Amaryllidaceae Alkaloids as Potential Glycogen Synthase Kinase-3β Inhibitors
Abstract
:1. Introduction
2. Results and Discussion
2.1. Amaryllidaceae Alkaloids
2.2. Potency of Amaryllidaceae Alkaloids to Inhibit GSK-3β
3. Experimental
3.1. Amaryllidaceae Alkaloids
3.2. GSK-3β Assay
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Saraswati, A.P.; Ali Hussaini, S.M.; Krishna, N.H.; Babu, B.N.; Kamal, A. Glykogen synthase kinase-3 and its inhibitors: Potential target for various therapeutics conditions. Eur. J. Med. Chem. 2018, 144, 843–858. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P.; Frame, S. The renaissance of GSK3. Nat. Rev. Mol. Cell Biol. 2001, 2, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Phukan, S.; Babu, V.S.; Kannoji, A.; Hariharan, R.; Balaji, V.N. GSK3β: Role in therapeutic landscape and development of modulators. Br. J. Pharmacol. 2010, 160, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Maqbool, M.; Mobashir, M.; Hoda, N. Pivotal role of glycogen synthase kinase-3: A therapeutic target for Alzheimer’s disease. Eur. J. Med. Chem. 2016, 107, 63–81. [Google Scholar] [CrossRef] [PubMed]
- Luo, J. Glycogen synthase kinase 3β (GSK3β) in tumorigenesis and cancer chemotherapy. Cancer Lett. 2009, 273, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, E.J.; Dokken, B.B. Role of glycogen synthase kinase-3 in insulin resistance and type 2 diabetes. Curr. Drug Targets 2006, 7, 1435–1441. [Google Scholar] [CrossRef] [PubMed]
- Lal, H.; Ahmad, F.; Woodgett, J.; Force, T. The GSK-3 family as therapeutic target for myocardial diseases. Circ. Res. 2015, 116, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Jope, R.S.; Roh, M.S. Glykogen synthase kinase-3 (GSK3) in psychiatric diseases and therapeutic interventions. Curr. Drug Targets 2006, 7, 1421–1434. [Google Scholar] [CrossRef] [PubMed]
- Plattner, F.; Angelo, M.; Giese, K.P. The roles of cyclin-dependent kinase 5 and glycogen synthase kinase 3 in tau hyperphosphorylation. J. Biol. Chem. 2006, 281, 25457–25465. [Google Scholar] [CrossRef] [PubMed]
- Palop, J.J.; Mucke, L. Amyloid-β induced neuronal dysfunction in Alzheimer’s disease: From synapses toward neural networks. Nat. Neurosci. 2010, 13, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Petanceska, S.S.; Seeger, M.; Checler, F. Mutant presenilin 1 increases the levels of Alzheimer amyloid β-peptide Aβ42 in late compartments of the constitutive secretory pathway. J. Neurochem. 2000, 74, 1878–1884. [Google Scholar] [CrossRef] [PubMed]
- Rockenstein, E.; Torrance, M.; Adame, A.; Mante, M.; Baron, P.; Rose, J.B.; Crews, L.; Masliah, E. Neuroprotective effects of regulators of the glycogen synthase kinase-3β signaling pathway in a transgenic model of Alzheimer’s disease are associated with reduced amyloid precursor protein phosphorylation. J. Neurosci. 2007, 27, 1981–1991. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A. Preclinical efficacy on GSK-3 inhibitors: Towards a future generation of powerful drugs. Med. Res. Rev. 2008, 28, 773–796. [Google Scholar] [CrossRef] [PubMed]
- Phiel, C.J.; Wilson, C.A.; Lee, V.M.; Klein, P.S. GSK-3α regulates production of Alzheimer’s disease amyloid-β peptides. Nature 2003, 423, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, J.M.; Fuertes, A.; Orozco, L.; del Monte-Millan, M.; Deldago, E.; Medina, M. Evidence for irreversible inhibition of glycogen synthase kinase-3β by tideglusib. J. Biol. Chem. 2012, 287, 893–904. [Google Scholar] [CrossRef] [PubMed]
- Del Ser, T.; Steinwachs, K.C.; Gertz, H.J.; Andress, M.V.; Gomez-Carrillo, B.; Medina, M.; Vericat, J.A.; Redondo, P.; Fleet, D.; Leon, T. Treatment of Alzheimer’s disease with the GSK-3 inhibitor tideglusib: A pilot study. J. Alzheimer Dis. 2013, 33, 205–215. [Google Scholar]
- Shimura, T. Acquired radioresistance of cancer and the AKT/GSK3β/cyclin D1 overexpression cycle. J. Radiat. Res. 2011, 52, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Jope, R.S.; Yuskaitis, C.J.; Beurel, E. Glycogen synthase kinase-3 (GSK3): Inflammation, diseases, and therapeutics. Neurochem. Res. 2007, 32, 577–595. [Google Scholar] [CrossRef] [PubMed]
- McCubrey, J.A.; Steelman, L.S.; Bertrand, F.E.; Davis, N.M.; Sokolosky, M.; Abrams, S.L.; Montalto, G.; D’Assoro, A.B.; Libra, M.; Nicoletti, F.; et al. GSK-3 as potential target for therapeutic intervention in cancer. Oncotarget 2014, 5, 2881–2911. [Google Scholar] [CrossRef] [PubMed]
- Hamann, M.; Alonso, D.; Martín-Aparicio, E.; Fuertes, A.; Pérez-Puerto, M.J.; Castro, A.; Morales, S.; Navarro, M.L.; Del Monte-Millán, M.; Medina, M.; et al. Glycogen synthase kinase-3 (GSK-3) inhibitory activity and structure-activity relationship (SAR) studies of the manzamine alkaloids. Potential for Alzheimer’s disease. J. Nat. Prod. 2007, 70, 1397–1405. [Google Scholar] [CrossRef] [PubMed]
- Witherington, J.; Bordas, V.; Garland, S.L.; Hickey, D.M.B.; Ife, R.J.; Liddle, J.; Saunders, M.; Smith, D.G.; Ward, R.W. 5-Aryl-pyrazolo[3,4-b]pyridines: Potent inhibitors of glycogen synthase kinase-3 (GSK-3). Bioorg. Med. Chem. 2003, 13, 1577–1580. [Google Scholar] [CrossRef]
- Naerum, L.; Norskov-Lauritsen, L.; Olesen, P.H. Scaffold hopping and optimization towards libraries of glycogen synthase kinase-3 inhibitors. Bioorg. Med. Chem. Lett. 2002, 12, 1525–1528. [Google Scholar] [CrossRef]
- Martinez, A.; Alonso, M.; Castro, A.; Perez, C.; Moreno, F.J. First non-ATP competitive glycogen synthase kinase 3 β (GSK-3β) inhibitors: Thiadiazolidinones (TDZD) as potential drugs for the treatment of Alzheimer’s disease. J. Med. Chem. 2002, 45, 1292–1299. [Google Scholar] [CrossRef] [PubMed]
- Coghlan, M.P.; Culbert, A.A.; Cross, D.A.E.; Corcoran, S.L.; Yates, J.D.; Pearce, N.J.; Rausch, O.L.; Murphy, G.J.; Carter, P.S.; Cox, L.R.; et al. Selective small molecule inhibitors of glycogen synthase kinase-3 modulate glycogen metabolism and gene transcription. Chem. Biol. 2000, 7, 793–803. [Google Scholar] [CrossRef]
- Leost, M.; Schultz, C.; Link, A.; Wu, Y.Z.; Biernat, J.; Man-Delkow, E.M.; Bibb, J.A.; Snyder, G.L.; Greengard, P.; Zaharevitz, D.W.; et al. Paullones are potent inhibitors of glycogen synthase kinase-3β and cyclin-dependent kinase 5/p25. Eur. J. Biochem. 2000, 267, 5983–5994. [Google Scholar] [CrossRef] [PubMed]
- Pandey, M.K.; DeGrado, T.R. Glycogen synthase kinase-3 (GSK-3)-targeted therapy and imaging. Theranostics 2016, 6, 571–593. [Google Scholar] [CrossRef] [PubMed]
- Doskočil, I.; Hošťálková, A.; Šafratová, M.; Benešová, N.; Havlík, J.; Havelek, R.; Kuneš, J.; Královec, K.; Chlebek, J.; Cahlíková, L. Cytotoxic activities of Amaryllidaceae alkaloids against gastrointestinal cancer cells. Phytochem. Lett. 2015, 13, 394–398. [Google Scholar] [CrossRef]
- Ago, Y.; Koda, K.; Takuma, K.; Matsuda, T. Pharmacological aspects of the acetylcholinesterase inhibitor galantamine. J. Pharm. Sci. 2011, 116, 6–17. [Google Scholar] [CrossRef]
- Cahlíková, L.; Pérez, D.I.; Štěpánková, Š.; Chlebek, J.; Šafratová, M.; Hošťálková, A.; Opletal, L. In vitro inhibitory effects of 8-O-demethylmaritidine and undulatine on acetylcholinesterase and their predicted penetration across the blood-brain barrier. J. Nat. Prod. 2015, 78, 1189–1192. [Google Scholar] [CrossRef] [PubMed]
- Cedrón, J.C.; Ravelo, A.G.; León, L.G.; Padrón, J.M.; Estévez-Braun, A. Antiproliferative and structure activity relationships of Amaryllidaceae alkaloids. Molecules 2015, 20, 13854–13863. [Google Scholar] [CrossRef] [PubMed]
- Van Goietsenoven, G.; Hutton, J.; Becker, J.P.; Lallemand, B.; Robert, F.; Lefranc, F.; Pirker, C.; Vandenbussche, G.; Van Antwerpen, P.; Evidente, A.; et al. Targeting of eEF1A with Amaryllidaceae isocarbostyrils as a strategy to combat melanomas. FASEB J. 2010, 24, 4575–4584. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Pignanelli, C.; Tarade, D.; Gilbert, T.; Noel, M.; Mansour, F.; Adams, S.; Dowhayko, K.; Vshyvenko, S.; Hudlicky, T.; et al. Cancer cell mitochondria targeting by pancratistatin analogs is dependent on functional complex II and III. Sci. Rep. 2017, 7, 42957. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, M.; Kunitomo, J.; Kimura, E.; Hayase, Y.; Kobayashi, H.; Uchiyama, N.; Kawamoto, T.; Tanaka, T.; Mol, C.; Dougan, D.R. Design, synthesis and structure-activity relationships of 1,3,4-oxadiazole derivatives as novel inhibitors of glycogen synthase kinase-3β. Bioorg. Med. Chem. 2009, 17, 2017–2029. [Google Scholar] [CrossRef] [PubMed]
- Meijer, L.; Thunnissen, A.-M.W.H.; White, A.W.; Garnier, M.; Nikolic, M.; Tsai, L.-H.; Walter, J.; Cleverley, K.E.; Salinas, P.C.; Wu, Y.-Z.; et al. Inhibition of cyclin-dependent kinases, GSK-3β and CK1 by hymenialdisine, a marine sponge constituent. Chem. Biol. 2000, 7, 51–63. [Google Scholar] [CrossRef]
- Kitagawa, I.; Kobayashi, M.; Kitanaka, K.; Kido, M.; Kyogoku, Y. Marine natural products XII. On the chemical constituents of the Okinawan marine sponge Hymeniacidon aldis. Chem. Pharm. Bull. 1983, 31, 2321–2328. [Google Scholar] [CrossRef]
- Cimino, G.; de Rosa, S.; de Stefano, S.; Mazzarella, L.; Puliti, R.; Sodano, G. Isolation and X-ray crystal structure of a novel bromo-compound from two marine sponges. Tetrahedron Lett. 1982, 23, 767–768. [Google Scholar] [CrossRef]
- Gompel, M.; Leost, M.; Bal De Kier, J.E.; Puricelli, L.; Hernandez, F.L.; Palermo, J.; Meijer, L. Meridianins, a new family of protein kinase inhibitors isolated from the Ascidian Aplidium meridianum. Bioorg. Med. Chem. Lett. 2004, 14, 1703–1707. [Google Scholar] [CrossRef] [PubMed]
- Shiono, Y.; Miyazaki, N.; Murayyma, T.; Harizon, T.K.; Katja, D.G.; Supratman, U.; Nakata, J.; Kakihara, Y.; Saeki, M.; Yoshida, J.; et al. GSK-3β inhibitory activities of novel dichlororesorcinol derivatives from Cosmopora vilior isolated from mangrove plant. Phytochem. Lett. 2016, 18, 122–127. [Google Scholar] [CrossRef]
- Kulhánková, A.; Cahlíková, L.; Novák, Z.; Macáková, K.; Kuneš, J.; Opletal, L. Alkaloids from Zephyranthes robusta Baker and their acetylcholinesterase and butyrylcholinesterase-inhibitory activity. Chem. Biodivers. 2013, 10, 1120–1127. [Google Scholar] [CrossRef] [PubMed]
- Šafratová, M.; Novák, Z.; Kulhánková, A.; Kuneš, J.; Hrabinová, M.; Jun, D.; Macáková, K.; Opletal, L.; Cahlíková, L. Revised NMR data for 9-O-demethylgalanthine: An alkaloid from Zephyranthes robusta (Amaryllidaceae) and its biological activity. Nat. Prod. Commun. 2014, 9, 787–788. [Google Scholar] [PubMed]
- Cahlíková, L.; Hrabinová, M.; Kulhánková, A.; Benešová, N.; Chlebek, J.; Jun, D.; Novák, Z.; Kuča, K.; Macáková, K.; Opletal, L. Alkaloids from Chlidanthus fragrans and their acetylcholinesterase, butyrylcholinesterase and prolyl oligopeptidase activities. Nat. Prod. Commun. 2013, 8, 1541–1544. [Google Scholar] [PubMed]
- Vaněčková, N.; Hošťálková, A.; Šafratová, M.; Kuneš, J.; Hulcová, D.; Hrabinová, M.; Doskočil, I.; Štěpánková, Š.; Opletal, L.; Nováková, L.; et al. Isolation of Amaryllidaceae alkaloids from Nerine bowdenii W. Watson and their biological activities. RSC Adv. 2016, 6, 80114–80120. [Google Scholar] [CrossRef]
- Šafratová, M.; Hošťálková, A.; Hulcová, D.; Breiterová, K.; Hrabcová, V.; Machado, M.; Fontinha, D.; Prudêncio, M.; Kuneš, J.; Chlebek, J.; et al. Alkaloids from Narcissus poeticus cv. Pink Parasol of various structural types and their biological activity. Arch. Pharm. Res. 2017, 41, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Havlasová, J.; Šafratová, M.; Siatka, T.; Štěpánková, Š.; Ločárek, M.; Opletal, L.; Hrabinová, M.; Jun, D.; Benešová, N.; Novák, Z.; et al. Chemical composition of bioactive alkaloid extracts from some Narcissus species and varieties and their biological activity. Nat. Prod. Commun. 2014, 9, 1151–1155. [Google Scholar] [PubMed]
- Baki, A.; Bielik, A.; Molnár, L.; Szendrei, G.; Keserü, G.M. A high throughput luminescent assay for glycogen synthase kinase-3β inhibitors. ASSAY Drug. Dev. Technol. 2007, 5, 75–83. [Google Scholar]
Sample Availability: Samples of the compounds, except of 6, 10, 12, 13, 16, and 26, are available from the authors. |

| Structural Type | Alkaloid | % of Inhibition |
|---|---|---|
| Belladine | Beladine (1) | 34.4 ± 2.7 |
| Haemanthamine | Epimaritidine (2) | 45.2 ± 1.1 |
| Haemanthamine (3) | 52.4 ± 0.1 | |
| Haemanthidine (4) | 33.0 ± 2.2 | |
| Hamayne (5) | 33.9 ± 0.1 | |
| Seco-isopowellaminone (6) | 38.5 ± 0.8 | |
| Crinine | Ambelline (7) | 38.0 ± 0.8 |
| Crinine (8) | 39.6 ± 5.4 | |
| Undulatine (9) | 43.3 ± 4.0 | |
| Crinamidine (10) | 32.1 ± 7.9 | |
| Galanthamine | Chlidanthine (11) | 37.9 ± 9.5 |
| Narwedine (12) | 37.7 ± 0.3 | |
| Lycoraminone (13) | 38.9 ± 1.0 | |
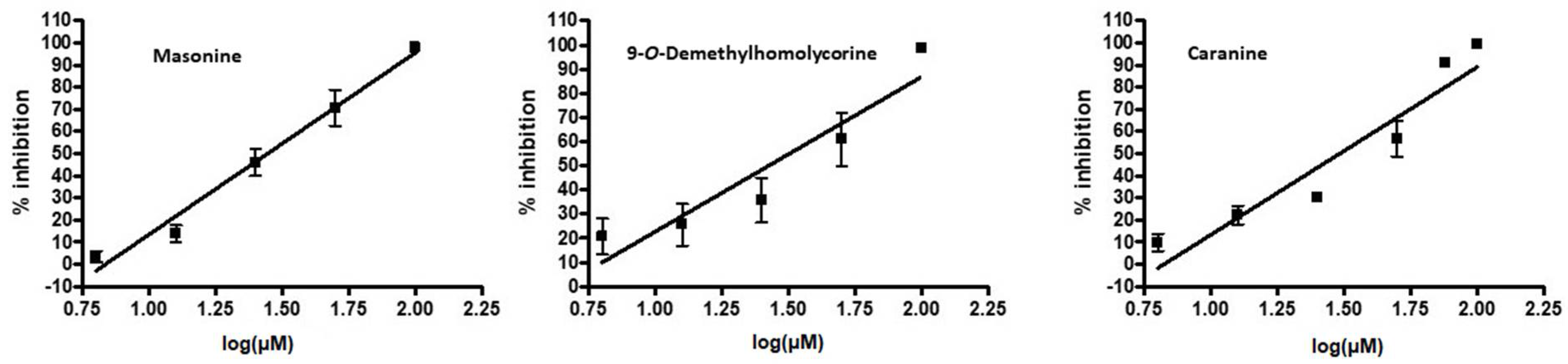
| Lycorine | Caranine (14) | 61.8 ± 9.2 |
| Lycorine (15) | 32.9 ± 0.2 | |
| 1-O-Acetyllycorine (16) | 49.9 ± 1.9 | |
| Galanthine (17) | 26.4 ± 7.7 | |
| 9-O-Demethylgalanthine (18) | 50.9 ± 8.9 | |
| Norpluviine (19) | 45.0 ± 4.3 | |
| Tazettine | Tazettine (20) | 49.2 ± 0.3 |
| Homolycorine | Hippeastrine (21) | 10.7 ± 2.5 |
| Homolycorine (22) | 54.4 ± 0.6 | |
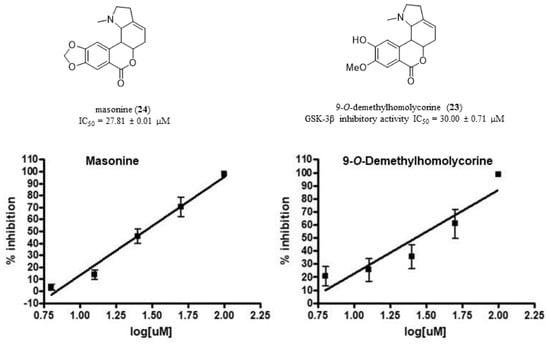
| 9-O-Demethylhomolycorine (23) | 63.6 ± 1.3 | |
| Masonine (24) | 66.0 ± 4.0 | |
| Lycorenine (25) | 47.6 ± 3.5 | |
| O-Ethyllycorenine (26) | 57.7 ± 3.5 | |
| Oduline (27) | 57.7 ± 4.4 | |
| Tetrahydromasonine (28) | 22.4 ± 0.2 |
| Alkaloid | IC50 (µM) * |
|---|---|
| Caranine (14) | 30.75 ± 0.04 |
| 9-O-Demethylhomolycorine (23) | 30.00 ± 0.71 |
| Masonine (24) | 27.81 ± 0.05 |
| SB-415286 ** | 70.00 nM |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hulcová, D.; Breiterová, K.; Siatka, T.; Klímová, K.; Davani, L.; Šafratová, M.; Hošťálková, A.; De Simone, A.; Andrisano, V.; Cahlíková, L. Amaryllidaceae Alkaloids as Potential Glycogen Synthase Kinase-3β Inhibitors. Molecules 2018, 23, 719. https://doi.org/10.3390/molecules23040719
Hulcová D, Breiterová K, Siatka T, Klímová K, Davani L, Šafratová M, Hošťálková A, De Simone A, Andrisano V, Cahlíková L. Amaryllidaceae Alkaloids as Potential Glycogen Synthase Kinase-3β Inhibitors. Molecules. 2018; 23(4):719. https://doi.org/10.3390/molecules23040719
Chicago/Turabian StyleHulcová, Daniela, Kateřina Breiterová, Tomáš Siatka, Kamila Klímová, Lara Davani, Marcela Šafratová, Anna Hošťálková, Angela De Simone, Vincenza Andrisano, and Lucie Cahlíková. 2018. "Amaryllidaceae Alkaloids as Potential Glycogen Synthase Kinase-3β Inhibitors" Molecules 23, no. 4: 719. https://doi.org/10.3390/molecules23040719