-yl)methyl-5-oxo-[1,2,4]triazole derivatives. Eur. J. Med. Chem. 2004, 39, 793–804. [Google Scholar] [CrossRef] [PubMed]
- Shawali, A.S. 1,3,4-Thiadiazoles of pharmacological interest: Recent trends in their synthesis via tandem 1,3-dipolar cycloaddition: Review. J. Adv. Res. 2014, 5, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Nieman, C. Influence of trace amounts of fatty acids on the growth of microorganisms. Bacteriol. Rev. 1954, 18, 147–163. [Google Scholar] [PubMed]
- Kabara, J.J.; Swieczkowski, D.M.; Conley, A.J.; Truant, J.P. Fatty Acids and Derivatives as Antimicrobial Agents. Antimicrob. Agents Chemother. 1972, 2, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.J.; Yoo, J.-S.; Lee, T.-G.; Cho, H.-Y.; Kim, Y.-H.; Kim, W.-G. Fatty acid synthesis is a target for antibacterial activity of unsaturated fatty acids. FEBS Lett. 2005, 579, 5157–5162. [Google Scholar] [CrossRef] [PubMed]
- Pohl, C.H.; Kock, J.L.F.; Thibane, V.S. Antifungal free fatty acids: A review. Sci. Microb. Pathog. 2011, 1, 61–71. [Google Scholar]
- Avis, T.J. Antifungal compounds that target fungal membranes: Applications in plant disease control. Can. J. Plant Pathol. 2007, 29, 323–329. [Google Scholar] [CrossRef]
- Ahmad, A.; Ahmad, A.; Varshney, H.; Rauf, A.; Rehan, M.; Subbarao, N.; Khan, A.U. Designing and synthesis of novel antimicrobial heterocyclic analogs of fatty acids. Eur. J. Med. Chem. 2013, 70, 887–900. [Google Scholar] [CrossRef] [PubMed]
- Fu, N.; Wang, S.; Zhang, Y.; Zhang, C.; Yang, D.; Weng, L.; Zhao, B.; Wang, L. Efficient click chemistry towards fatty acids containing 1,2,3-triazole: Design and synthesis as potential antifungal drugs for Candida albicans. Eur. J. Med. Chem. 2017, 136, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Soliman, H.M.; Basuny, A.M.; Arafat, S.M. Utilization of Stearic acid Extracted from Olive Pomace for Production of Triazoles, Thiadiazoles and Thiadiazines Derivatives of Potential Biological Activities. J. Oleo Sci. 2015, 64, 1019–1032. [Google Scholar] [CrossRef] [PubMed]
- Uto, Y.; Ueno, Y.; Kiyotsuka, Y.; Miyazawa, Y.; Kurata, H.; Ogata, T.; Yamada, M.; Deguchi, T.; Konishi, M.; Takagi, T.; et al. Synthesis and evaluation of novel stearoyl-CoA desaturase 1 inhibitors: 1’-{6-[5-(pyridin-3-ylmethyl)-1,3,4-oxadiazol-2-yl]pyridazin-3-yl}-3, 4-dihydrospiro[chromene-2,4’-piperidine] analogs. Eur. J. Med. Chem. 2010, 45, 4788–4796. [Google Scholar] [CrossRef] [PubMed]
- Yale, H.L.; Losee, K.; Martins, J.; Holsing, M.; Perry, F.M.; Bernstein, J. Chemotherapy of Experimental Tuberculosis. VIII. The Synthesis of Acid Hydrazides, their Derivatives and Related Compounds. J. Am. Chem. Soc. 1953, 75, 1933–1942. [Google Scholar] [CrossRef]
- Dobosz, M.; Struga, M.; Chodkowska, A.; Jagiełło-Wójtowicz, E.; Stępniak, K.; Koział, A.E. Synthesis and some pharmacological properties of 3-(4-phenyl-5-oxo-1,2,4-triazolin-1-ylmethyl)-1,2,4-triazolin-5-thione derivatives. Acta Pol. Pharm. 2002, 59, 281–290. [Google Scholar] [PubMed]
- Plech, T.; Kaproń, B.; Paneth, A.; Wujec, M.; Czarnomysy, R.; Bielawska, A.; Bielawski, K.; Trotsko, N.; Kuśmierz, E.; Paneth, P. Search for human DNA topoisomerase II poisons in the group of 2,5-disubstituted-1,3,4-thiadiazoles. J. Enzyme Inhib. Med. Chem. 2015, 30, 1021–1026. [Google Scholar] [CrossRef] [PubMed]
- Bielenica, A.; Stefańska, J.; Stępień, K.; Napiórkowska, A.; Augustynowicz-Kopeć, E.; Sanna, G.; Madeddu, S.; Boi, S.; Giliberti, G.; Wrzosek, M.; et al. Synthesis, cytotoxicity and antimicrobial activity of thiourea derivatives incorporating 3-(trifluoromethyl)phenyl moiety. Eur. J. Med. Chem. 2015, 101, 111–125. [Google Scholar] [CrossRef] [PubMed]
- Bielenica, A.; Stępień, K.; Napiórkowska, A.; Augustynowicz-Kopeć, E.; Krukowski, S.; Włodarczyk, M.; Struga, M. Synthesis and Antimicrobial Activity of 4-Chloro-3-Nitrophenylthiourea Derivatives Targeting Bacterial Type II Topoisomerases. Chem. Biol. Drug Des. 2016, 87, 905–917. [Google Scholar] [CrossRef] [PubMed]
- Patrick, G.L. An Introduction to Medicinal Chemistry, 5th ed.; Oxford University Press: Oxford, UK, 2009; Volume 40. [Google Scholar]
- Todeschini, R.; Consonni, V. Molecular Descriptors for Chemoinformatics; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Kubinyi, H. QSAR: Hansch Analysis and Related Approaches; John Wiley & Sons: Hoboken, NJ, USA, 1995; Volume 16. [Google Scholar]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and Computational Approaches to Estimate Solubility and Permeability in Drug Discovery and Development Settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Golbraikh, A.; Tropsha, A. Predictive QSAR modeling based on diversity sampling of experimental datasets for the training and test set selection. Mol. Divers. 2002, 16, 357–369. [Google Scholar]
- Filipowska, A.; Filipowski, W.; Tkacz, E.; Nowicka, G.; Struga, M. Statistical analysis of the impact of molecular descriptors on cytotoxicity of thiourea derivatives incorporating 2-aminothiazole scaffold. Chem. Pharm. Bull. 2016, 64, 1196–1202. [Google Scholar] [CrossRef] [PubMed]
- Kupcewicz, B.; Małecka, M.; Zapadka, M.; Krajewska, U.; Rozalski, M.; Budzisz, E. Quantitative relationships between structure and cytotoxic activity of flavonoid derivatives. An application of Hirshfeld surface derived descriptors. Bioorg. Med. Chem. Lett. 2016, 26, 3336–3341. [Google Scholar] [CrossRef] [PubMed]
- Roy, K.; Kar, S.; Das, R.N. A Primer on QSAR/QSPR Modeling Fundamental Concepts; SpringerBriefs in Molecular Science; Springer International Publishing AG.: Basel, Switzerland; 2015. [Google Scholar]
- Kumar, D.; Judge, V.; Narang, R.; Sangwan, S.; De Clercq, E.; Balzarini, J.; Narasimhan, B. Benzylidene/2-chlorobenzylidene hydrazides: Synthesis, antimicrobial activity, QSAR studies and antiviral evaluation. Eur. J. Med. Chem. 2010, 45, 2806–2816. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Kapoor, A.; Thangadurai, A.; Kumar, P.; Narasimhan, B. Synthesis, antimicrobial evaluation and QSAR studies of 3-ethoxy-4-hydroxybenzylidene/4-nitrobenzylidene hydrazides. Chin. Chem. Lett. 2011, 22, 1293–1296. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aeribically; Approved Standard M7-a7; CLSI: Wayne, PA, USA, 2006.
- Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Approved Standard M27-A3; CLSI: Wayne, PA, USA, 2008.
- Hypercube Inc. HyperChem(TM) Professional 7.5; Hypercube Inc.: Gainesville, FL, USA, 2007. [Google Scholar]
- Stefańska, J.; Nowicka, G.; Struga, M.; Szulczyk, D.; Koziol, A.E.; Augustynowicz-Kopec, E.; Napiorkowska, A.; Bielenica, A.; Filipowski, W.; Filipowska, A.; et al. Antimicrobial and anti-biofilm activity of thiourea derivatives incorporating a 2-aminothiazole scaffold. Chem. Pharm. Bull. 2015, 63, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Kuśmierz, E.; Siwek, A.; Kosikowska, U.; Malm, A.; Stefanska, J.; Dzitko, K.; Wujec, M. Antibacterial Activity and Structure-activity Relationship Studies of 4- substituted-5-(diphenylmethyl)-2,4-dihydro-3H-1,2,4-triazole-3-thiones. Lett. Drug Des. Discov. 2013, 10, 95–101. [Google Scholar] [CrossRef]
- Carbó-Dorca, R.; Robert, D.; Amat, L.; Girones, X.; Besalu, E. Molecular Quantum Similarity in QSAR and Drug Design, 1st ed.; Springer-Verlag: Berlin, Germany, 2000; pp. 27–28. [Google Scholar]
- Yee, L.C.; Wei, Y.C. Current Modeling Methods Used in QSAR/QSPR. In Statistical Modeling of Molecular Descriptors in QSAR/QSPR, 1st ed.; Dehme, M., Varmuza, K., Bonchev, D., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA.: Weinheim, Germany, 2012. [Google Scholar]
- Statsoft.com. STATISTICA, New Features in STATISTICA 12. 2016. Available online: http://www.statsoft.com/Products/STATISTICA-Features/Version-12 (accessed on 3 April 2018).
- Freund, R.; Wilson, W.; Sa, P. Regression Analysis: Statistical Modeling of a Response Variable, 2nd ed.; Academic Press: Burlington, VT, USA, 2006; pp. 73–115. [Google Scholar]
- Filipowska, A.; Filipowski, W.; Tkacz, E.; Wujec, M. Statistical Analysis of the Impact of Molecular Descriptors on Antimicrobial Activity of Thiourea Derivatives Incorporating 3-amino-1,2,4-triazole Scaffold. In Innovations in Biomedical Engineering. Advances in Intelligent Systems and Computing, 1st ed.; Gzik, M., Tkacz, E., Paszenda, Z., Piętka, E., Eds.; Springer: Cham, Switzerland, 2017; pp. 171–184. [Google Scholar]
- Shapiro, S.S.; Wilk, M.B. An Analysis of Variance Test for Normality (Complete Samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Filipowska, A.; Filipowski, W.; Tkacz, E. Study of structure-cytotoxicity relationships of thiourea derivatives containing the 2-aminothiazole moiety. In Innovations in Biomedical Engineering. Advances in Intelligent Systems and Computing, 1st ed.; Gzik, M., Tkacz, E., Paszenda, Z., Piętka, E., Eds.; Springer: Cham, Switzerland, 2017; pp. 276–285. [Google Scholar]
- Khaledian, S.; Saaidpour, S. Quantitative structure-property relationship modelling of distribution coefficients (logD7.4) of diverse drug by sub-structural molecular fragments method. Orient. J. Chem. 2015, 31, 1969–1976. [Google Scholar] [CrossRef]
- Goodarzi, M.; Funar-Timofei, S.; Heyden, Y.V. Towards better understanding of feature-selection or reduction techniques for Quantitative Structure–Activity Relationship models. TrAC Trends Anal. Chem. 2012, 42, 49–63. [Google Scholar] [CrossRef]
- Consonni, V.; Ballabio, D.; Todeschini, R. Evaluation of model predictive ability by external validation techniques. J. Chemom. 2010, 24, 194–201. [Google Scholar] [CrossRef]
- Golbraikh, A.; Tropsha, A. Beware of q2! J. Mol. Graph. Model. 2002, 20, 269–276. [Google Scholar] [CrossRef]
- Roy, K.; Chakraborty, P.; Mitra, I.; Ojha, P.K.; Kar, S.; Das, R.N. Some case studies on application of ‘rm2’ metrics for judging quality of quantitative structure-activity relationship predictions: Emphasis on scaling of response data. J. Comput. Chem. 2013, 34, 1071–1082. [Google Scholar] [CrossRef] [PubMed]
- Astel, A.; Biziuk, M.; Przyjazny, A.; Namieśnik, J. Chemometrics in monitoring spatial and temporal variations in drinking water quality. Water Res. 2006, 40, 1706–1716. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 1–10, 1a–10a, 1b–10b are available from the authors. |




| Compound | MIC/μg/mL | |||
|---|---|---|---|---|
| Candida albicans ATCC 10231 | Candida albicans ATCC 30028 | Candida albicans Clinical Isolate 26 | Candida albicans Clinical Isolate 18 | |
| 1 | 12.5 | 100 | >100 | >100 |
| 2 | 1.56 | 100 | >100 | 25 |
| 3 | 1.56 | 100 | 50 | 12.5 |
| 4 | 1.56 | 50 | 12.5 | 6.25 |
| 5 | 1.56 | 1.56 | 1.56 | 1.56 |
| 6 | 1.56 | 25 | 3.125 | 3.125 |
| 7 | 1.56 | >100 | 25 | 25 |
| 8 | 1.56 | 25 | 12.5 | 6.25 |
| 9 | 1.56 | 50 | 50 | 50 |
| 10 | 12.5 | 25 | 25 | 12.5 |
| 1a | 50 | >100 | >100 | >100 |
| 2a | >100 | >100 | >100 | >100 |
| 3a | >100 | >100 | >100 | >100 |
| 4a | >100 | >100 | >100 | >100 |
| 5a | 12.5 | 25 | 100 | 25 |
| 6a | 12.5 | 50 | >100 | >100 |
| 7a | >100 | >100 | >100 | >100 |
| 8a | 100 | 100 | 100 | >100 |
| 9a | 100 | >100 | >100 | >100 |
| 10a | 50 | 100 | 100 | >100 |
| 1b | >100 | >100 | >100 | >100 |
| 2b | >100 | >100 | >100 | >100 |
| 3b | >100 | >100 | >100 | >100 |
| 4b | >100 | >100 | >100 | >100 |
| 5b | >100 | >100 | >100 | >100 |
| 6b | >100 | >100 | >100 | >100 |
| 7b | >100 | >100 | >100 | >100 |
| 8b | >100 | >100 | >100 | >100 |
| 9b | >100 | >100 | >100 | >100 |
| 10b | >100 | >100 | >100 | >100 |
| Ref. * | 0.25–0.125 | 0.25–0.125 | 0.25–0.125 | 0.25–0.125 |
| C. albicans Strain | QSAR Equation | Modeling ntr = 24 | Internal Validation | External Validation ntest = 6 | Golbraikh&Tropsha |
|---|---|---|---|---|---|
| Candida albicans ATCC 10231 | log(1/MICC. alb.ATCC10231) = 0.0001(±0.00005)EIA + 0.19 (±0.05)Rf − 0.039(±0.006)HF − 11.84(±3.9) | R = 0.889 | Q2LOO = 0.733 R2Yscr = 0.146 Q2Yscr = −0.097 = 0.713 | Q2(F1) = 0.890 | k = 1.013 k’ = 0.964 R20 = 0.891 R’20 = 0.838 |
| R2 = 0.790 | Q2(F2) = 0.890 | ||||
| R2adj.= 0.758 | Q2(F3) = 0.867 | ||||
| F = 25.1 | RMSEext = 0.321 | ||||
| p = 5.6·10-7 | = 0.717 | ||||
| RMSEtr = 0.419 | Δr2m = 0.111 | ||||
| log(1/MICC. alb.ATCC10231) = −0.11(±0.01)logP2 + 0.00090(±0.0001)Rf2 + 0.030(±0.01)µ2 − 8.96(±1.8) | R = 0.901 | Q2LOO = 0.750 R2Yscr = 0.089 Q2Yscr = −0.167 = 0.766 | Q2(F1) = 0.879 | k = 1.005 k’ = 0.989 R20 = 0.845 R’20 = 0.778 | |
| R2 = 0.812 | Q2(F2) = 0.879 | ||||
| R2adj.= 0.784 | Q2(F3) = 0.904 | ||||
| F = 28.8 | RMSEext = 0.208 | ||||
| p = 1.9·10−7 | = 0.687 | ||||
| RMSEtr = 0.419 | Δr2m = 0.145 | ||||
| Candida albicans clinical isolate 18 | log(1/MICC. alb.clin.18) = −0.003(±0.0007)logP3 + 0.0003(±0.0001)Rf2 + 3.9·10−12(±1·10−12)ICC2 − 7.89 (±1.1) | R = 0.918 | Q2LOO = 0.774 R2Yscr = 0.122 Q2Yscr = −0.115 = 0.814 | Q2(F1) = 0.879 | k = 1.005 k’ = 0.989 R20 = 0.880 R’20 = 0.902 |
| R2 = 0.843 | Q2(F2) = 0.879 | ||||
| R2adj.= 0.819 | Q2(F3) = 0.904 | ||||
| F = 35.7 | RMSEext = 0.208 | ||||
| p = 3.2·10−8 | = 0.806 | ||||
| RMSEtr = 0.292 | Δr2m = 0.095 | ||||
| log(1/MICC. alb.clin.18) = 4.9·10−12(±7·10-13)EE2 − 6.94(±0.7) | R = 0.831 | Q2LOO = 0.673 R2Yscr = 0.057 Q2Yscr = −0.041 = 0.630 | Q2(F1) = 0.640 | k = 1.009 k’ = 0.967 R20 = 0.641 R’20 = 0.605 | |
| R2 = 0.691 | Q2(F2) = 0.641 | ||||
| R2adj.= 0.677 | Q2(F3) = 0.712 | ||||
| F = 49.2 | RMSEext = 0.360 | ||||
| p = 4.87·10−7 | = 0.539 | ||||
| RMSEtr = 0.390 | Δr2m = 0.064 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jóźwiak, M.; Stępień, K.; Wrzosek, M.; Olejarz, W.; Kubiak-Tomaszewska, G.; Filipowska, A.; Filipowski, W.; Struga, M. Synthesis, Structural Studies and Biological Evaluation of Connections of Thiosemicarbazide, 1,2,4-Triazole and 1,3,4-Thiadiazole with Palmitic Acid. Molecules 2018, 23, 822. https://doi.org/10.3390/molecules23040822
Jóźwiak M, Stępień K, Wrzosek M, Olejarz W, Kubiak-Tomaszewska G, Filipowska A, Filipowski W, Struga M. Synthesis, Structural Studies and Biological Evaluation of Connections of Thiosemicarbazide, 1,2,4-Triazole and 1,3,4-Thiadiazole with Palmitic Acid. Molecules. 2018; 23(4):822. https://doi.org/10.3390/molecules23040822
Chicago/Turabian StyleJóźwiak, Michał, Karolina Stępień, Małgorzata Wrzosek, Wioletta Olejarz, Grażyna Kubiak-Tomaszewska, Anna Filipowska, Wojciech Filipowski, and Marta Struga. 2018. "Synthesis, Structural Studies and Biological Evaluation of Connections of Thiosemicarbazide, 1,2,4-Triazole and 1,3,4-Thiadiazole with Palmitic Acid" Molecules 23, no. 4: 822. https://doi.org/10.3390/molecules23040822