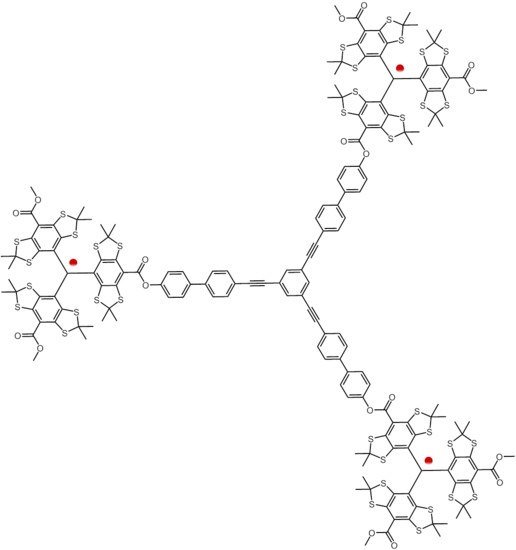
Synthesis of Nanometer Sized Bis- and Tris-trityl Model Compounds with Different Extent of Spin–Spin Coupling
Abstract
1. Introduction
1.1. Motivation
1.2. Electron–Electron Interaction in Different Coupling Regimes
2. Results and Discussion
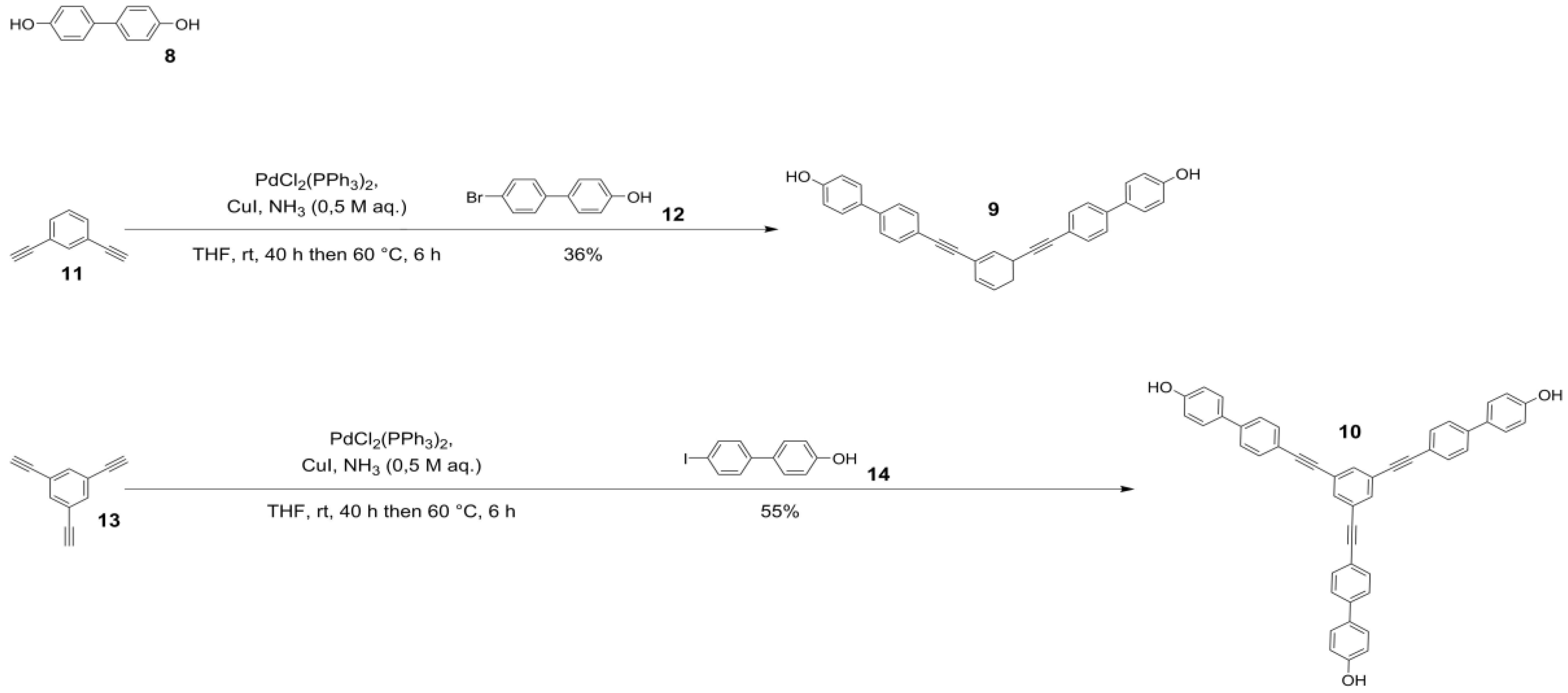
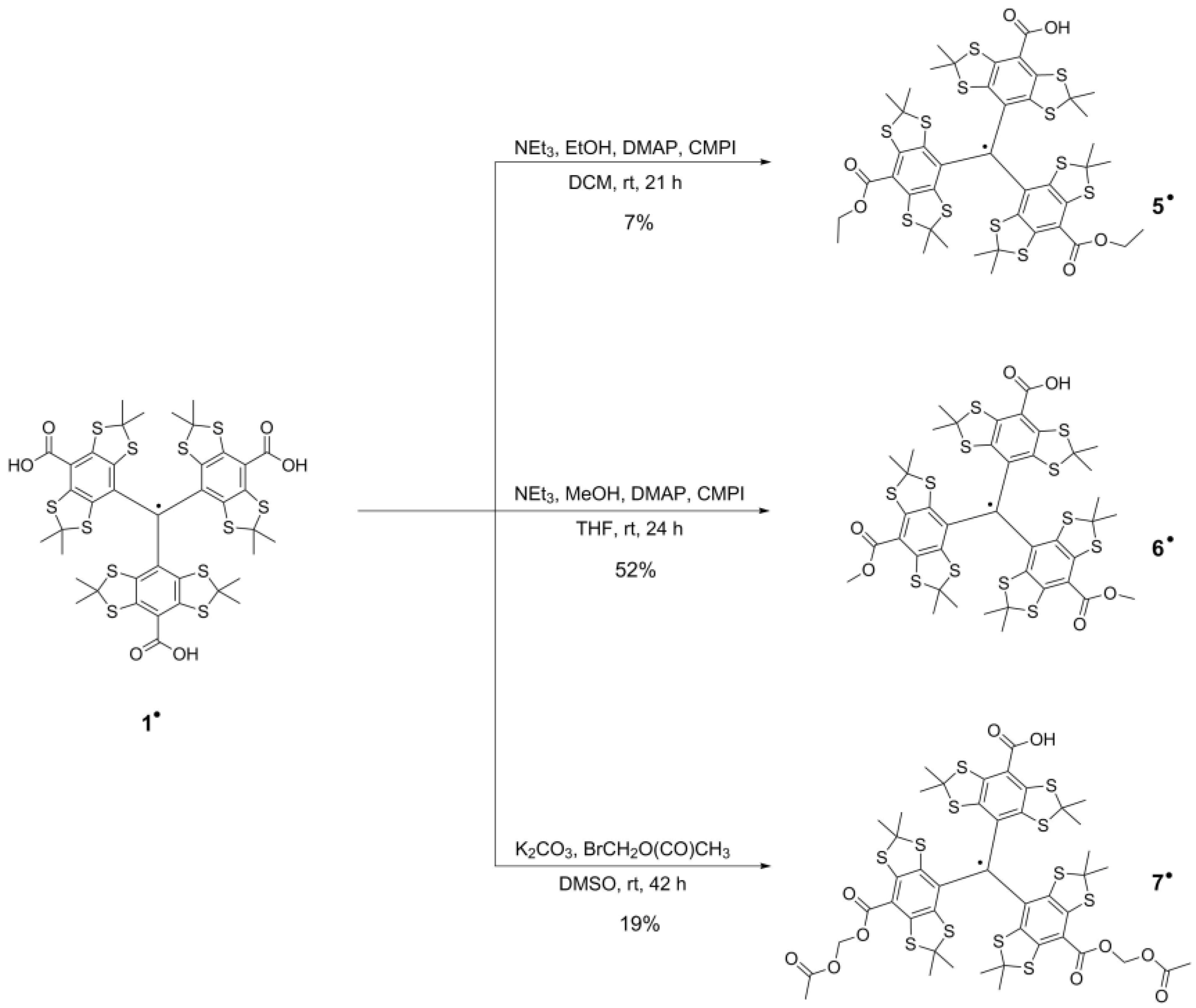
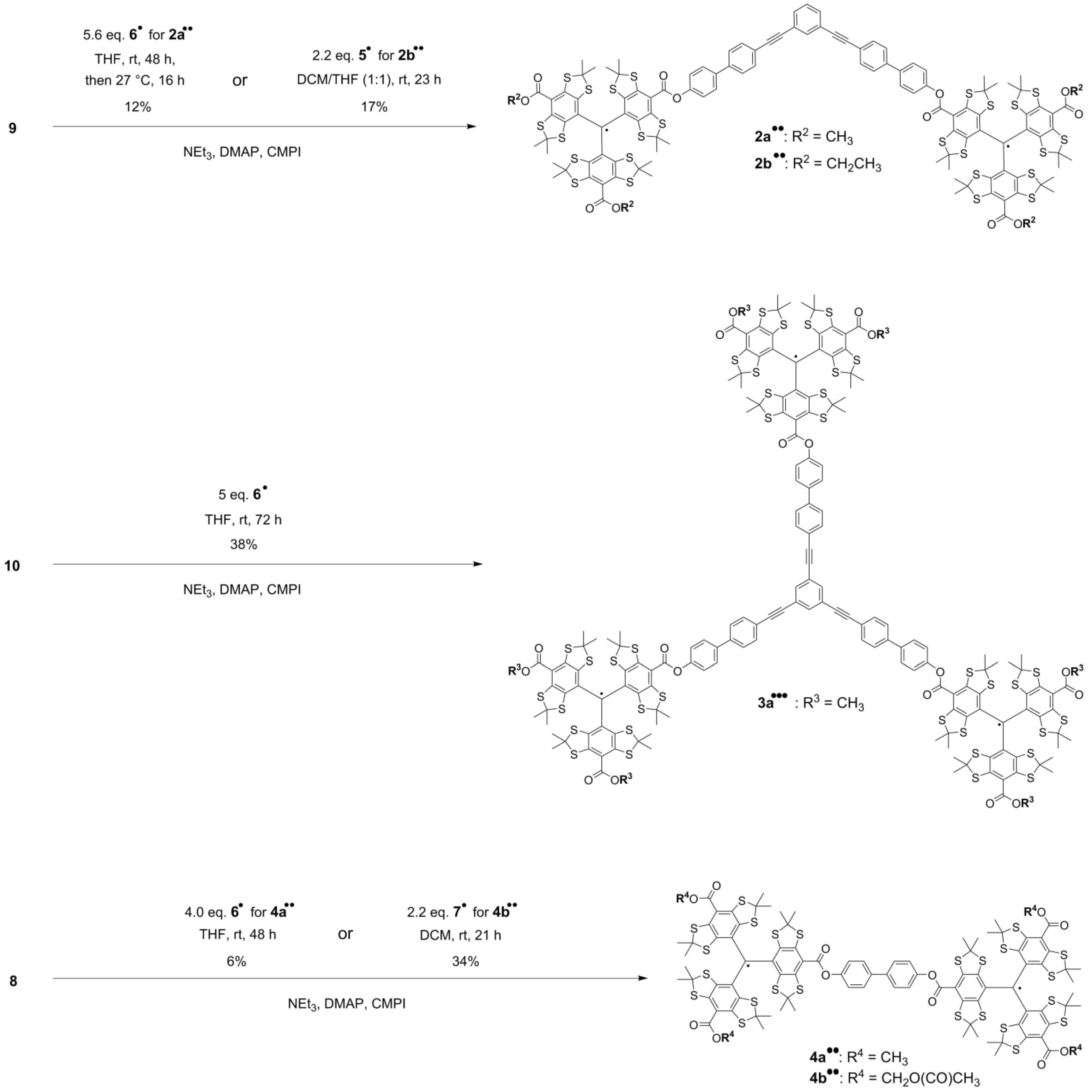
2.1. Syntheses
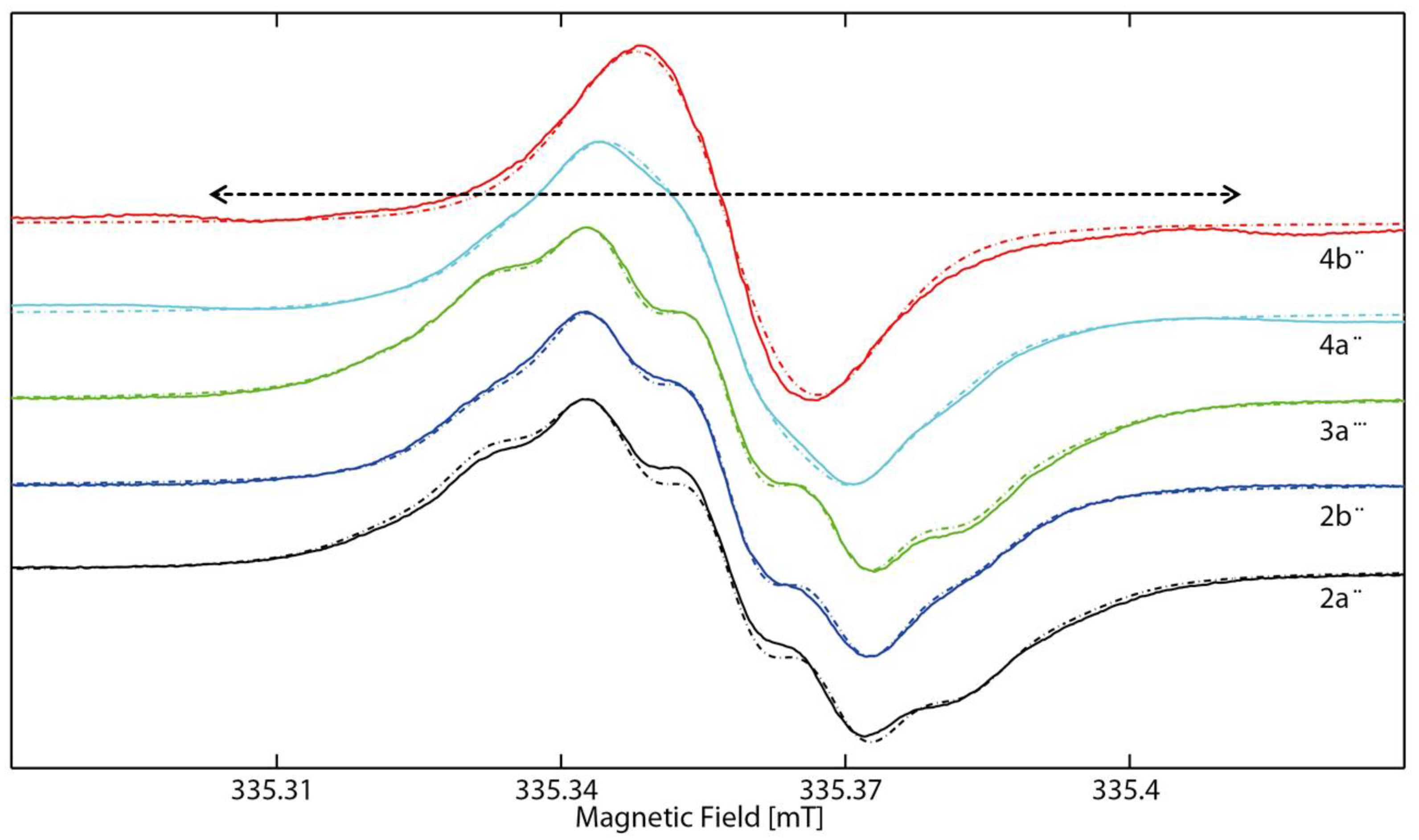
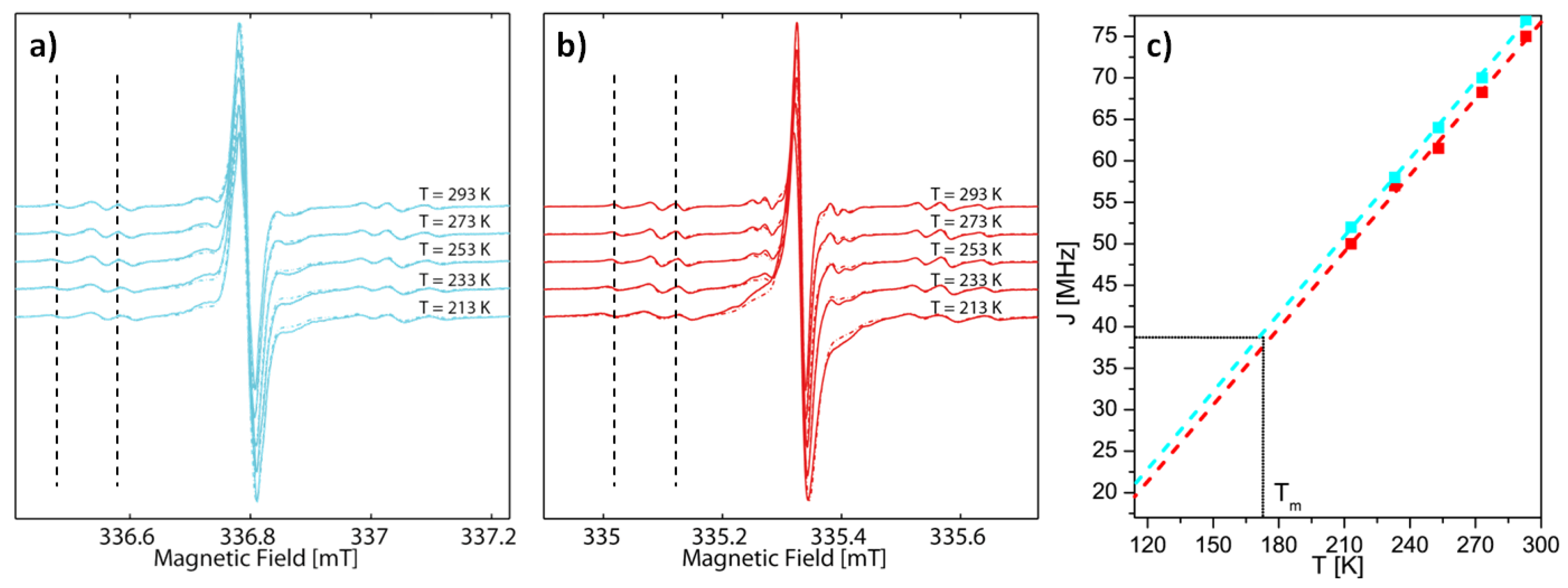
2.2. EPR Spectroscopy
2.3. DFT Calculations
3. Materials and Methods
3.1. General Procedures
3.2. Nuclear Magnetic Resonance Spectroscopy (NMR)
3.3. Mass Spectometry (MS)
3.4. EPR Sample Preparation
3.5. EPR Measurements
3.6. DFT Calculations
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Schiemann, O.; Prisner, T.F. Long-Range Distance Determinations in Biomacromolecules by EPR Spectroscopy. Q. Rev. Biophys. 2007, 40, 1–53. [Google Scholar] [CrossRef] [PubMed]
- Timmel, C.R.; Harmer, J.R. (Eds.) Structural Information from Spin-Labels and Intrinsic Paramagnetic Centres in the Biosciences, 1st ed.; Structure and Bonding 152; Springer: Berlin/Heidelberg, Germany, 2014; pp. 1–332. ISBN 978-3-642-39124-8. [Google Scholar]
- Misra, S.K. (Ed.) Distance Measurements: Continuous-Wave (CW) and Pulsed Dipolar EPR. In Multifrequency Electron Paramagnetic Resonance. Theory and Applications, 1st ed.; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2011; pp. 545–588. ISBN 978-3-527-40779-8. [Google Scholar]
- Bordignon, E.; Bleicken, S. New Limits of Sensitivity of Site-Directed Spin Labeling Electron Paramagnetic Resonance for Membrane Proteins. Biochim. Biophys. Acta-Biomembr. 2018, 1860, 841–853. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, G. DEER Distance Measurements on Proteins. Annu. Rev. Phys. Chem. 2012, 63, 419–446. [Google Scholar] [CrossRef] [PubMed]
- Joseph, B.; Sikora, A.; Cafiso, D.S. Ligand Induced Conformational Changes of a Membrane Transporter in E. Coli Cells Observed with DEER/PELDOR. J. Am. Chem. Soc. 2016, 138, 1844–1847. [Google Scholar] [CrossRef] [PubMed]
- Mchaourab, H.S.; Steed, P.R.; Kazmier, K. Toward the Fourth Dimension of Membrane Protein Structure: Insight into Dynamics from Spin-Labeling EPR Spectroscopy. Structure 2011, 19, 1549–1561. [Google Scholar] [CrossRef] [PubMed]
- Pliotas, C.; Ward, R.; Branigan, E.; Rasmussen, A.; Hagelueken, G.; Huang, H.; Black, S.S.; Booth, I.R.; Schiemann, O.; Naismith, J.H. Conformational State of the MscS Mechanosensitive Channel in Solution Revealed by Pulsed Electron–electron Double Resonance (PELDOR) Spectroscopy. Proc. Natl. Acad. Sci. USA 2012, 109, E2675–E2682. [Google Scholar] [CrossRef] [PubMed]
- Klare, J.P.; Steinhoff, H.J. Spin Labeling Studies of Transmembrane Signaling and Transport: Applications to Phototaxis, ABC Transporters and Symporters. Methods Enzymol. 2015, 564, 315–347. [Google Scholar] [PubMed]
- Schmidt, M.J.; Borbas, J.; Drescher, M.; Summerer, D. A Genetically Encoded Spin Label for Electron Paramagnetic Resonance Distance Measurements. J. Am. Chem. Soc. 2014, 136, 1238–1241. [Google Scholar] [CrossRef] [PubMed]
- Martorana, A.; Bellapadrona, G.; Feintuch, A.; Di Gregorio, E.; Aime, S.; Goldfarb, D. Probing Protein Conformation in Cells by EPR Distance Measurements using Gd3+ Spin Labeling. J. Am. Chem. Soc. 2014, 136, 13458–13465. [Google Scholar] [CrossRef] [PubMed]
- Marko, A.; Denysenkov, V.; Margraf, D.; Cekan, P.; Schiemann, O.; Sigurdsson, S.T.; Prisner, T.F. Conformational Flexibility of DNA. J. Am. Chem. Soc. 2011, 133, 13375–13379. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.; Qin, P.Z. RNA Dynamics: Perspectives from Spin Labels. Wiley Interdiscip. Rev. RNA 2012, 3, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Prisner, T.F.; Marko, A.; Sigurdsson, S.T. Conformational Dynamics of Nucleic Acid Molecules Studied by PELDOR Spectroscopy with Rigid Spin Labels. J. Magn. Reson. 2015, 252, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Reginsson, G.W.; Shelke, S.A.; Rouillon, C.; White, M.F.; Sigurdsson, S.T.; Schiemann, O. Protein-Induced Changes in DNA Structure and Dynamics Observed with Noncovalent Site-Directed Spin Labeling and PELDOR. Nucleic Acids Res. 2012, 41, e11. [Google Scholar] [CrossRef] [PubMed]
- Halbmair, K.; Seikowski, J.; Tkach, I.; Höbartner, C.; Sezer, D.; Bennati, M. High-Resolution Measurement of Long-Range Distances in RNA: Pulse EPR Spectroscopy with TEMPO-Labeled Nucleotides. Chem. Sci. 2016, 7, 3172–3180. [Google Scholar] [CrossRef]
- Schiemann, O.; Fritscher, J.; Kisseleva, N.; Sigurdsson, S.T.; Prisner, T.F. Structural Investigation of a High-Affinity MnII Binding Site in the Hammerhead Ribozyme by EPR Spectroscopy and DFT Calculations. Effects of Neomycin B on Metal-Ion Binding. ChemBioChem 2003, 4, 1057–1065. [Google Scholar] [CrossRef] [PubMed]
- Duss, O.; Yulikov, M.; Jeschke, G.; Allain, F.H.T. EPR-Aided Approach for Solution Structure Determination of Large RNAs or Protein-RNA Complexes. Nat. Commun. 2014, 5, 3669–3678. [Google Scholar] [CrossRef] [PubMed]
- Duss, O.; Yulikov, M.; Allain, F.H.; Jeschke, G. Combining NMR and EPR to Determine Structures of Large RNAs and Protein–RNA Complexes in Solution. Methods Enzymol. 2015, 558, 279–331. [Google Scholar] [CrossRef] [PubMed]
- Ji, M.; Ruthstein, S.; Saxena, S. Paramagnetic Metal Ions in Pulsed ESR Distance Distribution Measurements. Acc. Chem. Res. 2013, 47, 688–695. [Google Scholar] [CrossRef] [PubMed]
- Fielding, A.J.; Heaven, G.; Hollas, M.A. New Developments in Spin Labels for Pulsed Dipolar EPR. Molecules 2014, 19, 16998–17025. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, T.F.; Putterman, M.R.; Desai, A.; Horne, W.S.; Saxena, S. The Double-Histidine Cu2+-Binding Motif: A Highly Rigid, Site-Specific Spin Probe for Electron Spin Resonance Distance Measurements. Angew. Chem. Int. Ed. 2015, 54, 6330–6334. [Google Scholar] [CrossRef] [PubMed]
- Di Valentin, M.; Albertini, M.; Zurlo, E.; Gobbo, M.; Carbonera, D. Porphyrin Triplet State as a Potential Spin Label for Nanometer Distance Measurements by PELDOR Spectroscopy. J. Am. Chem. Soc. 2014, 136, 6582–6585. [Google Scholar] [CrossRef] [PubMed]
- Qi, M.; Gross, A.; Jeschke, G.; Godt, A.; Drescher, M. Gd(III)-PyMTA Label is Suitable for In-Cell EPR. J. Am. Chem. Soc. 2014, 136, 15366–15378. [Google Scholar] [CrossRef] [PubMed]
- Kerzhner, M.; Abdullin, D.; Więcek, J.; Matsuoka, H.; Hagelueken, G.; Schiemann, O.; Famulok, M. Post-synthetic Spin-Labeling of RNA through Click Chemistry for PELDOR Measurements. Chem. Eur. J. 2016, 22, 12113–12121. [Google Scholar] [CrossRef] [PubMed]
- Lueders, P.; Jäger, H.; Hemminga, M.A.; Jeschke, G.; Yulikov, M. Distance Measurements on Orthogonally Spin-Labeled Membrane Spanning WALP23 Polypeptides. J. Phys. Chem. B 2013, 117, 2061–2068. [Google Scholar] [CrossRef] [PubMed]
- Rajca, A.; Kathirvelu, V.; Roy, S.K.; Pink, M.; Rajca, S.; Sarkar, S.; Eaton, S.S.; Eaton, G.R. A Spirocyclohexyl Nitroxide Amino Acid Spin Label for Pulsed EPR Spectroscopy Distance Measurements. Chem. Eur. J. 2010, 16, 5778–5782. [Google Scholar] [CrossRef] [PubMed]
- Reginsson, G.W.; Kunjir, N.C.; Sigurdsson, S.T.; Schiemann, O. Trityl Radicals: Spin Labels for Nanometer-Distance Measurements. Chem. Eur. J. 2012, 18, 13580–13584. [Google Scholar] [CrossRef] [PubMed]
- Kunjir, N.C.; Reginsson, G.W.; Schiemann, O.; Sigurdsson, S.T. Measurements of Short Distances between Trityl Spin Labels with CW EPR, DQC and PELDOR. Phys. Chem. Chem. Phys. 2013, 15, 19673–19685. [Google Scholar] [CrossRef] [PubMed]
- Shevelev, G.Y.; Gulyak, E.L.; Lomzov, A.A.; Kuzhelev, A.A.; Krumkacheva, O.A.; Kupryushkin, M.S.; Tormyshev, V.M.; Fedin, M.V.; Bagryanskaya, E.G.; Pyshnyi, D.V. A Versatile Approach to Attachment of Triarylmethyl Labels to DNA for Nanoscale Structural EPR Studies at Physiological Temperatures. J. Phys. Chem. B 2017, 122, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Likhtenshtein, G.I.; Yamauchi, J.; Nakatsuji, S.; Smirnov, A.I.; Tamura, R. Nitroxides: Applications in Chemistry, Biomedicine, and Materials Science, 1st ed.; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2008; ISBN 978-3-527-31889-6. [Google Scholar]
- Tidwell, T.T. Tetrathiatriarylmethyl (TAM) and related triarylmethyl radicals. In Stable Radicals, 1st ed.; Hicks, R.G., Ed.; John Wiley & Sons Ltd.: Chichester, UK, 2010; pp. 16–20. ISBN 978-0-470-77083-2. [Google Scholar]
- Jassoy, J.J.; Berndhäuser, A.; Duthie, F.; Kühn, S.P.; Hagelueken, G.; Schiemann, O. Versatile Trityl Spin Labels for Nanometer Distance Measurements on Biomolecules in Vitro and within Cells. Angew. Chem. Int. Ed. 2017, 56, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Kuzhelev, A.A.; Trukhin, D.V.; Krumkacheva, O.A.; Strizhakov, R.K.; Rogozhnikova, O.Y.; Troitskaya, T.I.; Fedin, M.V.; Tormyshev, V.M.; Bagryanskaya, E.G. Room-Temperature Electron Spin Relaxation of Triarlymethyl Radicals at X- and Q-Bands. J. Phys. Chem. B 2015, 119, 13630–13640. [Google Scholar] [CrossRef] [PubMed]
- Owenius, R.; Eaton, G.R.; Eaton, S.S. Frequency (250 MHz to 9.2 GHz) and Viscosity Dependence of Electron Spin Relaxation of Triarylmethyl Radicals at Room Temperature. J. Magn. Reson. 2005, 172, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Kulik, L.; Dzuba, S.; Grigoryev, I.; Tsvetkov, Y.D. Electron Dipole–dipole Interaction in ESEEM of Nitroxide Biradicals. Chem. Phys. Lett. 2001, 343, 315–324. [Google Scholar] [CrossRef]
- Milikisyants, S.; Scarpelli, F.; Finiguerra, M.G.; Ubbink, M.; Huber, M. A Pulsed EPR Method to Determine Distances between Paramagnetic Centers with Strong Spectral Anisotropy and Radicals: The Dead-Time Free RIDME Sequence. J. Magn. Reson. 2009, 201, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Abdullin, D.; Duthie, F.; Meyer, A.; Müller, E.S.; Hagelueken, G.; Schiemann, O. Comparison of PELDOR and RIDME for Distance Measurments between Nitroxides and Low Spin Fe(III) Ions. J. Phys. Chem. B 2015, 119, 13534–13542. [Google Scholar] [CrossRef] [PubMed]
- Borbat, P.P.; Freed, J.H. Multiple-Quantum ESR and Distance Measurements. Chem. Phys. Lett. 1999, 313, 145–154. [Google Scholar] [CrossRef]
- Saxena, S.; Freed, J.H. Double Quantum Two-Dimensional Fourier Transform Electron Spin Resonance: Distance Measurements. Chem. Phys. Lett. 1996, 251, 102–110. [Google Scholar] [CrossRef]
- Saxena, S.; Freed, J.H. Theory of Double Quantum Two-Dimensional Electron Spin Resonance with Application to Distance Measurements. J. Chem. Phys. 1997, 107, 1317–1340. [Google Scholar] [CrossRef]
- Jeschke, G.; Pannier, M.; Godt, A.; Spiess, H.W. Dipolar Spectroscopy and Spin Alignment in Electron Paramagnetic Resonance. Chem. Phys. Lett. 2000, 331, 243–252. [Google Scholar] [CrossRef]
- Krumkacheva, O.; Bagryanskaya, E. EPR-Based Distance Measurements at Ambient Temperature. J. Magn. Reson. 2017, 280, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Liu, Y.; Borbat, P.; Zweier, J.L.; Freed, J.H.; Hubbell, W.L. Pulsed ESR Dipolar Spectroscopy for Distance Measurements in Immobilized Spin Labeled Proteins in Liquid Solution. J. Am. Chem. Soc. 2012, 134, 9950–9952. [Google Scholar] [CrossRef] [PubMed]
- Shevelev, G.Y.; Krumkacheva, O.A.; Lomzov, A.A.; Kuzhelev, A.A.; Rogozhnikova, O.Y.; Trukhin, D.V.; Troitskaya, T.I.; Tormyshev, V.M.; Fedin, M.V.; Pyshnyi, D.V. Physiological-Temperature Distance Measurement in Nucleic Acid Using Triarylmethyl-Based Spin Labels and Pulsed Dipolar EPR Spectroscopy. J. Am. Chem. Soc. 2014, 136, 9874–9877. [Google Scholar] [CrossRef] [PubMed]
- Andersson, S.; Radner, F.; Rydbeek, A.; Servin, R.; Wistrand, L.-G. Free Radicals. U.S. Patent Application No. 5530140A, 25 June 1996. [Google Scholar]
- Saigo, K.; Usui, M.; Kikuchi, K.; Shimada, E.; Mukaiyama, T. New Method for the Preparation of Carboxylic Esters. Bull. Chem. Soc. Japan 1977, 50, 1863–1866. [Google Scholar] [CrossRef]
- Bunz, U.H.F. Poly(aryleneethynylene)s: Syntheses, Properties, Structures, and Applications. Chem. Rev. 2000, 100, 1605–1644. [Google Scholar] [CrossRef] [PubMed]
- Bode, B.E.; Margraf, D.; Plackmeyer, J.; Dürner, G.; Prisner, T.F.; Schiemann, O. Counting the Monomers in Nanometer-Sized Oligomers by Pulsed Electron-Electron Double Resonance. J. Am. Chem. Soc. 2007, 129, 6736–6745. [Google Scholar] [CrossRef] [PubMed]
- Valera, S.; Taylor, J.E.; Daniels, D.S.B.; Dawson, D.M.; Athukorala Arachchige, K.S.; Ashbrook, S.E.; Slawin, A.M.Z.; Bode, B.E. A Modular Approach for the Synthesis of Nanometer-Sized Polynitroxide Multi-Spin Systems. J. Org. Chem. 2014, 79, 8313–8323. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, G.; Sajid, M.; Schulte, M.; Ramezanian, N.; Volkov, A.; Zimmermann, H.; Godt, A. Flexibility of Shape-Persistent Molecular Building Blocks Composed of p-Phenylene and Ethynylene Units. J. Am. Chem. Soc. 2010, 132, 10107–10117. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, G.; Sajid, M.; Schulte, M.; Godt, A. Three-Spin Correlations in Double Electron–electron Resonance. Phys. Chem. Chem. Phys. 2009, 11, 6580–6591. [Google Scholar] [CrossRef] [PubMed]
- Atherton, N.M. Principles of Electron Spin Resonance; Ellis Horwood Ltd.: New York, NY, USA, 1993. [Google Scholar]
- Eaton, G.R.; Eaton, S.S. Spin Labeling:Theory and Applications. In Biological Magnetic Resonance; Berliner, L.J., Reuben, J., Eds.; Springer: Berlin, Germany, 1989; Volume 8, pp. 339–397. [Google Scholar] [CrossRef]
- Möbius, K.; Savitsky, A. High-Field EPR Spectroscopy on Proteins and Their Model Systems: Characterization of Transient Paramagnetic States; Royal Society of Chemistry: London, UK, 2008; ISBN 978-0-85404-368-2. [Google Scholar]
- Weber, A.; Schiemann, O.; Bode, B.E.; Prisner, T.F. PELDOR at S- and X-Band Frequencies and the Separation of Exchange Coupling from Dipolar Coupling. J. Magn. Reson. 2002, 157, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Margraf, D.; Cekan, P.; Prisner, T.F.; Sigurdsson, S.T.; Schiemann, O. Ferro- and Antiferromagnetic Exchange Coupling Constants in PELDOR Spectra. Phys. Chem. Chem. Phys. 2009, 11, 6708–6714. [Google Scholar] [CrossRef] [PubMed]
- Brandsma, L.; Vasilevsky, S.F.; Verkruijsse, H.D. Application of Transition Metal Catalysis in Organic Synthesis; Springer: Berlin, Germany, 1998; Chapter 10; ISBN 3-540-65550-6. [Google Scholar]
- Liu, Y.; Villamena, F.A.; Sun, J.; Xu, Y.; Dhimitruka, I.; Zweier, J.L. Synthesis and Characterization of Ester-Derivatized Tetrathiatriarylmethyl Radicals as intracellular Oxygen Probes. J. Org. Chem. 2008, 73, 1490–1497. [Google Scholar] [CrossRef] [PubMed]
- Stoll, S.; Schweiger, A. EasySpin, a Comprehensive Software Package for Spectral Simulation and Analysis in EPR. J. Magn. Reson. 2006, 178, 42–55. [Google Scholar] [CrossRef] [PubMed]
- Kuzhelev, A.A.; Tormyshev, V.M.; Rogozhnikova, O.Y.; Trukhin, D.V.; Troitskaya, T.I.; Strizhakov, R.K.; Krumkacheva, O.A.; Fedin, M.V.; Bagryanskaya, E.G. Triarylmethyl Radicals: An EPR Study of 13C Hyperfine Coupling Constants. Phys. Chem. 2017, 231, 777–794. [Google Scholar] [CrossRef] [PubMed]
- Lloveras, V.; Vidal-Gancedo, J.; Figueira-Duarte, T.M.; Nierengarten, J.-F.; Novoa, J.J.; Mota, F.; Ventosa, N.; Veciana, J.; Rovira, C. Tunneling versus Hopping in Mixed-Valence Oligo-p-phenylenevinylene Polychlorinated Bis(triphenylmethyl) Radical Anions. J. Am. Chem. Soc. 2011, 133, 5818–5833. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Villamena, F.A.; Rockenbauer, A.; Song, Y.; Zweier, J.L. Structural Factors Controlling the Spin-Spin Exchange Coupling: EPR Spectroscopic Studies of Highly Asymmetric Trityl-Nitroxide Biradicals. J. Am. Chem. Soc. 2013, 135, 2350–2356. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Brugh, A.; Rawson, J.; Therien, M.J.; Forbes, M.D.E. Alkyne-Bridged Multi[Copper(II)Porphyrin] Structures: Nuances of Orbital Symmetry in Long-Range, Through-Bond Mediated, Isotropic Spin Exchange Interactions. J. Am. Chem. Soc. 2017, 139, 9759–9762. [Google Scholar] [CrossRef] [PubMed]
- Alba-Simionesco, C.; Fan, J.; Angell, A. Thermodynamic aspects of the glass transition phenomen. II. Molecular liquids with variable interactions. J. Chem. Phys. 1999, 110, 5262–5272. [Google Scholar] [CrossRef]
- Akhmetzyanov, D.; Schöps, P.; Marko, A.; Kunjir, N.; Sigurdsson, S.T.; Prisner, T.F. Pulsed EPR Dipolar Spectroscopy at Q-and G-Band on a Trityl Biradical. Phys. Chem. Chem. Phys. 2015, 17, 24446–24451. [Google Scholar] [CrossRef] [PubMed]
- Meyer, A.; Schnakenburg, G.; Schiemann, O. The crystal structure of 4′-((4-(2,2′:6′,2′′-terpyridyl)phenyl)ethynyl)biphen-4-yl-(2,2,5,5-tetramethyl-N-oxyl-pyrroline)-3-formate. Acta Crystallogr. E 2015, 71, 1245–1249. [Google Scholar] [CrossRef] [PubMed]
- Meyer, A.; Abdullin, D.; Schnakenburg, G.; Schiemann, O. Single and Double Nitroxide Labeled Bis(terpyridine)copper(II): The Influence of Multispin and Jahn-Teller Effects on PELDOR and RIDME. Phys. Chem. Chem. Phys. 2016, 18, 9262–9271. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D. Density-Functional Exchange-Energy Approximation with Correct Asymptotic Behavior. Phys. Rev. A 1988, 38, 3098. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A Consistent and Accurate Ab Initio Parametrization of Density Functional Dispersion Correction (DFT-D) for the 94 Elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed]
- Neese, F. The ORCA Program System. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Perdew, J.P. Density-Functional Approximation for the Correlation Energy of the Inhomogeneous Electron Gas. Phys. Rev. B 1986, 33, 8822. [Google Scholar] [CrossRef]
- Schäfer, A.; Horn, H.; Ahlrichs, R. Fully Optimized Contracted Gaussian Basis Sets for Atoms Li to Kr. J. Chem. Phys. 1992, 97, 2571–2577. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
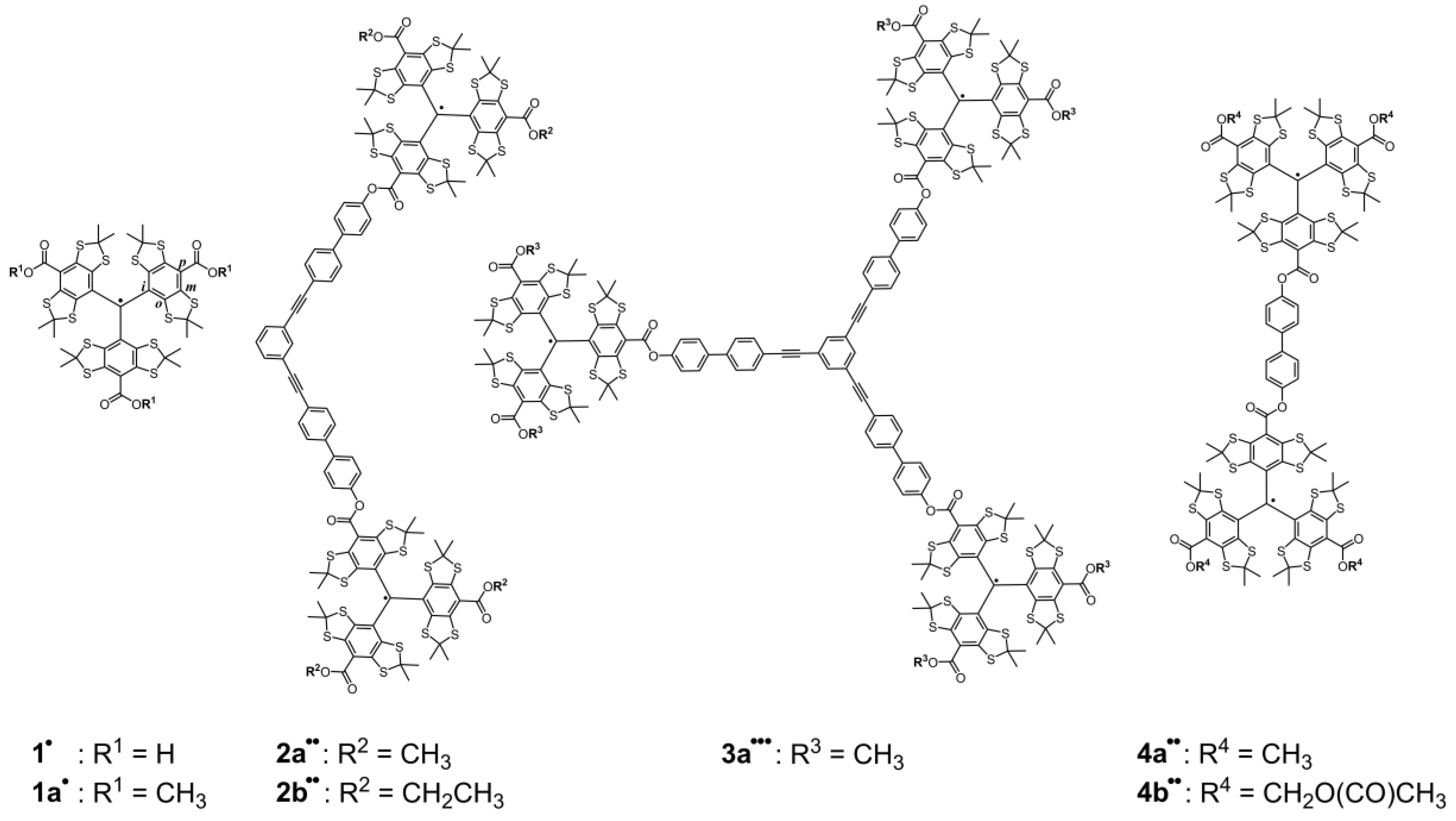



| Compound | 2a•• | 2b•• | 3a••• | 4a•• | 4b•• |
|---|---|---|---|---|---|
| giso | 2.00348 | 2.00344 | 2.00344 | 2.00351 | 2.00346 |
| aα-H a [MHz] | 0.3 | 0.3 | 0.3 | 0.15 | 0.13 |
| nα-H b | 6 | 4 | 6 | 12 | 8 |
| Lwpp c [MHz] | 0.22/0.15 | 0.22/0.17 | 0.22/0.17 | 0.17/0.15 | 0.22/0.15 |
| Compound | 1a• | 2a•• | 2b•• | 3a••• | 4a•• | 4b•• |
|---|---|---|---|---|---|---|
| g a | 2.00355 2.00355 2.00339 | 2.00354 2.00354 2.00338 | 2.00353 2.00353 2.00337 | 2.00351 2.00351 2.00335 | 2.00355 2.00355 2.00339 | 2.00351 2.00351 2.00335 |
| D b [MHz] | - | 1.2 | 1.2 | 1.2 (×2) | 7.0 | 7.0 |
| Lwpp c [MHz] | 2.36/1.00 | 2.36/1.00 | 1.57/1.00 | 2.36/1.00 | 2.36/1.00 | 1.26/1.26 |
| Source | i-13C | o-13C | m-13C | p-13C |
|---|---|---|---|---|
| Kuzhelev a | 31.1 | 25.2 | 6.7 | 9.2 |
| This work, exp | 31.3 | 25.0 | 6.2 | 8.4 |
| This work, DFT b | −37.3 | 34.6 | −10.6 | 15.8 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jassoy, J.J.; Meyer, A.; Spicher, S.; Wuebben, C.; Schiemann, O. Synthesis of Nanometer Sized Bis- and Tris-trityl Model Compounds with Different Extent of Spin–Spin Coupling. Molecules 2018, 23, 682. https://doi.org/10.3390/molecules23030682
Jassoy JJ, Meyer A, Spicher S, Wuebben C, Schiemann O. Synthesis of Nanometer Sized Bis- and Tris-trityl Model Compounds with Different Extent of Spin–Spin Coupling. Molecules. 2018; 23(3):682. https://doi.org/10.3390/molecules23030682
Chicago/Turabian StyleJassoy, J. Jacques, Andreas Meyer, Sebastian Spicher, Christine Wuebben, and Olav Schiemann. 2018. "Synthesis of Nanometer Sized Bis- and Tris-trityl Model Compounds with Different Extent of Spin–Spin Coupling" Molecules 23, no. 3: 682. https://doi.org/10.3390/molecules23030682
APA StyleJassoy, J. J., Meyer, A., Spicher, S., Wuebben, C., & Schiemann, O. (2018). Synthesis of Nanometer Sized Bis- and Tris-trityl Model Compounds with Different Extent of Spin–Spin Coupling. Molecules, 23(3), 682. https://doi.org/10.3390/molecules23030682