Effects of Exogenous Application of Protocatechuic Acid and Vanillic Acid to Chlorophylls, Phenolics and Antioxidant Enzymes of Rice (Oryza sativa L.) in Submergence
Abstract
:1. Introduction
2. Results
2.1. Effects of Exogenous Application of PA and VA on Rice Growth
2.2. Effects of Exogenous Application of PA and VA Photosynthetic Pigments and Lipid Peroxidation
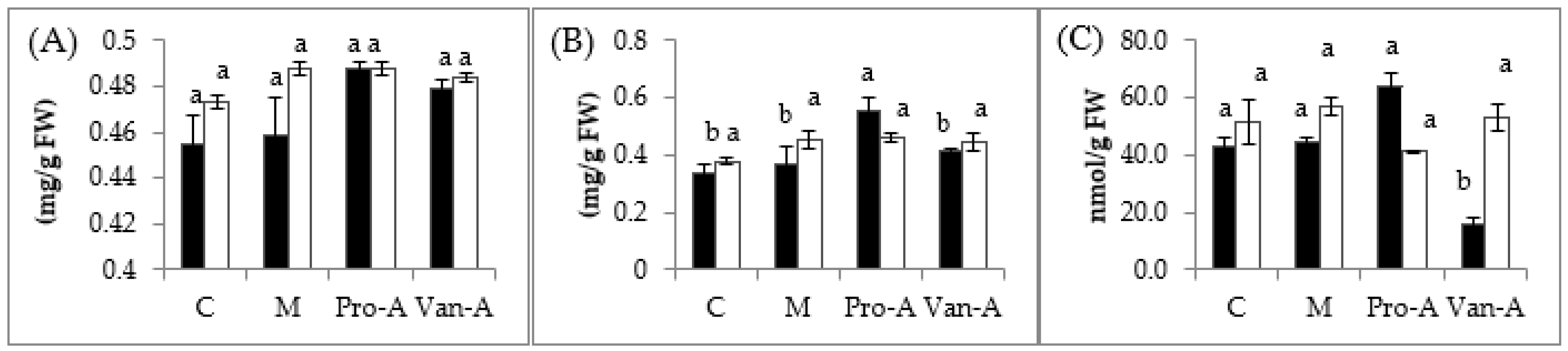
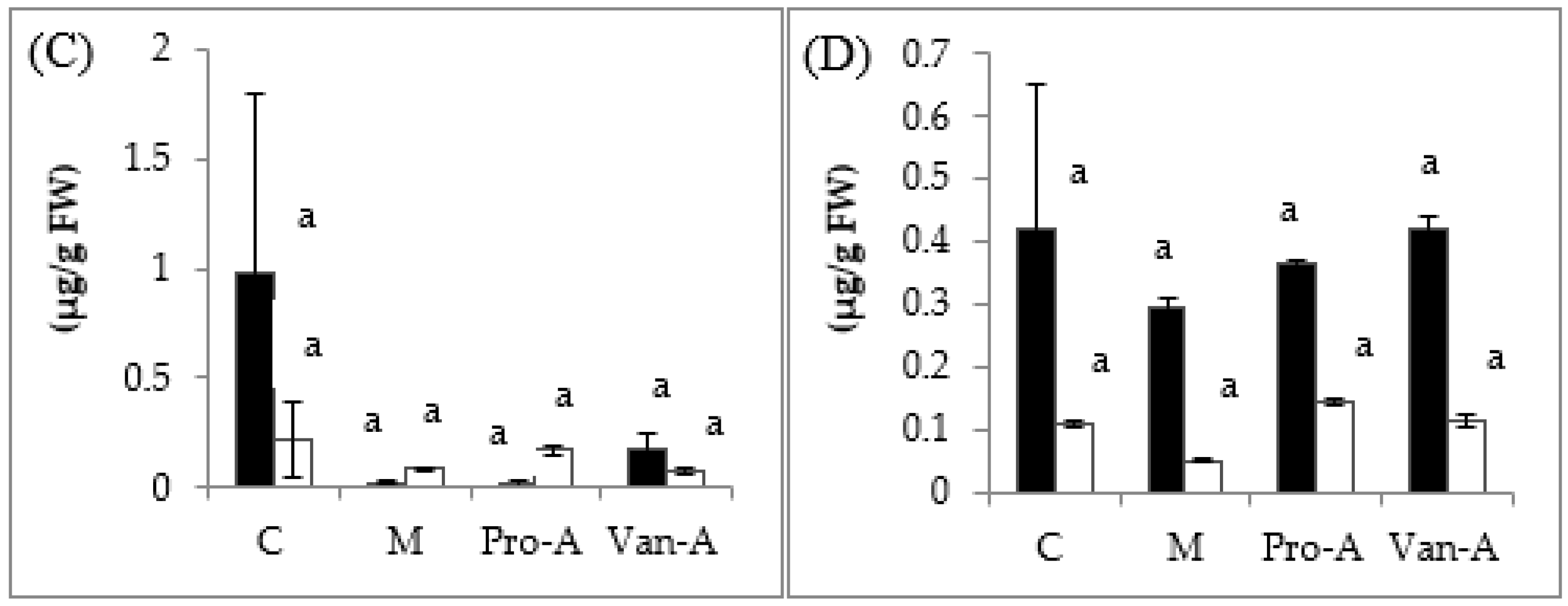
2.3. Effect of Exogenous Application of PA and VA on Total Contents of Phenolics and Flavonoids, and Endogenous PA and VA
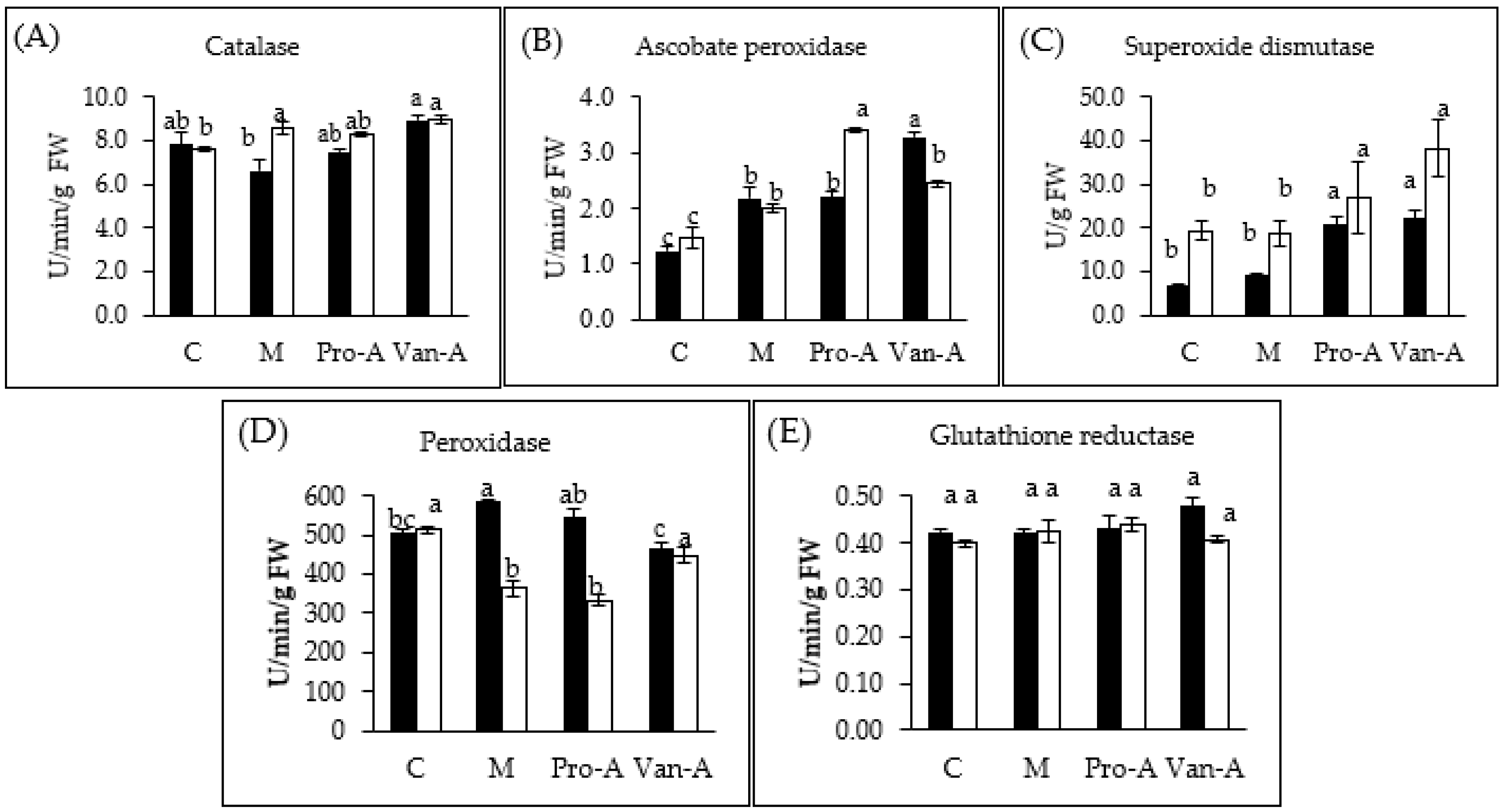
2.4. Effect of Exogenous Phenolics on Antioxidant Enzymes
2.5. Coefficient Correlations among Contents of Total Phenols, Flavonoids, Chlorophylls a and b, Lipid Peroxidation, and Gene Expression of Antioxidant Enzymes
3. Discussion
4. Materials and Methods
4.1. Plant Material and Treatments
4.2. Lipid Peroxidation Measurement
4.3. Measurement of Chlorophyll Contents
4.4. Estimation of Total Phenolic and Flavonoid Contents
4.5. Identification and Quantification of Protocatechuic Acid and Vanillic Acid
4.6. Enzyme Extraction and Assays
4.7. Gene Expression of Antioxidant Enzymes Using Quantitative Real-Time PCR
4.8. Statistical Analyses
5. Conclusions
Author Contributions
Conflicts of Interest
Appendix A
| Scale | Survival (%) |
|---|---|
| 1 | 100 |
| 3 | 95–99 |
| 5 | 75–94 |
| 7 | 50–75 |
| 9 | 0–49 |
References
- Ismail, A.M.; Singh, U.S.; Sing, S.; Dar, M.H.; Mackill, D.J. The contribution of submergence-tolerant (Sub1) rice varieties to food security in flood-prone rainfed lowland areas in Asia. Field Crops Res. 2013, 152, 83–93. [Google Scholar] [CrossRef]
- Mackill, D.J.; Ismail, A.M.; Pamplona, A.M.; Sanchez, D.L.; Carandang, J.J.; Septiningsih, E.M. Stress tolerant rice varieties for adaptation to a changing climate. Crop Environ. Bioinform. 2010, 7, 250–259. [Google Scholar]
- Wassmann, R.; Jagadish, S.V.K.; Sumfleth, K.; Pathak, H.; Howell, G.; Ismail, A.; Heuer, S. Chapter 3: Regional vulnerability of climate change impacts on Asian rice production and scope for adaptation. Adv. Agron. 2009, 102, 91–133. [Google Scholar]
- Xu, K.; Mackill, D.J. A major locus for submergence tolerance mapped on rice chromosome 9. Mol. Breed. 1996, 2, 219–224. [Google Scholar] [CrossRef]
- Xu, K.; Xia, X.; Fukao, T.; Canlas, P.; Maghirang-Rodriguez, R.; Heuer, S.; Ismail, A.M.; Bailey-Serres, J.; Ronald, P.C.; Mackill, D.J. Sub1A is an ethylene response factor-like gene that confers submergence tolerance to rice. Nature 2006, 442, 705–708. [Google Scholar] [CrossRef] [PubMed]
- Hattori, Y.; Nagai, K.; Furukawa, S.; Song, X.J.; Kawano, R.; Sakakibara, H.; Wu, J.Z.; Matsumoto, T.; Yoshimura, A.; Kitano, H.; et al. The ethylene response factors SNORKEL1 and SNORKEL2 allow rice to adapt to deep water. Nature 2009, 460, 1026–1031. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.R.; Ghosh, A. Submergence tolerance and yield performance of lowland rice as affected by agronomic management practices in eastern India. Field Crops Res. 1999, 63, 187–198. [Google Scholar] [CrossRef]
- Septiningsih, E.M.; Pamplona, A.M.; Sanchez, D.L.; Neeraja, C.N.; Vergara, G.V.; Heuer, S.; Mackill, D.J. Development of submergence-tolerant rice cultivars: The Sub1 locus and beyond. Ann. Bot. 2009, 103, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Chu, M.; Ding, Y.; Wang, S.; Liu, Z.; Tang, S.; Li, G. Exogenous spermidine alleviates oxidative damage and reduce yield loss in rice submerged at tillering stage. Front. Plant Sci. 2015, 6, 919. [Google Scholar] [CrossRef] [PubMed]
- Gratão, P.L.; Polle, A.; Lea, P.J.; Azevedo, R.A. Making the life of heavy metal-stressed plants a little easier. Funct. Plant Biol. 2005, 32, 481–494. [Google Scholar] [CrossRef]
- Alyemeni, M.N.; Hayat, Q.; Wijaya, L.; Hayat, S. Effect of salicylic acid on the growth, photosynthetic efficiency and enzyme activities of leguminous plant under cadmium stress. Not. Bot. Horti Agrobot. 2014, 42, 440–445. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Redox sensing and signalling associated with reactive oxygen in chloroplasts, peroxisomes and mitochondria. Physiol. Plant 2003, 119, 355–364. [Google Scholar] [CrossRef]
- Alscher, R.G.; Erturk, N.; Heath, L.S. Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J. Exp. Bot. 2002, 53, 1331–1341. [Google Scholar] [CrossRef] [PubMed]
- Bowler, C.; Montagu, M.V.; Inze, D. Superoxide dismutase and stress tolerance. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1992, 43, 83–116. [Google Scholar] [CrossRef]
- Asada, K. The water–water cycle in chloroplasts: Scavenging of active oxygens and dissipation of excess photons. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 601–639. [Google Scholar] [CrossRef] [PubMed]
- Damanik, R.I.; Maziah, M.; Ismail, M.R.; Ahmad, S.; Zain, A.M. Responses of the antioxidative enzymes in Malaysian rice (Oryza sativa L.) cultivars under submergence condition. Acta Physiol. Plant 2010, 32, 739–747. [Google Scholar] [CrossRef]
- Anandan, A.; Arunachalam, P. Relative proportion of antioxidative enzyme activity in locally grown Indian rice cultivars (Oryza sativa L.) under submergence condition. J. Plant Interact. 2012, 7, 183–192. [Google Scholar] [CrossRef]
- Cheynier, V. Phenolic compounds: From plants to foods. Phytochem. Rev. 2012, 11, 153–177. [Google Scholar] [CrossRef]
- Clé, C.; Hill, L.M.; Niggeweg, R.; Martin, C.R.; Guisez, Y.; Prinsen, E.; Jansen, M.A.K. Modulation of chlorogenic acid biosynthesis in Solanum lycopersicum; consequences for phenolic accumulation and UV-tolerance. Phytochemistry 2008, 69, 2149–2156. [Google Scholar] [CrossRef] [PubMed]
- Schmitz-Hoerner, R.; Weissenbock, G. Contribution of phenolic compounds to the UV-B screening capacity of developing barley primary leaves in relation to DNA damage and repair under elevated UV-B levels. Phytochemistry 2003, 64, 243–255. [Google Scholar] [CrossRef]
- Yan, K.; Zhao, S.; Bian, L.; Chen, Z. Saline stress enhanced accumulation of leaf phenolics in honeysuckle (Lonicera japonica Thunb.) without induction of oxidative stress. Plant Physiol. Biochem. 2017, 112, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Minh, L.T.; Khang, D.T.; Ha, P.T.T.; Tuyen, P.T.; Minh, T.N.; Quan, N.V.; Xuan, T.D. Effects of salinity stress on growth and phenolics of rice (Oryza sativa L.). Int. Lett. Nat. Sci. 2016, 57, 1–10. [Google Scholar] [CrossRef]
- Quan, N.T.; Anh, L.H.; Khang, D.T.; Tuyen, P.T.; Toan, N.P.; Minh, T.N.; Xuan, T.D. Involvement of secondary metabolites in response to drought stress of rice (Oryza sativa L.). Agriculture 2016, 6, 23. [Google Scholar] [CrossRef]
- Khang, D.T.; Ha, P.T.T.; Lang, N.T.; Tuyen, P.T.; Minh, L.T.; Minh, T.N.; Xuan, T.D. Involvement of phenolic compounds in anaerobic flooding germination of rice (Oryza sativa L.). Int. Lett. Nat. Sci. 2016, 56, 73–81. [Google Scholar] [CrossRef]
- Gautam, P.; Nayak, A.K.; Lal, B.; Bhattacharyya, P.; Tripathi, R.; Shahid, M.; Mohanty, M.; Raja, R.; Panda, B.B. Submergence tolerance in relation to application time of nitrogen and phosphorus in rice (Oryza sativa L.). Environ. Exp. Bot. 2014, 99, 159–166. [Google Scholar] [CrossRef]
- Metraux, J.P.; Kende, H. The role of ethylene in the growth response of submerged deep water rice. Plant Physiol. 1983, 72, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Ella, E.S.; Ismail, A.M. Seedling nutrient status before submergence affects survival after submergence in rice. Crop Sci. 2006, 46, 1673–1681. [Google Scholar] [CrossRef]
- Ella, E.S.; Kawano, N.; Yamauchi, Y.; Ismail, A.M. Blocking ethylene perception enhances flooding tolerance in rice seedlings. Funct. Plant Biol. 2003, 30, 813–819. [Google Scholar] [CrossRef]
- Ahmed, S.; Nawata, E.; Hosokawa, M.; Domae, Y.; Sakuratani, T. Alterations in photosynthesis and some antioxidant enzymatic activities of mungbean subjected to waterlogging. Plant Sci. 2002, 163, 117–123. [Google Scholar] [CrossRef]
- Voesenek, L.A.C.J.; Colmer, T.D.; Pierik, R.; Millenaar, F.F.; Peeters, A.J.M. How plants cope with complete submergence. New Phytol. 2006, 170, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Hattori, Y.; Nagai, K.; Ashikari, M. Rice growth adapting to deepwater. Curr. Opin. Plant Biol. 2011, 14, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, W. Aeration in higher plants. Adv. Bot. Res. 1979, 7, 225–332. [Google Scholar]
- Sarkar, R.K.; Das, S.; Ravi, I. Changes in certain antioxidative enzymes and growth parameters as a result of complete submergence and subsequent re-aeration of rice cultivars differing in submergence tolerance. J. Agron. Crop Sci. 2001, 187, 69–74. [Google Scholar] [CrossRef]
- Sarkar, R.K.; Reddy, J.N.; Sharma, S.G.; Ismail, A.M. Physiological basis of submergence tolerance in rice and implications for crop improvement. Curr. Sci. 2006, 91, 899–906. [Google Scholar]
- Wu, Y.S.; Yang, C.Y. Physiological responses and expression profile of NADPH oxidase in rice (Oryza sativa) seedlings under different levels of submergence. Rice 2016, 9, 2. [Google Scholar] [CrossRef] [PubMed]
- Belkhadi, A.; Hediji, H.; Abbes, Z.; Nouairi, I.; Barhoumi, Z.; Zarrouk, M.; Djebali, W. Effects of exogenous salicylic acid pre-treatment on cadmium toxicity and leaf lipid content in Linum usitatissimum L. Ecotoxicol. Environ. Saf. 2010, 73, 1004–1011. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Bouwkamp, J.C.; Solomos, T. Chlorophyllase activities and chlorophyll degradation during leaf senescence in non-yellowing mutant and wild type of Phaseolus vulgaris L. J. Exp. Bot. 1998, 49, 503–510. [Google Scholar]
- Yang, C.M.; Chang, I.F.; Lin, S.J.; Chou, C.H. Effects of three allelopathic phenolics on chlorophyll accumulation of rice (Oryza sativa) seedlings: II. Stimulation of consumption-orientation. Bot. Bull Acad. Sin. 2004, 45, 119–125. [Google Scholar]
- Davey, M.W.; Stals, E.; Panis, B.; Keulemans, J.; Swennen, R.L. High-throughput determination of malondialdehyde in plant tissues. Anal. Biochem. 2005, 347, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Sang, W.G.; Ma, K.P. Differential responses of the activities of antioxidant enzymes to thermal stresses between two invasive Eupatorium species in China. J. Integr. Plant Biol. 2008, 50, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Michalak, A. Phenolic compounds and their antioxidant activity in plants growing under heavy metal stress. Pol. J. Environ. Stud. 2006, 15, 523–530. [Google Scholar]
- Milić, B.L.; Djilas, S.M.; Čanadanović-Brunet, J.M. Antioxidative activity of phenolic compounds on the metal-ion breakdown of lipid peroxidation system. Food Chem. 1998, 61, 443–447. [Google Scholar] [CrossRef]
- Chou, T.H.; Ding, H.Y.; Hung, W.J.; Liang, C.H. Antioxidative characteristics and inhibition of α-melanocyte-stimulating hormone-stimulated melanogenesis of vanillin and vanillic acid from Origanum vulgare. Exp. Dermatol. 2010, 19, 742–750. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Chen, S.; Mi, Y.; Zhou, Z.; Ahammed, G.J. Effects of hypoxia stress and different level of Mn2+ on antioxidant enzyme of tomato seedlings. Am. J. Plant Sci. 2010, 1, 24–31. [Google Scholar] [CrossRef]
- Cavalcanti, F.R.; Oliveira, J.T.A.; Martins-Miranda, A.S.; Viégas, R.A.; Silveira, J.A.G. Superoxide dismutase, catalase and peroxidase activities do not confer protection against oxidative damage in salt-stressed cowpea leaves. New Phytol. 2004, 163, 563–571. [Google Scholar] [CrossRef]
- Michiels, C.; Raes, M.; Toussaint, O.; Remacle, J. Importance of Se-glutathione peroxidase, catalase, and Cu/Zn-SOD for cell survival against oxidative stress. Free Radic. Biol. Med. 1994, 17, 235–248. [Google Scholar] [CrossRef]
- Espinosa-Diez, C.; Miguel, V.; Mennerich, D.; Kietzmann, T.; Sánchez-Pérez, P.; Cadenas, S.; Lamas, S. Antioxidant responses and cellular adjustments to oxidative stress. Redox Biol. 2015, 6, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Setter, T.L.; Laureles, E.V. The beneficial effect of reduced elongation growth on submergence tolerance of rice. J. Exp. Bot. 1996, 47, 1551–1559. [Google Scholar] [CrossRef]
- Elanchezhian, R.; Haris, A.A.; Kumar, S.; Singh, S.S. Positive impact of paclobutrazol on gas exchange, chlorophyll fluorescence and yield parameters under submergence stress in rice. Indian J. Plant Physiol. 2015, 20, 111–115. [Google Scholar] [CrossRef]
- Xing, J.; Wu, H.; Zhang, Y.; Zhang, Y.; Wang, Y.; Li, Z.; Lin, H.; Chen, H.; Zhang, J.; Zhu, D. Transcriptomic analysis of gibberellin- and paclobutrazol-treated rice seedlings under submergence. Int. J. Mol. Sci. 2017, 18, 2225. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.H.; Tsou, C.C.; Hwang, S.Y.; Chen, L.F.; Lo, H.F. Paclobutrazol lead to enhanced antioxidative protection of sweet potato under flooding stress. Bot. Stud. 2008, 49, 9–18. [Google Scholar]
- Bailey-Serres, J.; Voesenek, L.A.C.J. Flooding stress: Acclimations and genetic diversity. Annu. Rev. Plant Biol. 2008, 59, 313–339. [Google Scholar] [CrossRef] [PubMed]
- Bailey-Serres, J.; Fukao, T.; Ronald, P.; Ismail, A.; Heuer, S.; Mackill, D. Submergence tolerant rice: SUB1′s journey from landrace to modern cultivar. Rice 2010, 3, 138–147. [Google Scholar] [CrossRef]
- Anandan, A.; Rajiv, G.; Ramarao, A.; Prakash, M. Internode elongation pattern and differential response of rice genotypes to varying levels of flood water. Funct. Plant Biol. 2012, 39, 137–145. [Google Scholar] [CrossRef]
- Catling, D. Rice in Deep Water; International Rice Research Institute: Manila, Philippines, 1992; 542p. [Google Scholar]
- IRRI (International Rice Research Institute). Standard Evaluation System for Rice, 3rd ed.; International Rice Research Institute: Los Banos, Philippines, 2002; 56p, Available online: http://www.knowledgebank.irri.org/images/docs/rice-standard-evaluation-system.pdf) (accessed on 15 December 2017).
- Demiral, T.; Türkan, İ. Comparative lipid peroxidation, antioxidant defense systems and proline content in roots of two rice cultivars differing in salt tolerance. Environ. Exp. Bot. 2005, 53, 247–257. [Google Scholar] [CrossRef]
- Quan, N.V.; Khang, D.T.; Dep, L.T.; Minh, T.N.; Nobukazu, N.; Xuan, T.D. The potential use of a food-dyeing plant Peristrophe bivalvis (L.) Merr. in Northern Vietnam. Int. J. Pharmacol. Phytochem. Ethnomed. 2016, 4, 14–26. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Herzog, V.; Fahimi, H. Determination of the activity of peroxidase. Anal. Biochem. 1973, 55, 554–562. [Google Scholar] [CrossRef]
- Foyer, C.H.; Halliwell, B. The presence of glutathione and glutathione reductase in chloroplasts: A proposed role in ascorbic acid metabolism. Planta 1976, 133, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]

| Treatments | Shoot Height(cm) | Survival Percentage | Scale |
|---|---|---|---|
| Control | 5.19 ± 0.08 c (0.0) | 75.56 ± 6.46 d | 5 |
| PA 0.01 | 4.56 ± 0.38 c (12.1) | 83.33 ± 4.35 c | 5 |
| PA 0.10 | 5.63 ± 0.38 b (−8.48) | 95.56 ± 3.51 b | 3 |
| PA 1.00 | 6.27 ± 0.28 ab (−20.8) | 100.00 ± 0.00 a | 1 |
| VA 0.01 | 4.45 ± 0.36 c (14.26) | 85.19 ± 5.67 c | 5 |
| VA 0.10 | 5.46 ± 0.17 b (−5.20) | 96.30 ± 2.65 b | 3 |
| VA 1.00 | 6.37 ± 1.41 ab (−22.4) | 96.67 ± 1.33 b | 3 |
| M 0.01 | 6.01 ± 0.61 ab (−15.7) | 97.78 ± 0.23 b | 3 |
| M 0.10 | 6.16 ± 1.03 ab (−18.69) | 100.00 ± 0.00 a | 1 |
| M 1.00 | 6.33 ± 2.96 a (−21.97) | 100.00 ± 0.00 a | 1 |
| Phenolics | Flavonoids | MDA | Chla | Chlb | CAT | SOD | APX | POD | GR | |
|---|---|---|---|---|---|---|---|---|---|---|
| Phenolics | 1 | |||||||||
| Flavonoids | 0.579 * | 1 | ||||||||
| MDA | −0.158 | 0.309 | 1 | |||||||
| Chla | 0.579 * | 0.640 * | 0.030 | 1 | ||||||
| Chlb | 0.521 | 0.797 ** | 0.364 | 0.832 ** | 1 | |||||
| CAT | 0.228 | 0.216 | −0.565 | −0.120 | −0.187 | 1 | ||||
| SOD | 0.818 ** | 0.793 ** | −0.188 | 0.644 * | 0.566 | 0.413 | 1 | |||
| APX | 0.865 ** | 0.291 | −0.542 | 0.322 | 0.178 | 0.484 | 0.721 ** | 1 | ||
| POD | −0.090 | −0.219 | 0.680 * | −0.100 | 0.044 | −0.773 ** | −0.398 | −0.274 | 1 | |
| GR | 0.364 | 0.208 | −0.630 * | 0.336 | 0.135 | 0.470 | 0.564 | 0.510 | −0.702 * | 1 |
| Genes | Primer Sequences |
|---|---|
| SOD | Forward: GGCTTGCATACAAACCTGAA Reverse: CTGACTGCTTCCCATGACACCAT |
| CAT | Forward: GTCGATTGGTGTTGAACAGG Reverse: AGGACGACAAGGATCAAACC |
| APX | Forward: GACTCTTGGAGCCCATTAGG Reverse: AGGGTGAAAGGGAACATCAG |
| POD | Forward: TTAGGGAGCAGTTTCCCACT Reverse: AGGGTGAAAGGGAACATCAG |
| GR | Forward: TTGGTGGAACGTGTGTTCTT Reverse: TCTCATTCACTTCCCATCCA |
| Actin | Forward: TGGTCGGAATGGGACAGAAG Reverse: CTCAGTCAGGAGAACAGGGT |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xuan, T.D.; Khang, D.T. Effects of Exogenous Application of Protocatechuic Acid and Vanillic Acid to Chlorophylls, Phenolics and Antioxidant Enzymes of Rice (Oryza sativa L.) in Submergence. Molecules 2018, 23, 620. https://doi.org/10.3390/molecules23030620
Xuan TD, Khang DT. Effects of Exogenous Application of Protocatechuic Acid and Vanillic Acid to Chlorophylls, Phenolics and Antioxidant Enzymes of Rice (Oryza sativa L.) in Submergence. Molecules. 2018; 23(3):620. https://doi.org/10.3390/molecules23030620
Chicago/Turabian StyleXuan, Tran Dang, and Do Tan Khang. 2018. "Effects of Exogenous Application of Protocatechuic Acid and Vanillic Acid to Chlorophylls, Phenolics and Antioxidant Enzymes of Rice (Oryza sativa L.) in Submergence" Molecules 23, no. 3: 620. https://doi.org/10.3390/molecules23030620






