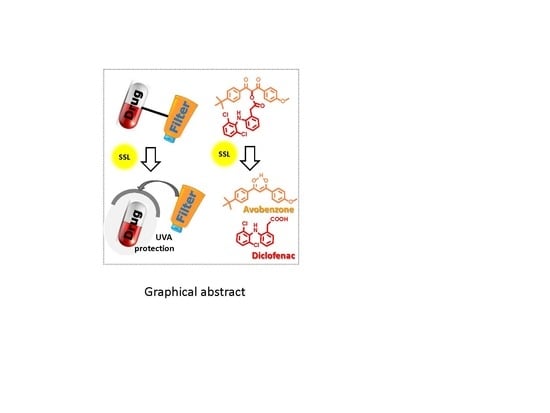
Sunscreen-Based Photocages for Topical Drugs: A Photophysical and Photochemical Study of A Diclofenac-Avobenzone Dyad
Abstract
:1. Introduction
2. Results
2.1. Synthesis
2.2. Photorelease Experiments
2.2.1. UV-Vis Absorption
2.2.2. HPLC Analysis
2.2.3. Fluorescence Analysis
2.3. Time-Resolved Experiments
3. Materials and Methods
3.1. Reagents and Solvents
3.2. Characterization
3.3. Synthesis
3.4. Photophysical/Photochemical Instrumentation
3.4.1. UV-Vis Absorption
3.4.2. Fluorescence Emission
3.4.3. Phosphorescence Emission
3.4.4. Laser Flash Photolysis (LFP)
3.4.5. Steady-State Photolysis
3.4.6. HPLC Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Division of Cancer Prevention and Control. 2016. Available online: https://www.cdc.gov/cancer/skin/statistics/trends.htm (accessed on 22 February 2018).
- Curtius, K.; Wright, N.A.; Graham, T.A. An evolutionary perspective on field cancerization. Nat. Rev. Cancer 2017, 18, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Brem, R.; Guven, M.; Karran, P. Oxidatively-generated damage to DNA and proteins mediated by photosensitized UVA. Free Rad. Biol. Med. 2017, 107, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Lhiaubet-Vallet, V.; Miranda, M.A. CRC Handbook of Organic Photochemistry and Photobiology, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2012; Volume 2, pp. 1541–1555. ISBN 9781439899335. [Google Scholar]
- Epe, B. DNA damage spectra induced by photosensitization. Photochem. Photobiol. Sci. 2012, 11, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Karran, P.; Brem, R. Protein oxidation, UVA and human DNA repair. DNA Repair 2016, 44, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Montoro, J.; Rodríguez, M.; Díaz, M.; Bertomeu, F.C.D. Photoallergic contact dermatitis due to diclofenac. Contact Dermat. 2003, 48, 115. [Google Scholar] [CrossRef]
- Fernández-Jorge, B.; Goday-Buján, J.J.; Murga, M.; Molina, F.P.; Pérez-Varela, L.; Fonseca, E. Photoallergic contact dermatitis due to diclofenac with crossreaction to aceclofenac: Two case reports. Contact Dermat. 2009, 61, 236–237. [Google Scholar] [CrossRef] [PubMed]
- Jenerowicz, D.; Jakubowicz, O.; Polańska, A.; Sadowska-Przytocka, A.; Dańczak-Pazdrowska, A.; Żaba, R. Photosensitivity to selected topical nonsteroidal anti-inflammatory drugs preparations—A review of literature data and author’s own experience. Cent. Eur. J. Immunol. 2011, 36, 197–203. [Google Scholar]
- Przybilla, B.; Ring, J.; Schwab, U.; Galosi, A.; Dorn, M.; Braun-Falco, O. Photosensibilisierende Eigenschaften nichtsteroidaler Antirheumatika im Photopatch-Test. Hautarzt 1987, 38, 18–25. [Google Scholar] [PubMed]
- Monteiro, A.F.; Rato, M.; Martins, C. Drug-induced photosensitivity: Photoallergic and phototoxic reactions. Clin. Dermatol. 2016, 34, 571–581. [Google Scholar] [CrossRef] [PubMed]
- Akat, P.B. Severe photosensitivity reaction induced by topical diclofenac. Indian J. Pharmacol. 2013, 45, 408–409. [Google Scholar] [CrossRef] [PubMed]
- Kowalzick, L.; Ziegler, H. Photoallergic contact dermatitis from topical diclofenac in Solaraze gel. Contact Dermat. 2006, 54, 348–349. [Google Scholar] [CrossRef] [PubMed]
- Encinas, S.; Bosca, F.; Miranda, M.A. Photochemistry of 2,6-dichlorodiphenylamine and 1-chlorocarbazole, the photoactive chromophores of diclofenac, meclofenamic acid and their major photoproducts. Photochem. Photobiol. 1998, 68, 640–645. [Google Scholar] [CrossRef]
- Encinas, S.; Bosca, F.; Miranda, M.A. Phototoxicity associated with diclofenac: A photophysical, photochemical, and photobiological study on the drug and its photoproducts. Chem. Res. Toxicol. 1998, 11, 946–952. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.E.; Roberts-Thomson, S.; Zhen, D.; Duke, C.C. Photochemical studies on the antiinflammatory drug diclofenac. Photochem. Photobiol. 1990, 52, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Ioele, G.; De Luca, M.; Tavano, L.; Ragno, G. The difficulties for a photolabile drug in topical formulations: The case of diclofenac. Int. J. Pharm. 2014, 465, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Ioele, G.; Tavano, L.; De Luca, M.; Ragno, G.; Picci, N.; Muzzalupo, R. Photostability and ex-vivo permeation studies on diclofenac in topical niosomal formulations. Int. J. Pharm. 2015, 494, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Aparici-Espert, I.; Cuquerella, M.C.; Paris, C.; Lhiaubet-Vallet, V.; Miranda, M.A. Photocages for protection and controlled release of bioactive compounds. Chem. Commun. 2016, 52, 14215–14218. [Google Scholar] [CrossRef] [PubMed]
- Klán, P.; Šolomek, T.; Bochet, C.G.; Blanc, A.; Givens, R.; Rubina, M.; Popik, V.; Kostikov, A.; Wirz, J. Photoremovable protecting groups in chemistry and biology: Reaction mechanisms and efficacy. Chem. Rev. 2013, 113, 119–191. [Google Scholar] [CrossRef] [PubMed]
- Šolomek, T.; Wirz, J.; Klán, P. Searching for improved photoreleasing abilities of organic molecules. Acc. Chem. Res. 2015, 48, 3064–3072. [Google Scholar] [CrossRef] [PubMed]
- Young, D.D.; Deiters, A. Photochemical control of biological processes. Org. Biomol. Chem. 2007, 5, 999–1005. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Li, J.; Wu, D.; Qiu, Z.; Zhang, Y. Chemistry and biological applications of photo-labile organic molecules. Chem. Soc. Rev. 2010, 39, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Pravst, I.; Zupan, M.; Stavber, S. Solvent-free bromination of 1,3-diketones and β-keto esters with NBS. Green Chem. 2006, 8, 1001–1005. [Google Scholar] [CrossRef]
- Paris, C.; Lhiaubet-Vallet, V.; Jiménez, O.; Trullas, C.; Miranda, M.Á. A blocked diketo form of avobenzone: Photostability, photosensitizing properties and triplet quenching by a triazine-derived UVB-filter. Photochem. Photobiol. 2009, 85, 178–184. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |











© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aparici-Espert, I.; Miranda, M.A.; Lhiaubet-Vallet, V. Sunscreen-Based Photocages for Topical Drugs: A Photophysical and Photochemical Study of A Diclofenac-Avobenzone Dyad. Molecules 2018, 23, 673. https://doi.org/10.3390/molecules23030673
Aparici-Espert I, Miranda MA, Lhiaubet-Vallet V. Sunscreen-Based Photocages for Topical Drugs: A Photophysical and Photochemical Study of A Diclofenac-Avobenzone Dyad. Molecules. 2018; 23(3):673. https://doi.org/10.3390/molecules23030673
Chicago/Turabian StyleAparici-Espert, Isabel, Miguel A. Miranda, and Virginie Lhiaubet-Vallet. 2018. "Sunscreen-Based Photocages for Topical Drugs: A Photophysical and Photochemical Study of A Diclofenac-Avobenzone Dyad" Molecules 23, no. 3: 673. https://doi.org/10.3390/molecules23030673