AIEgen-Based Fluorescent Nanomaterials: Fabrication and Biological Applications
Abstract
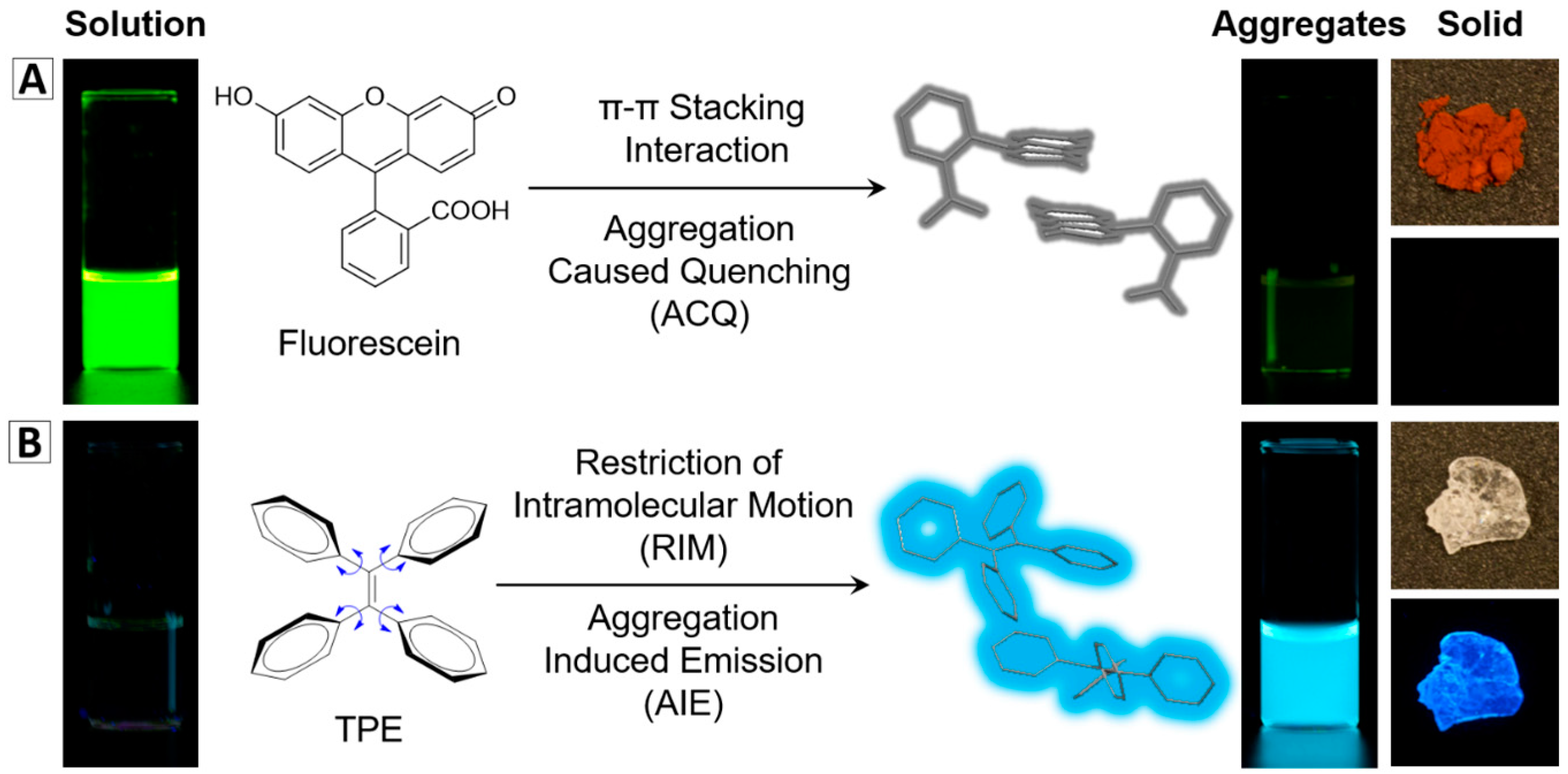
:1. Introduction
2. Synthesis and Properties of AIEgen-Based Nanomaterials
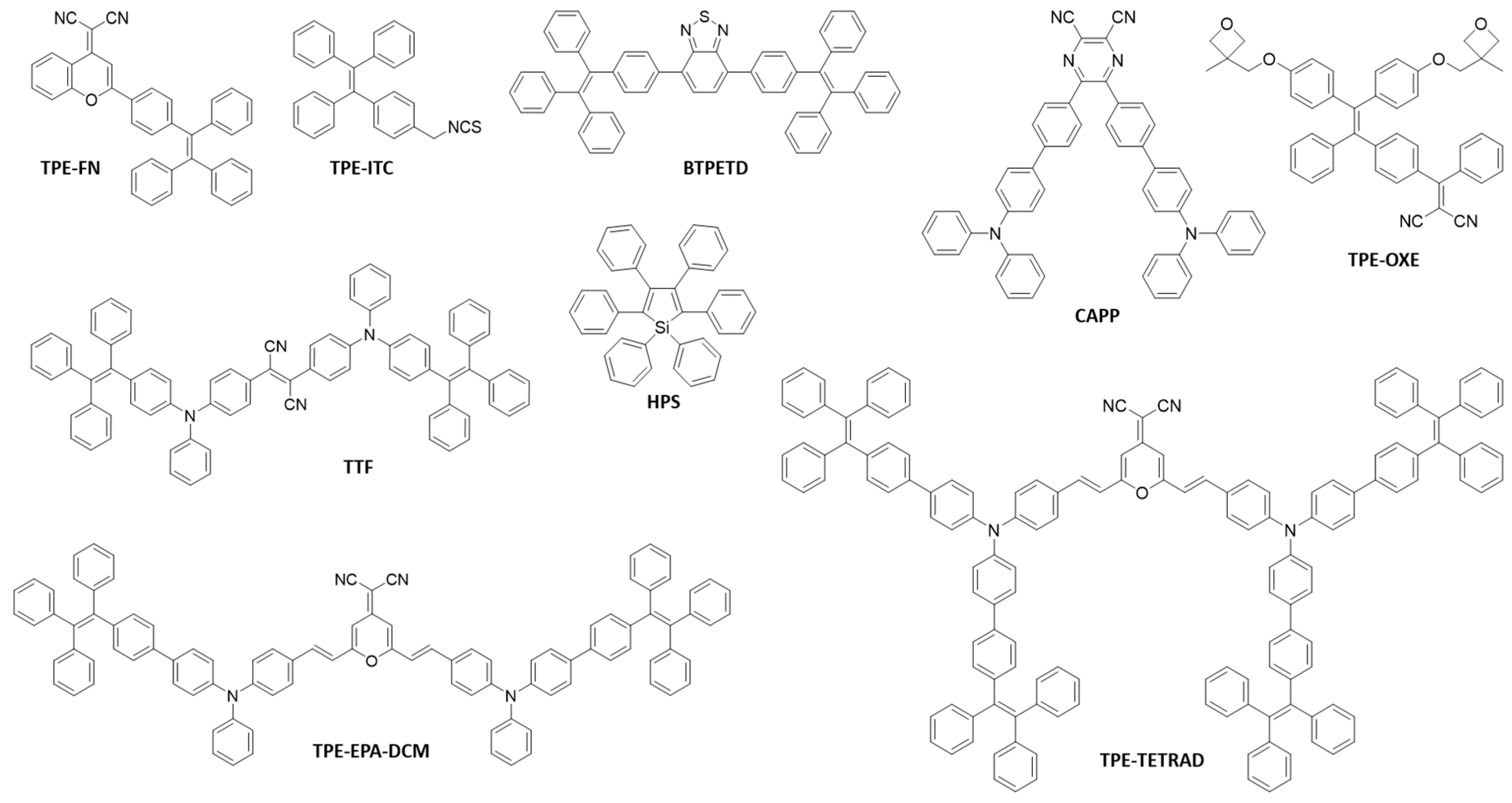
2.1. Examples of AIEgens Used for Fluorescent Nanomaterials Fabrication
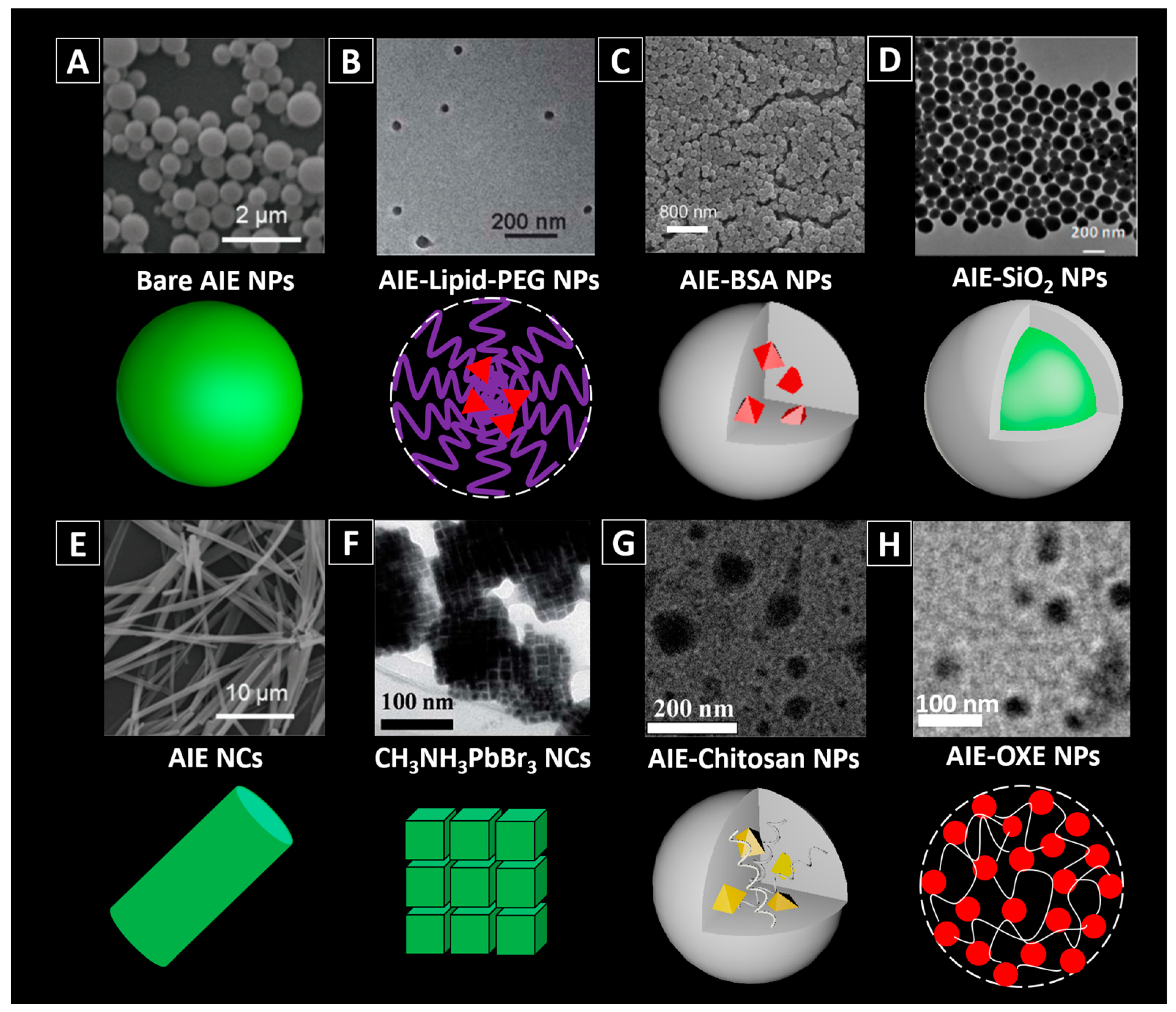
2.2. Fabrication of AIEgen-Based Nanomaterials
2.2.1. Non-Covalent Binding
Amorphous AIE NPs
AIE Nanocrystals
2.2.2. Covalent Binding
2.3. Functionalization of the AIEgen-Based Nanomaterials
3. Biological Applications
3.1. Bioimaging—In Vitro and In Vivo
3.1.1. Cell Imaging
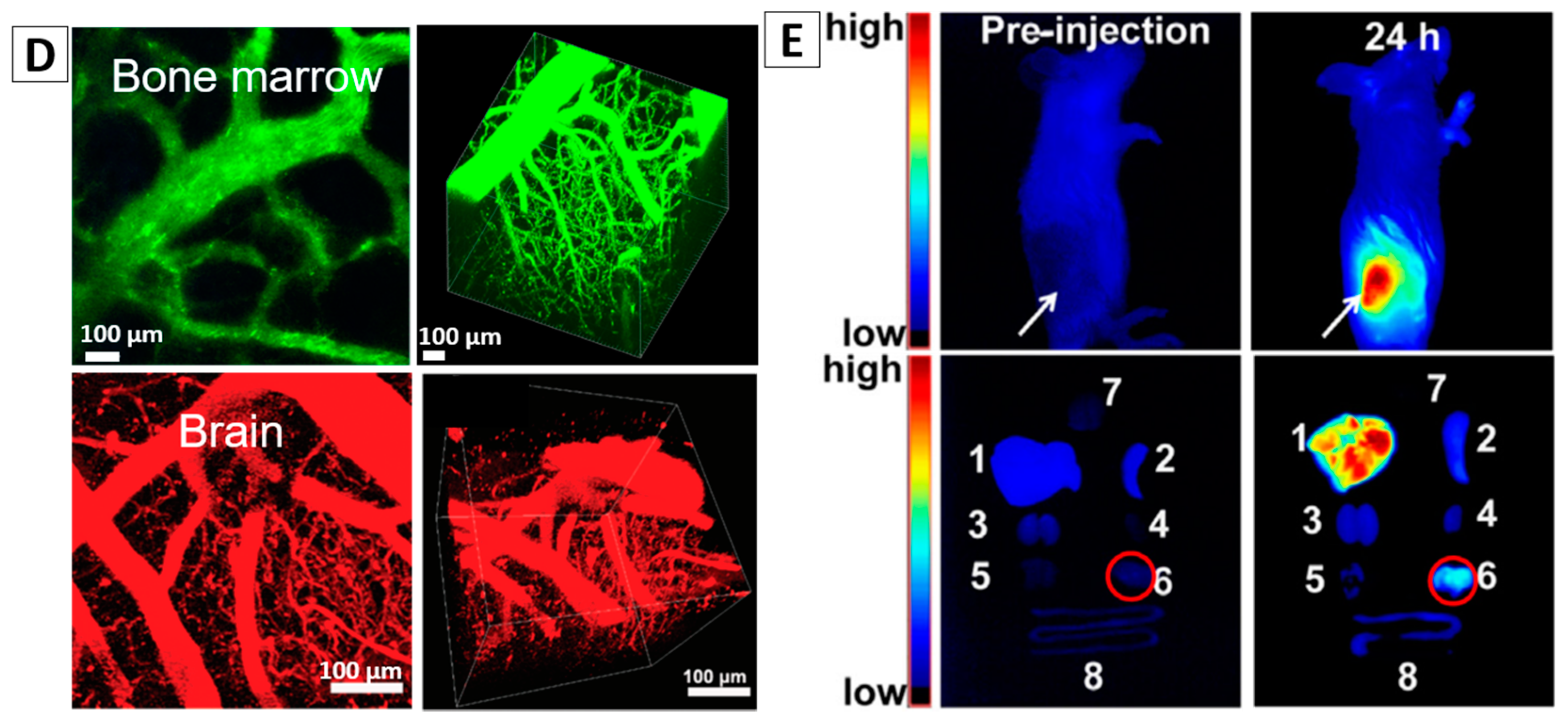
3.1.2. Vascular Imaging
3.1.3. Tumor Imaging
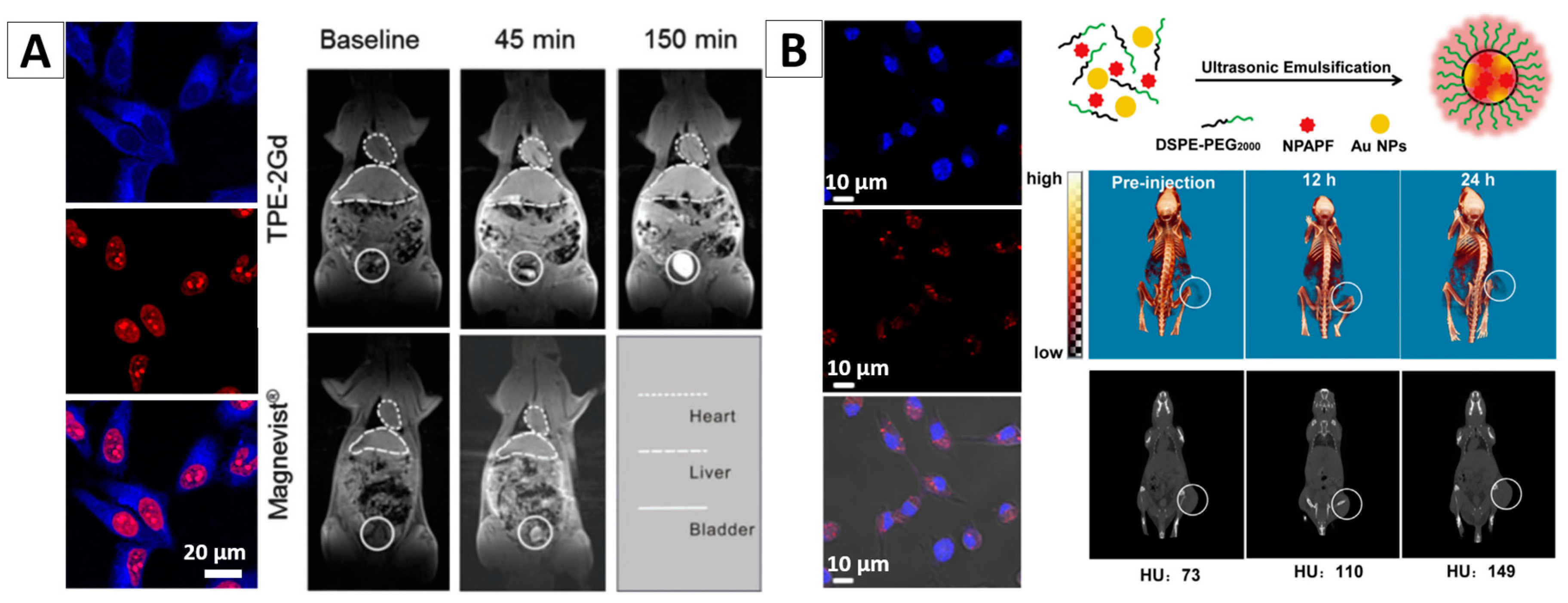
3.1.4. Dual-Modality Imaging
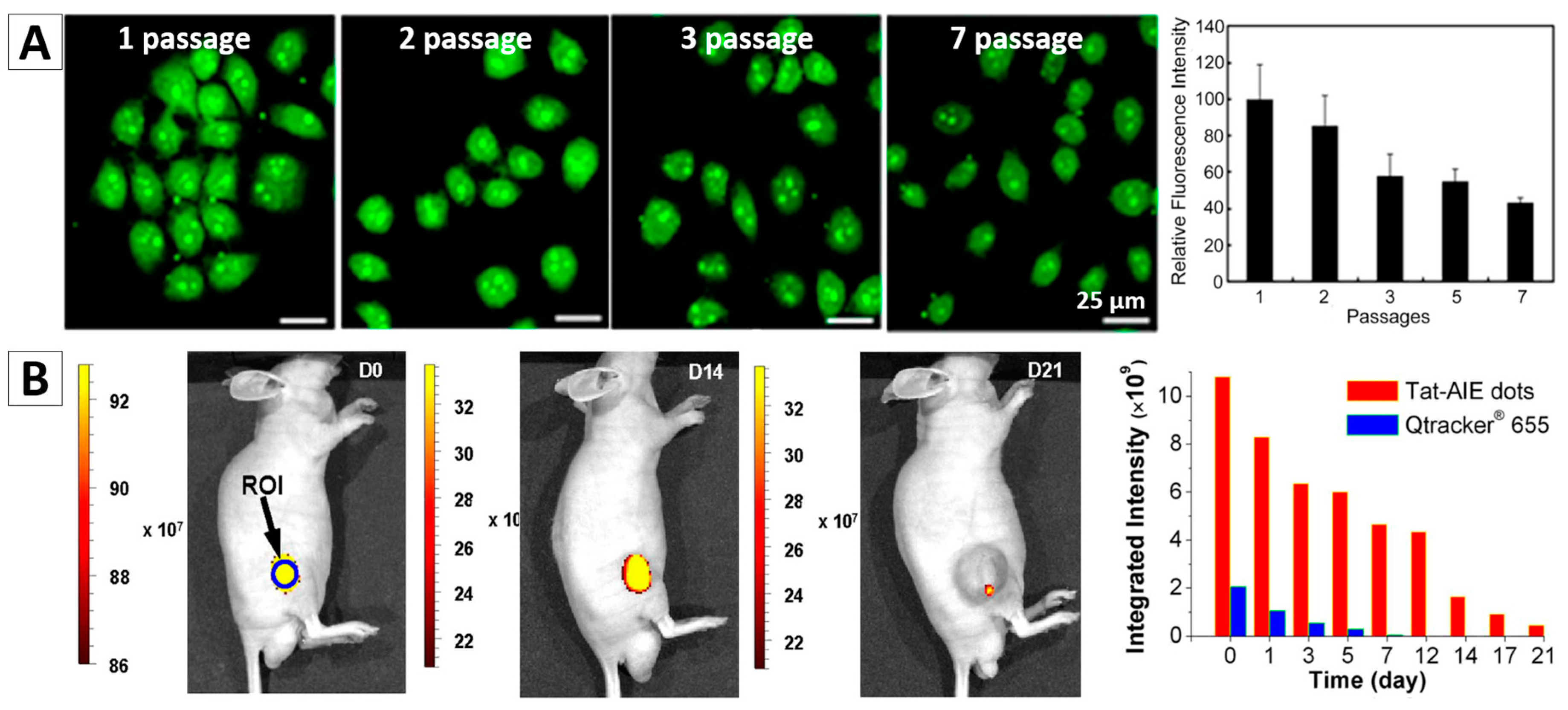
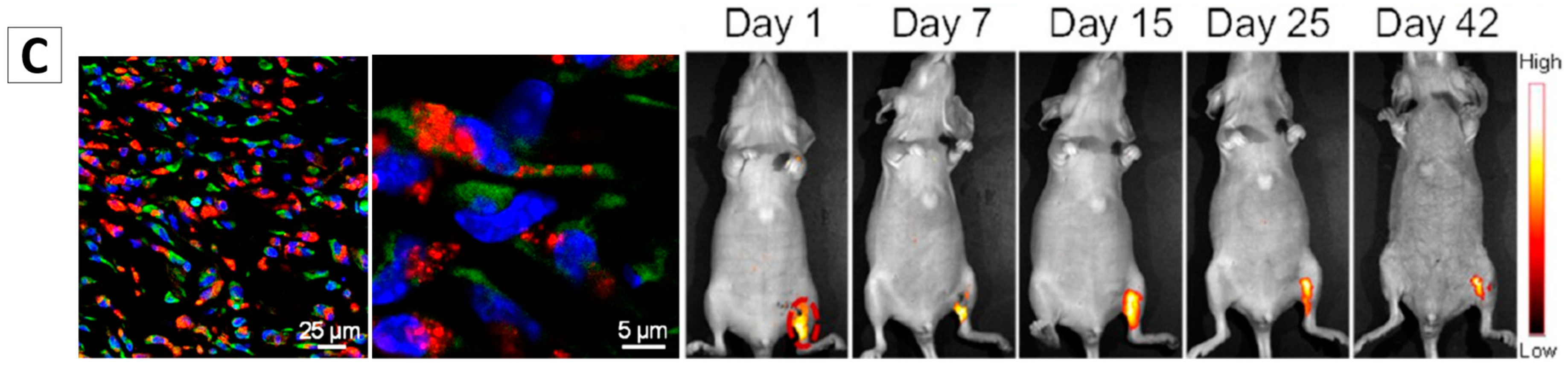
3.2. Cell Tracing
3.3. Theranosis
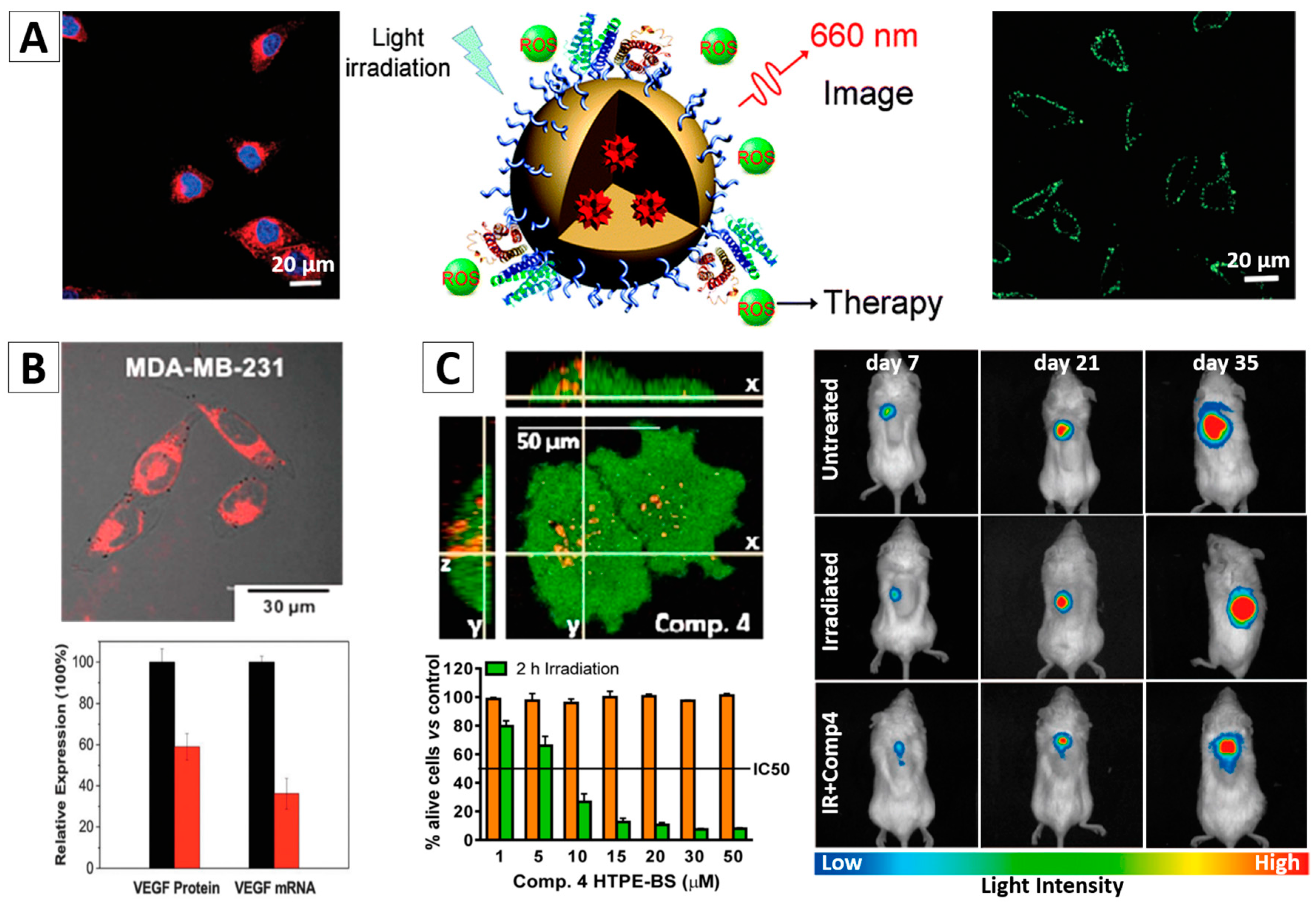
3.3.1. Photodynamic Therapy (PDT)
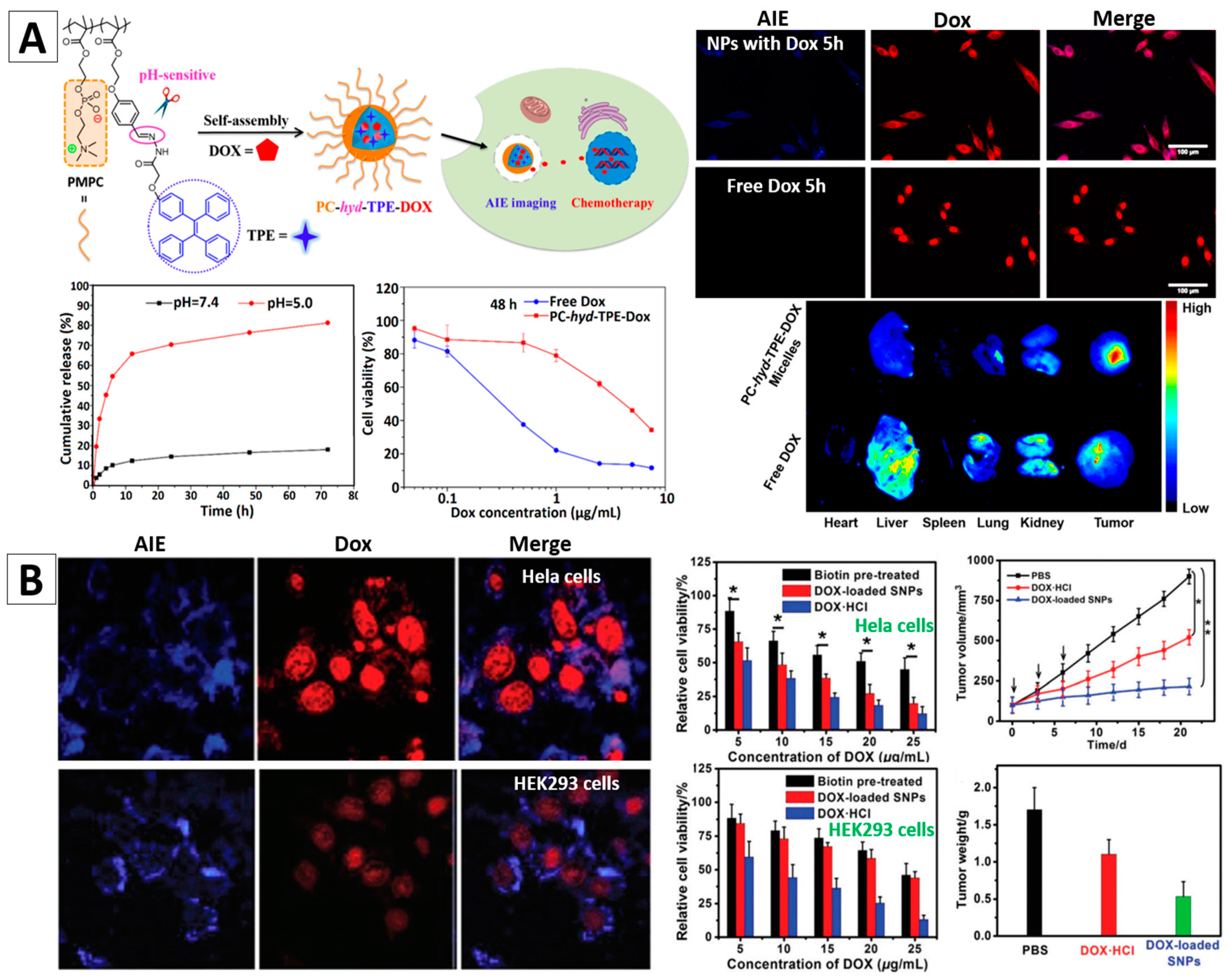
3.3.2. Drug Delivery
4. Conclusions and Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Liu, B. Aggregation-induced emission: Materials and biomedical applications. MRS Bull. 2017, 42, 458–463. [Google Scholar] [CrossRef]
- Chen, S.; Wang, H.; Hong, Y.; Tang, B.Z. Fabrication of fluorescent nanoparticles based on AIE luminogens (AIE dots) and their applications in bioimaging. Mater. Horiz. 2016, 3, 283–293. [Google Scholar] [CrossRef]
- Algarra, M.; Perez-Martin, M.; Cifuentes-Rueda, M.; Jimenez-Jimenez, J.; da Silva, J.C.G.E.; Bandosz, T.J.; Rodriguez-Castellon, E.; Navarrete, J.T.L.; Casado, J. Carbon dots obtained using hydrothermal treatment of formaldehyde. Cell imaging in vitro. Nanoscale 2014, 6, 9071–9077. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.S.; Chai, L.J.; Huang, Y.Y.; Tang, C.; Shen, J.J.; Chen, J.R.; Feng, H. A real-time fluorescent assay for the detection of alkaline phosphatase activity based on carbon quantum dots. Biosens. Bioelectron. 2015, 68, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Xie, Z.; Lam, J.W.Y.; Cheng, L.; Tang, B.Z.; Chen, H.; Qiu, C.; Kwok, H.S.; Zhan, X.; Liu, Y.; et al. Aggregation-induced emission of 1-methyl-1,2,3,4,5-pentaphenylsilole. Chem. Commun. 2001, 18, 1740–1741. [Google Scholar] [CrossRef]
- Mei, J.; Hong, Y.; Lam, J.W.Y.; Qin, A.; Tang, Y.; Tang, B.Z. Aggregation-Induced Emission: The Whole Is More Brilliant than the Parts. Adv. Mater. 2014, 26, 5429–5479. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.Y.; Zhang, X.Q.; Yang, B.; Deng, F.J.; Li, Z.; Wei, J.C.; Zhang, X.Y.; Wei, Y. Water dispersible, non-cytotoxic, cross-linked luminescent AIE dots: Facile preparation and bioimaging applications. Appl. Surf. Sci. 2014, 322, 155–161. [Google Scholar] [CrossRef]
- Leung, N.L.; Xie, N.; Yuan, W.; Liu, Y.; Wu, Q.; Peng, Q.; Miao, Q.; Lam, J.W.; Tang, B.Z. Restriction of intramolecular motions: The general mechanism behind aggregation-induced emission. Chemistry 2014, 20, 15349–15353. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.E.; Yao, Q.C.; Xia, M. How is the AIE mechanism profoundly changed in an ESIPT family: The novel introduction of a tetraphenylethene group onto (Z)-3-(quinolin-2-ylmethylene)-3,4-dihydroquinoxalin-2(1H)-one. Phys. Chem. Chem. Phys. 2015, 17, 3287–3294. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.; Wu, W.; Xu, S.; Liu, B. Far Red/Near-Infrared AIE Dots for Image-Guided Photodynamic Cancer Cell Ablation. ACS Appl. Mater. Interfaces 2016, 8, 21193–21200. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Chen, S.S.; Zhu, D.D.; Lu, X.M.; Lu, Q.H. Preparation of aggregation-induced emission dots for long-term two-photon cell imaging. J. Mater. Chem. B 2015, 3, 3091–3097. [Google Scholar] [CrossRef]
- Fang, X.; Chen, X.; Li, R.; Liu, Z.; Chen, H.; Sun, Z.; Ju, B.; Liu, Y.; Zhang, S.X.; Ding, D.; et al. Multicolor Photo-Crosslinkable AIEgens toward Compact Nanodots for Subcellular Imaging and STED Nanoscopy. Small 2017, 13, 1702128. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Feng, G.; He, B.; Goh, C.; Xu, S.; Ramos-Ortiz, G.; Aparicio-Ixta, L.; Zhou, J.; Ng, L.; Zhao, Z.; et al. Silole-Based Red Fluorescent Organic Dots for Bright Two-Photon Fluorescence In vitro Cell and In vivo Blood Vessel Imaging. Small 2016, 12, 782–792. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Tang, B.Z.; Liu, B. Quantum dot-sized organic fluorescent dots for long-term cell tracing. In Proceedings of the Medical Imaging 2014: Biomedical Applications in Molecular, Structural, and Functional Imaging, San Diego, CA, USA, 15–20 February 2014; Volume 9038. [Google Scholar]
- Feng, G.; Tay, C.Y.; Chui, Q.X.; Liu, R.; Tomczak, N.; Liu, J.; Tang, B.Z.; Leong, D.T.; Liu, B. Ultrabright organic dots with aggregation-induced emission characteristics for cell tracking. Biomaterials 2014, 35, 8669–8677. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Zhu, Z.; Cai, P.; Liu, R.; Tomczak, N.; Ding, D.; Liu, J.; Qin, W.; Zhao, Z.; Hu, Y.; et al. Organic Dots with Aggregation-Induced Emission (AIE Dots) Characteristics for Dual-Color Cell Tracing. Chem. Mater. 2013, 25, 4181–4187. [Google Scholar] [CrossRef]
- Zhang, C.; Jin, S.; Li, S.; Xue, X.; Liu, J.; Huang, Y.; Jiang, Y.; Chen, W.Q.; Zou, G.; Liang, X.J. Imaging intracellular anticancer drug delivery by self-assembly micelles with aggregation-induced emission (AIE micelles). ACS Appl. Mater. Interfaces 2014, 6, 5212–5220. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Zhao, Y.; Dai, L.; Zhang, X.; Hao, X.; Zhang, C.; Huo, S.; Liu, J.; Liu, C.; Kumar, A.; et al. Spatiotemporal drug release visualized through a drug delivery system with tunable aggregation-induced emission. Adv. Mater. 2014, 26, 712–717. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.Z. AIE dots for bioimaging, diagnosis and therapy. Nanomedicine 2016, 12, 462. [Google Scholar] [CrossRef]
- Qin, W.; Ding, D.; Liu, J.; Yuan, W.Z.; Hu, Y.; Liu, B.; Tang, B.Z. Biocompatible Nanoparticles with Aggregation-Induced Emission Characteristics as Far-Red/Near-Infrared Fluorescent Bioprobes for In vitro and In vivo Imaging Applications. Adv. Funct. Mater. 2012, 22, 771–779. [Google Scholar] [CrossRef]
- Zhao, Z.J.; Lu, P.; Lam, J.W.Y.; Wang, Z.M.; Chan, C.Y.K.; Sung, H.H.Y.; Williams, I.D.; Ma, Y.G.; Tang, B.Z. Molecular anchors in the solid state: Restriction of intramolecular rotation boosts emission efficiency of luminogen aggregates to unity. Chem. Sci. 2011, 2, 672–675. [Google Scholar] [CrossRef]
- Yu, G.; Yin, S.; Liu, Y.; Chen, J.; Xu, X.; Sun, X.; Ma, D.; Zhan, X.; Peng, Q.; Shuai, Z.; et al. Structures, Electronic States, Photoluminescence, and Carrier Transport Properties of 1,1-Disubstituted 2,3,4,5-Tetraphenylsiloles. J. Am. Chem. Soc. 2005, 127, 6335–6346. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.P.; Haeussler, M.; Tang, B.Z.; Dong, Y.; Sin, K.; Mar, W.; Trau, D.; Seydack, M.; Renneberg, R. Silole nanocrystals as novel biolabels. J. Immunol. Methods 2004, 295, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Li, K.; Chen, S.; Qin, A.; Ding, D.; Zhang, S.; Liu, Y.; Liu, B.; Sun, J.Z.; Tang, B.Z. Aggregation-induced red-NIR emission organic nanoparticles as effective and photostable fluorescent probes for bioimaging. J. Mater. Chem. 2012, 22, 15128–15135. [Google Scholar] [CrossRef]
- Wang, D.; Qian, J.; Qin, W.; Qin, A.; Tang, B.Z.; He, S. Biocompatible and photostable AIE dots with red emission for in vivo two-photon bioimaging. Sci. Rep. 2014, 4, 4279. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.J.; Chen, S.M.; Lam, J.W.Y.; Jim, C.K.W.; Chan, C.Y.K.; Wang, Z.M.; Lu, P.; Deng, C.M.; Kwok, H.S.; Ma, Y.G.; et al. Steric Hindrance, Electronic Communication, and Energy Transfer in the Photo- and Electroluminescence Processes of Aggregation-Induced Emission Luminogens. J. Phys. Chem. C 2010, 114, 7963–7972. [Google Scholar] [CrossRef]
- Zhao, Z.; Deng, C.; Chen, S.; Lam, J.W.; Qin, W.; Lu, P.; Wang, Z.; Kwok, H.S.; Ma, Y.; Qiu, H.; et al. Full emission color tuning in luminogens constructed from tetraphenylethene, benzo-2,1,3-thiadiazole and thiophene building blocks. Chem. Commun. 2011, 47, 8847–8849. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Feng, C.; Hong, Y.; Liu, J.; Chen, S.; Ng, K.M.; Luo, K.Q.; Tang, B.Z. Cytophilic fluorescent bioprobes for long-term cell tracking. Adv. Mater. 2011, 23, 3298–3302. [Google Scholar] [CrossRef] [PubMed]
- Fateminia, S.M.; Wang, Z.; Goh, C.C.; Manghnani, P.N.; Wu, W.; Mao, D.; Ng, L.G.; Zhao, Z.; Tang, B.Z.; Liu, B. Nanocrystallization: A Unique Approach to Yield Bright Organic Nanocrystals for Biological Applications. Adv. Mater. 2017, 29, 1604100. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Qin, W.; Ding, D.; Tomczak, N.; Geng, J.; Liu, R.; Liu, J.; Zhang, X.; Liu, H.; Liu, B.; et al. Photostable fluorescent organic dots with aggregation-induced emission (AIE dots) for noninvasive long-term cell tracing. Sci. Rep. 2013, 3, 1150. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Li, K.; Feng, G.X.; Li, M.; Yang, Z.Y.; Liu, B.; Tang, B.Z. Bright and Photostable Organic Fluorescent Dots with Aggregation-Induced Emission Characteristics for Noninvasive Long-Term Cell Imaging. Adv. Funct. Mater. 2014, 24, 635–643. [Google Scholar] [CrossRef]
- Geng, J.; Li, K.; Ding, D.; Zhang, X.; Qin, W.; Liu, J.; Tang, B.Z.; Liu, B. Lipid-PEG-folate encapsulated nanoparticles with aggregation induced emission characteristics: Cellular uptake mechanism and two-photon fluorescence imaging. Small 2012, 8, 3655–3663. [Google Scholar] [CrossRef] [PubMed]
- Yadav, R.; Kumar, D.; Kumari, A.; Yadav, S.K. Encapsulation of Catechin and Epicatechin on Bsa Nps Improved Their Stability and Antioxidant Potential. EXCLI J. 2014, 13, 331–346. [Google Scholar] [PubMed]
- Liu, L.; Zhang, F.; Xu, B.; Tian, W. Silica nanoparticles based on an AIE-active molecule for ratiometric detection of RNS in vitro. J. Mater. Chem. B 2017, 5, 9197–9203. [Google Scholar] [CrossRef]
- Li, D.; Qin, W.; Xu, B.; Qian, J.; Tang, B.Z. AIE Nanoparticles with High Stimulated Emission Depletion Efficiency and Photobleaching Resistance for Long-Term Super-Resolution Bioimaging. Adv. Mater. 2017, 29, 1703643. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Song, P.; Peng, L.; Tong, A.; Xiang, Y. Aggregation-Induced Emission Luminogen-Embedded Silica Nanoparticles Containing DNA Aptamers for Targeted Cell Imaging. ACS Appl. Mater. Interfaces 2016, 8, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhang, J.; Song, M.; Yin, J.; Yu, G.A.; Liu, S.H. A novel fluorene-based aggregation-induced emission (AIE)-active gold(i) complex with crystallization-induced emission enhancement (CIEE) and reversible mechanochromism characteristics. Chem. Commun. 2015, 51, 326–329. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhou, T.; Liu, G.; Shi, J.; Zhong, H.; Dong, Y. Tetraphenylethylene derivative capped CH3NH3PbBr3 nanocrystals: AIE-activated assembly into superstructures. Faraday Discuss. 2017, 196, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Wan, Q.; Liu, M.; Xu, D.; Mao, L.; Tian, J.; Huang, H.; Gao, P.; Deng, F.; Zhang, X.; Wei, Y. Fabrication of aggregation induced emission active luminescent chitosan nanoparticles via a “one-pot” multicomponent reaction. Carbohydr. Polym. 2016, 152, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Liu, D.; Mahtab, F.; Xin, L.; Shen, Z.; Yu, Y.; Chan, C.Y.; Lu, P.; Lam, J.W.; Sung, H.H.; et al. Synthesis, structure, aggregation-induced emission, self-assembly, and electron mobility of 2,5-bis(triphenylsilylethynyl)-3,4-diphenylsiloles. Chemistry 2011, 17, 5998–6008. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, S.; Lam, J.W.; Qin, W.; Kwok, R.T.; Xie, N.; Hu, Q.; Tang, B.Z. Long-term fluorescent cellular tracing by the aggregates of AIE bioconjugates. J. Am. Chem. Soc. 2013, 135, 8238–8245. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Zhang, X.Y.; Zhang, X.Q.; Huang, Z.F.; Wei, Y.; Tao, L. Fabrication of aggregation-induced emission based fluorescent nanoparticles and their biological imaging application: Recent progress and perspectives. Mater. Today 2016, 19, 284–291. [Google Scholar] [CrossRef]
- Ma, C.; Xie, G.; Zhang, X.; Yang, L.; Li, Y.; Liu, H.; Wang, K.; Wei, Y. Ring-opening crosslinking PEGylation of an AIE epoxy monomer towards biocompatible fluorescent nanoparticles. J. Mater. Chem. B 2016, 4, 8009–8015. [Google Scholar] [CrossRef]
- Li, M.; Hong, Y.; Wang, Z.; Chen, S.; Gao, M.; Kwok, R.T.; Qin, W.; Lam, J.W.; Zheng, Q.; Tang, B.Z. Fabrication of chitosan nanoparticles with aggregation-induced emission characteristics and their applications in long-term live cell imaging. Macromol. Rapid Commun. 2013, 34, 767–771. [Google Scholar] [CrossRef] [PubMed]
- Faisal, M.; Hong, Y.; Liu, J.; Yu, Y.; Lam, J.W.; Qin, A.; Lu, P.; Tang, B.Z. Fabrication of fluorescent silica nanoparticles hybridized with AIE luminogens and exploration of their applications as nanobiosensors in intracellular imaging. Chemistry 2010, 16, 4266–4272. [Google Scholar] [CrossRef] [PubMed]
- Cheon, J.; Lee, J.H. Synergistically integrated nanoparticles as multimodal probes for nanobiotechnology. Acc. Chem. Res. 2008, 41, 1630–1640. [Google Scholar] [CrossRef] [PubMed]
- Nicol, A.; Kwok, R.T.K.; Chen, C.; Zhao, W.; Chen, M.; Qu, J.; Tang, B.Z. Ultrafast Delivery of Aggregation-Induced Emission Nanoparticles and Pure Organic Phosphorescent Nanocrystals by Saponin Encapsulation. J. Am. Chem. Soc. 2017, 139, 14792–14799. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Ding, D.; Prashant, C.; Qin, W.; Yang, C.-T.; Tang, B.Z.; Liu, B. Gadolinium-Functionalized Aggregation-Induced Emission Dots as Dual-Modality Probes for Cancer Metastasis Study. Adv. Healthc. Mater. 2013, 2, 1600–1605. [Google Scholar] [CrossRef] [PubMed]
- Mahtab, F.; Yu, Y.; Lam, J.W.Y.; Liu, J.; Zhang, B.; Lu, P.; Zhang, X.; Tang, B.Z. Fabrication of Silica Nanoparticles with Both Efficient Fluorescence and Strong Magnetization and Exploration of Their Biological Applications. Adv. Funct. Mater. 2011, 21, 1733–1740. [Google Scholar] [CrossRef]
- Yu, C.J.; Wu, S.M.; Tseng, W.L. Magnetite nanoparticle-induced fluorescence quenching of adenosine triphosphate-BODIPY Conjugates: Application to adenosine triphosphate and pyrophosphate sensing. Anal. Chem. 2013, 85, 8559–8565. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xu, B.; Zhang, L.; Zhang, J.; Ma, T.; Zhang, J.; Fu, X.; Tian, W. Folic acid-functionalized mesoporous silica nanospheres hybridized with AIE luminogens for targeted cancer cell imaging. Nanoscale 2013, 5, 2065–2072. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, X.; Wang, S.; Liu, M.; Tao, L.; Wei, Y. Surfactant modification of aggregation-induced emission material as biocompatible nanoparticles: Facile preparation and cell imaging. Nanoscale 2013, 5, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Sharma, A.; Qi, J.; Peng, X.; Lee, D.Y.; Hu, R.; Lin, D.; Qu, J.; Kim, J.S. Super-resolution fluorescent materials: An insight into design and bioimaging applications. Chem. Soc. Rev. 2016, 45, 4651–4667. [Google Scholar] [CrossRef] [PubMed]
- Leung, C.W.; Hong, Y.; Chen, S.; Zhao, E.; Lam, J.W.; Tang, B.Z. A photostable AIE luminogen for specific mitochondrial imaging and tracking. J. Am. Chem. Soc. 2013, 135, 62–65. [Google Scholar] [CrossRef] [PubMed]
- Matela, G.; Gao, P.; Guigas, G.; Eckert, A.F.; Nienhaus, K.; Nienhaus, G.U. A far-red emitting fluorescent marker protein, mGarnet2, for microscopy and STED nanoscopy. Chem. Commun. 2017, 53, 979–982. [Google Scholar] [CrossRef] [PubMed]
- Hanne, J.; Falk, H.J.; Gorlitz, F.; Hoyer, P.; Engelhardt, J.; Sahl, S.J.; Hell, S.W. STED nanoscopy with fluorescent quantum dots. Nat. Commun. 2015, 6, 7127. [Google Scholar] [CrossRef] [PubMed]
- Hong, G.; Lee, J.C.; Robinson, J.T.; Raaz, U.; Xie, L.; Huang, N.F.; Cooke, J.P.; Dai, H. Multifunctional in vivo vascular imaging using near-infrared II fluorescence. Nat. Med. 2012, 18, 1841–1846. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Knopp, M.V. Quantifying tumor vascular heterogeneity with dynamic contrast-enhanced magnetic resonance imaging: A review. J. Biomed. Biotechnol. 2011, 2011, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Annapragada, A. Advances in nanoparticle imaging technology for vascular pathologies. Annu. Rev. Med. 2015, 66, 177–193. [Google Scholar] [CrossRef] [PubMed]
- Oheim, M.; Michael, D.J.; Geisbauer, M.; Madsen, D.; Chow, R.H. Principles of two-photon excitation fluorescence microscopy and other nonlinear imaging approaches. Adv. Drug. Deliv. Rev. 2006, 58, 788–808. [Google Scholar] [CrossRef] [PubMed]
- So, P.T.; Dong, C.Y.; Masters, B.R.; Berland, K.M. Two-photon excitation fluorescence microscopy. Annu. Rev. Biomed. Eng. 2000, 2, 399–429. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.; Cai, X.; Lou, X.; Feng, G.; Min, X.; Luo, W.; He, B.; Goh, C.C.; Ng, L.G.; Zhou, J.; et al. Biocompatible Green and Red Fluorescent Organic Dots with Remarkably Large Two-Photon Action Cross Sections for Targeted Cellular Imaging and Real-Time Intravital Blood Vascular Visualization. ACS Appl. Mater. Interfaces 2015, 7, 14965–14974. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.; Goh, C.C.; Feng, G.; Zhao, Z.; Liu, J.; Liu, R.; Tomczak, N.; Geng, J.; Tang, B.Z.; Ng, L.G.; et al. Ultrabright organic dots with aggregation-induced emission characteristics for real-time two-photon intravital vasculature imaging. Adv. Mater. 2013, 25, 6083–6088. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Alifu, N.; Jiang, T.; Zhu, Z.; Wang, Y.; Hua, J.; Qian, J. Biocompatible aggregation-induced emission nanoparticles with red emission for in vivo three-photon brain vascular imaging. J. Mater. Chem. B 2017, 5, 2757–2762. [Google Scholar] [CrossRef]
- Zhang, J.M.; Li, C.; Zhang, X.; Huo, S.D.; Jin, S.B.; An, F.F.; Wang, X.D.; Xue, X.; Okeke, C.I.; Duan, G.Y.; et al. In vivo tumor-targeted dual-modal fluorescence/CT imaging using a nanoprobe co-loaded with an aggregation-induced emission dye and gold nanoparticles. Biomaterials 2015, 42, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Bandla, A.; Mao, D.; Feng, G.; Qin, W.; Liao, L.D.; Thakor, N.; Tang, B.Z.; Liu, B. Biocompatible Red Fluorescent Organic Nanoparticles with Tunable Size and Aggregation-Induced Emission for Evaluation of Blood-Brain Barrier Damage. Adv. Mater. 2016, 28, 8760–8765. [Google Scholar] [CrossRef] [PubMed]
- McGuire, S. World Cancer Report 2014. Geneva, Switzerland: World Health Organization, International Agency for Research on Cancer, WHO Press, 2015. Adv. Nutr. 2016, 7, 418–419. [Google Scholar] [CrossRef] [PubMed]
- Fass, L. Imaging and cancer: A review. Mol. Oncol. 2008, 2, 115–152. [Google Scholar] [CrossRef] [PubMed]
- Shao, A.; Xie, Y.; Zhu, S.; Guo, Z.; Zhu, S.; Guo, J.; Shi, P.; James, T.D.; Tian, H.; Zhu, W.-H. Far-Red and Near-IR AIE-Active Fluorescent Organic Nanoprobes with Enhanced Tumor-Targeting Efficacy: Shape-Specific Effects. Angew. Chem. Int. Ed. Engl. 2015, 127, 7383–7388. [Google Scholar] [CrossRef]
- Ding, D.; Li, K.; Qin, W.; Zhan, R.; Hu, Y.; Liu, J.; Tang, B.Z.; Liu, B. Conjugated polymer amplified far-red/near-infrared fluorescence from nanoparticles with aggregation-induced emission characteristics for targeted in vivo imaging. Adv. Healthc. Mater. 2013, 2, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Singhal, A.; Zheng, J.; Wang, C.; Chan, W.C.W. Optimizing the Synthesis of Red- to Near-IR-Emitting CdS-Capped CdTexSe1-xAlloyed Quantum Dots for Biomedical Imaging. Chem. Mater. 2006, 18, 4845–4854. [Google Scholar] [CrossRef]
- Cutler, C.S.; Hennkens, H.M.; Sisay, N.; Huclier-Markai, S.; Jurisson, S.S. Radiometals for combined imaging and therapy. Chem. Rev. 2013, 113, 858–883. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Al Zaki, A.; Hui, J.Z.; Muzykantov, V.R.; Tsourkas, A. Multifunctional nanoparticles: Cost versus benefit of adding targeting and imaging capabilities. Science 2012, 338, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.Y.; Li, J.; Zhu, Z.P.; Liu, Q.; Xue, Q.; Ding, D. In vivo cancer research using aggregation-induced emission organic nanoparticles. Drug Discov. Today 2017, 22, 1412–1420. [Google Scholar] [CrossRef] [PubMed]
- Nam, T.; Park, S.; Lee, S.Y.; Park, K.; Choi, K.; Song, I.C.; Han, M.H.; Leary, J.J.; Yuk, S.A.; Kwon, I.C.; et al. Tumor targeting chitosan nanoparticles for dual-modality optical/MR cancer imaging. Bioconjug. Chem. 2010, 21, 578–582. [Google Scholar] [CrossRef] [PubMed]
- Geng, J.; Liao, L.-D.; Qin, W.; Tang, B.Z.; Thakor, N.; Liu, B. Fluorogens with Aggregation Induced Emission: Ideal Photoacoustic Contrast Reagents Due to Intramolecular Rotation. J. Nanosci. Nanotechnol. 2015, 15, 1864–1868. [Google Scholar] [CrossRef] [PubMed]
- Xing, H.; Bu, W.; Zhang, S.; Zheng, X.; Li, M.; Chen, F.; He, Q.; Zhou, L.; Peng, W.; Hua, Y. Multifunctional nanoprobes for upconversion fluorescence, MR and CT trimodal imaging. Biomaterials 2012, 33, 1079–1089. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, M.; Hong, Y.; Lam, J.W.; Zheng, Q.; Tang, B.Z. Dual-modal MRI contrast agent with aggregation-induced emission characteristic for liver specific imaging with long circulation lifetime. ACS Appl. Mater. Interfaces 2014, 6, 10783–10791. [Google Scholar] [CrossRef] [PubMed]
- Jing, L.; Liang, X.; Deng, Z.; Feng, S.; Li, X.; Huang, M.; Li, C.; Dai, Z. Prussian blue coated gold nanoparticles for simultaneous photoacoustic/CT bimodal imaging and photothermal ablation of cancer. Biomaterials 2014, 35, 5814–5821. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Liu, X.; Liao, L.D.; Bandla, A.; Ling, J.M.; Liu, Y.H.; Thakor, N.; Bazan, G.C.; Liu, B. Encapsulated Conjugated Oligomer Nanoparticles for Real-Time Photoacoustic Sentinel Lymph Node Imaging and Targeted Photothermal Therapy. Small 2016, 12, 4873–4880. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Liu, B. Polymer-encapsulated organic nanoparticles for fluorescence and photoacoustic imaging. Chem. Soc. Rev. 2014, 43, 6570–6597. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.; Liu, B. Multifunctional AIEgens for Future Theranostics. Small 2016, 12, 6528–6535. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Liu, J.; Liew, W.H.; Duan, Y.; Geng, J.; Thakor, N.; Yao, K.; Liao, L.-D.; Liu, B. Organic molecules with propeller structures for efficient photoacoustic imaging and photothermal ablation of cancer cells. Mater. Chem. Front. 2017, 1, 1556–1562. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, X.; Tao, L.; Chi, Z.; Xu, J.; Wei, Y. Aggregation induced emission-based fluorescent nanoparticles: Fabrication methodologies and biomedical applications. J Mater. Chem. B 2014, 2, 4398–4414. [Google Scholar] [CrossRef]
- Schroeder, T. Imaging stem-cell-driven regeneration in mammals. Nature 2008, 453, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Sapelkin, A.V.; Titirici, M.M.; Sukhorukov, G.B. In Situ Synthesis of Fluorescent Carbon Dots/Polyelectrolyte Nanocomposite Microcapsules with Reduced Permeability and Ultrasound Sensitivity. ACS Nano 2016, 10, 9608–9615. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.G.; Liang, J.; Wong, W.L.; Chisholm, V. Green fluorescent protein as a second selectable marker for selection of high producing clones from transfected CHO cells. Gene 2000, 242, 201–207. [Google Scholar] [CrossRef]
- Wang, Z.; Yong, T.Y.; Wan, J.; Li, Z.H.; Zhao, H.; Zhao, Y.; Gan, L.; Yang, X.L.; Xu, H.B.; Zhang, C. Temperature-sensitive fluorescent organic nanoparticles with aggregation-induced emission for long-term cellular tracing. ACS Appl. Mater. Interfaces 2015, 7, 3420–3425. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.; Mao, D.; Li, K.; Wang, X.; Qin, W.; Liu, R.; Chiam, D.S.; Tomczak, N.; Yang, Z.; Tang, B.Z.; et al. Precise and long-term tracking of adipose-derived stem cells and their regenerative capacity via superb bright and stable organic nanodots. ACS Nano 2014, 8, 12620–12631. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Zhang, C.J.; Gao, M.; Zhang, R.; Tang, B.Z.; Liu, B. Specific light-up bioprobe with aggregation-induced emission and activatable photoactivity for the targeted and image-guided photodynamic ablation of cancer cells. Angew. Chem. Int. Ed. Engl. 2015, 54, 1780–1786. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Huang, Y.; Zhang, G.; Zhao, R.; Yang, H.; Zhang, D. Targeted bioimaging and photodynamic therapy of cancer cells with an activatable red fluorescent bioprobe. Anal. Chem. 2014, 86, 7987–7995. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Feng, G.; Qin, W.; Tang, B.Z.; Liu, B. Targeted and image-guided photodynamic cancer therapy based on organic nanoparticles with aggregation-induced emission characteristics. Chem. Commun. 2014, 50, 8757–8760. [Google Scholar] [CrossRef] [PubMed]
- Jayaram, D.T.; Ramos-Romero, S.; Shankar, B.H.; Garrido, C.; Rubio, N.; Sanchez-Cid, L.; Gomez, S.B.; Blanco, J.; Ramaiah, D. In vitro and in vivo Demonstration of Photodynamic Activity and Cytoplasm Imaging through TPE Nanoparticles. ACS Chem. Biol. 2016, 11, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Jin, G.; Feng, G.; Qin, W.; Tang, B.Z.; Liu, B.; Li, K. Multifunctional organic nanoparticles with aggregation-induced emission (AIE) characteristics for targeted photodynamic therapy and RNA interference therapy. Chem. Commun. 2016, 53, 2752–2755. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Han, H.; Tong, H.; Chen, T.; Wang, H.; Ji, J.; Jin, Q. Zwitterionic Phosphorylcholine-TPE Conjugate for pH-Responsive Drug Delivery and AIE Active Imaging. ACS Appl. Mater. Interfaces 2016, 8, 21185–21192. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.C.; Cook, T.R.; Li, Y.; Yan, X.Z.; Wu, D.; Shao, L.; Shen, J.; Tang, G.P.; Huang, F.H.; Chen, X.Y.; et al. Tetraphenylethene-based highly emissive metallacage as a component of theranostic supramolecular nanoparticles. Proc. Natl. Acad. Sci. USA 2016, 113, 13720–13725. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, G.; Dong, S.; Xiong, J.; Du, Z.; Cheng, X. A pH-responsive AIE nanoprobe as a drug delivery system for bioimaging and cancer therapy. J. Mater. Chem. B 2015, 3, 7401–7407. [Google Scholar] [CrossRef]
- Liow, S.S.; Dou, Q.; Kai, D.; Li, Z.; Sugiarto, S.; Yu, C.Y.; Kwok, R.T.; Chen, X.; Wu, Y.L.; Ong, S.T.; et al. Long-Term Real-Time In vivo Drug Release Monitoring with AIE Thermogelling Polymer. Small 2017, 13, 1603404. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.C.; Zhao, R.; Wu, D.; Zhang, F.W.; Shao, L.; Zhou, J.; Yang, J.; Tang, G.P.; Chen, X.Y.; Huang, F.H. Pillar[5]arene-based amphiphilic supramolecular brush copolymers: Fabrication, controllable self-assembly and application in self-imaging targeted drug delivery. Polym. Chem. 2016, 7, 6178–6188. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Zhang, Y.; Xu, B.; Tian, W. Fluorescent nanoparticles based on AIE fluorogens for bioimaging. Nanoscale 2016, 8, 2471–2487. [Google Scholar] [CrossRef] [PubMed]


| AIEgens | λEx (nm) | λEm (nm) | Quantum Yield |
|---|---|---|---|
| TPE [26] | 299 | 462 (solid aggregate) | 49% in film state |
| TPEPy [21] | 350 | 468 (film) | 100% in film state |
| BTPETD [27] | 350 | 539 (film) | 89% in film state |
| HPS [22] | 363 | 497 (film) | 78% in film state |
| TPE-OXE [12] | 365 | 620 (solid powder) | 57% in solid state |
| TPE-EPA-DCM [20] | 480 | 633 (THF/water mixture) | 12% in BSA matrix with a loading ratio of 3.07% TPE-EPA-DCM |
| TTF [28] | 500 | 660 (THF/water mixture) | 52.5% in solid state |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, H.; Zhao, X.; Chen, S. AIEgen-Based Fluorescent Nanomaterials: Fabrication and Biological Applications. Molecules 2018, 23, 419. https://doi.org/10.3390/molecules23020419
Gao H, Zhao X, Chen S. AIEgen-Based Fluorescent Nanomaterials: Fabrication and Biological Applications. Molecules. 2018; 23(2):419. https://doi.org/10.3390/molecules23020419
Chicago/Turabian StyleGao, Hui, Xin Zhao, and Sijie Chen. 2018. "AIEgen-Based Fluorescent Nanomaterials: Fabrication and Biological Applications" Molecules 23, no. 2: 419. https://doi.org/10.3390/molecules23020419





