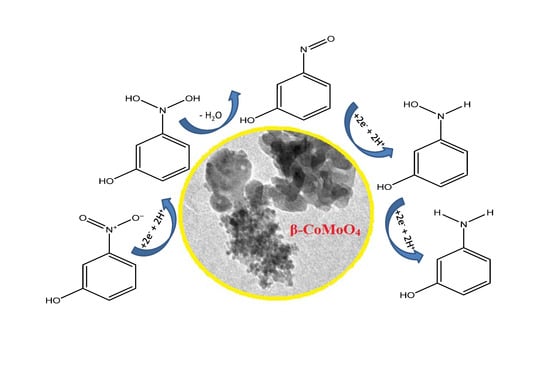
High Catalytic Efficiency of Nanostructured β-CoMoO4 in the Reduction of the Ortho-, Meta- and Para-Nitrophenol Isomers
Abstract
:1. Introduction
2. Material and Methods
2.1. Materials
2.2. Catalyst Preparation
2.3. Characterization
2.4. General Procedure for the Reduction of Nitrophenol Isomers
3. Results and Discussion
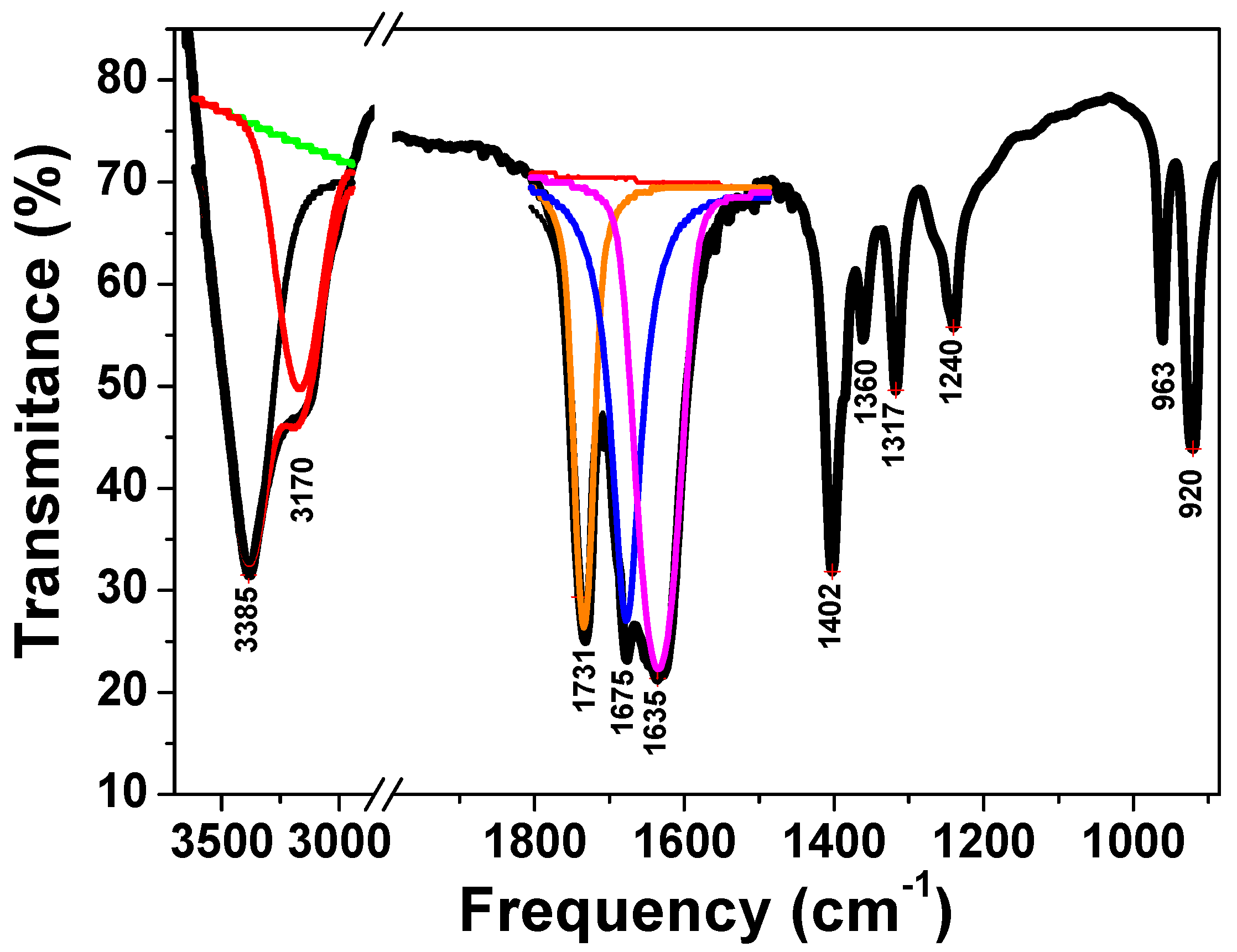
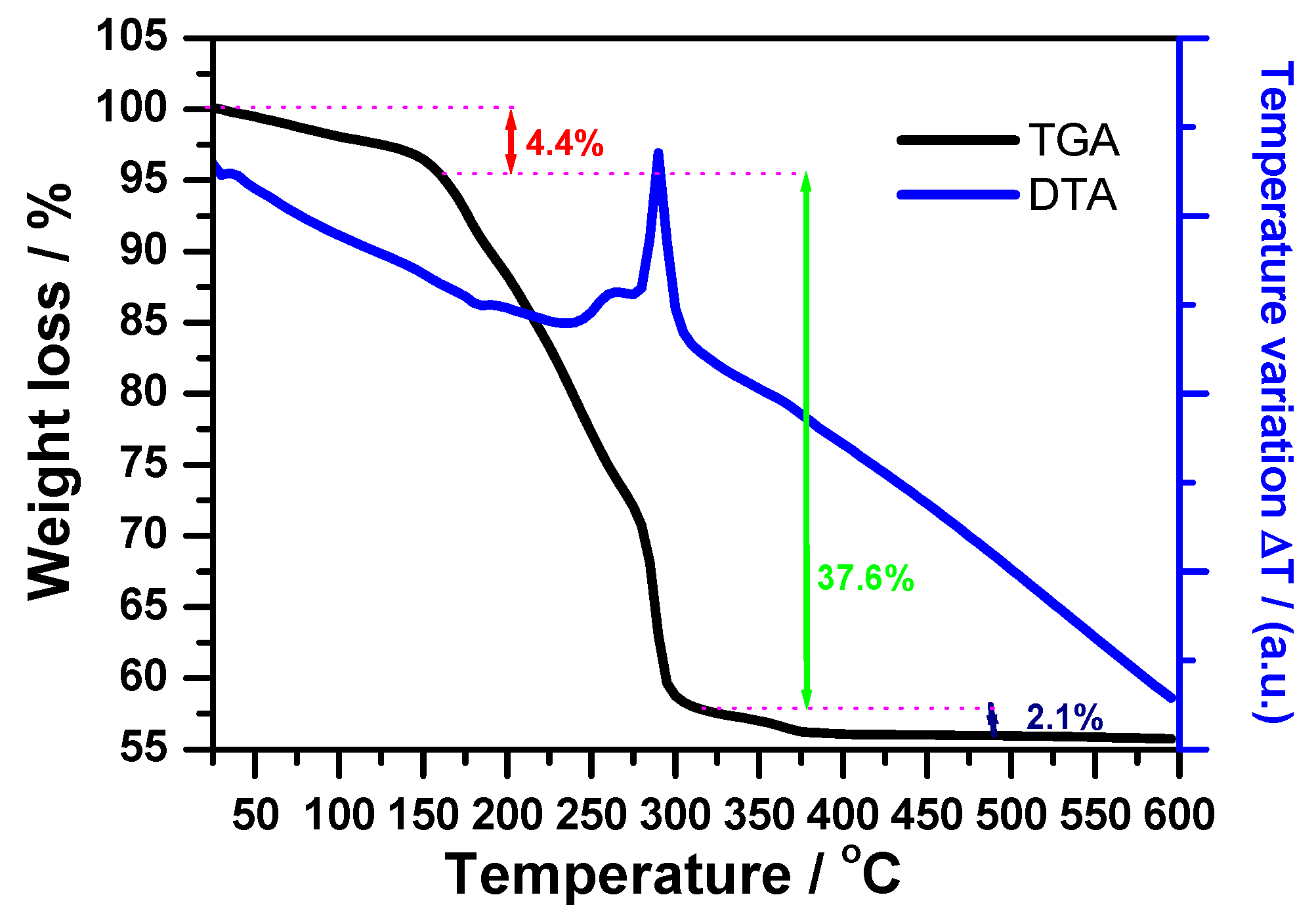
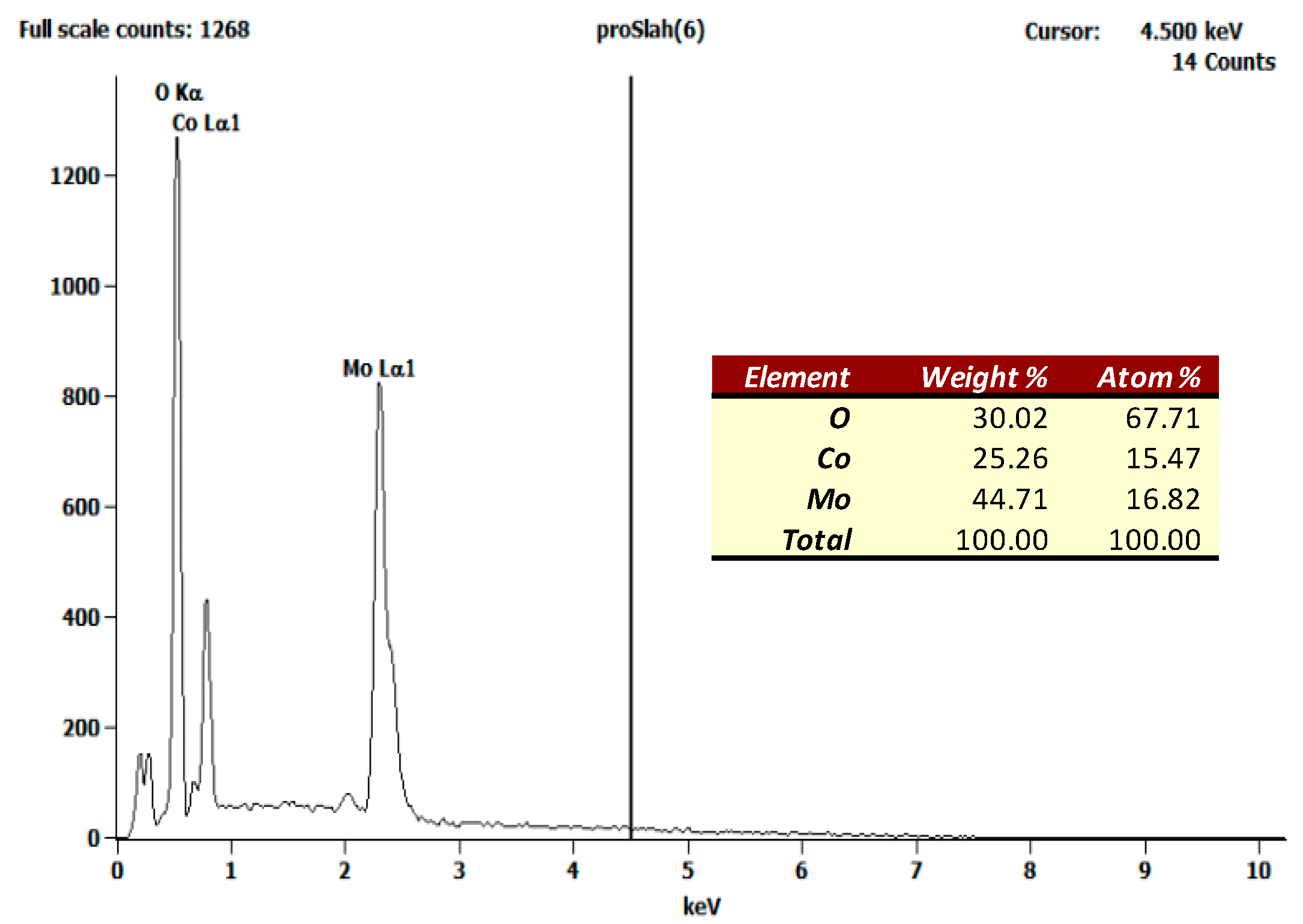
3.1. Complex Precursor Characterizations
3.2. Cobalt Molybdate Characterization
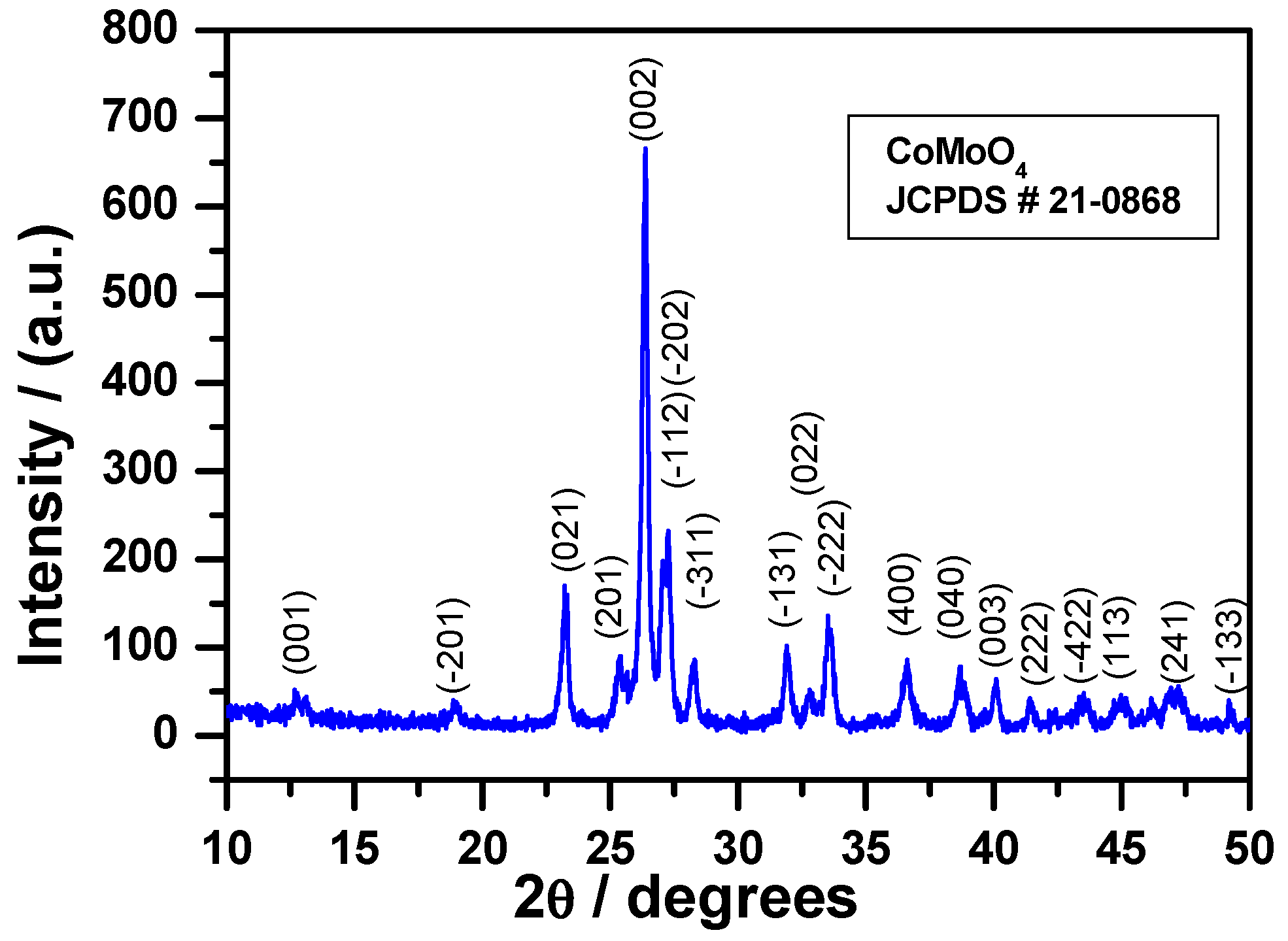
3.2.1. X-ray Diffraction
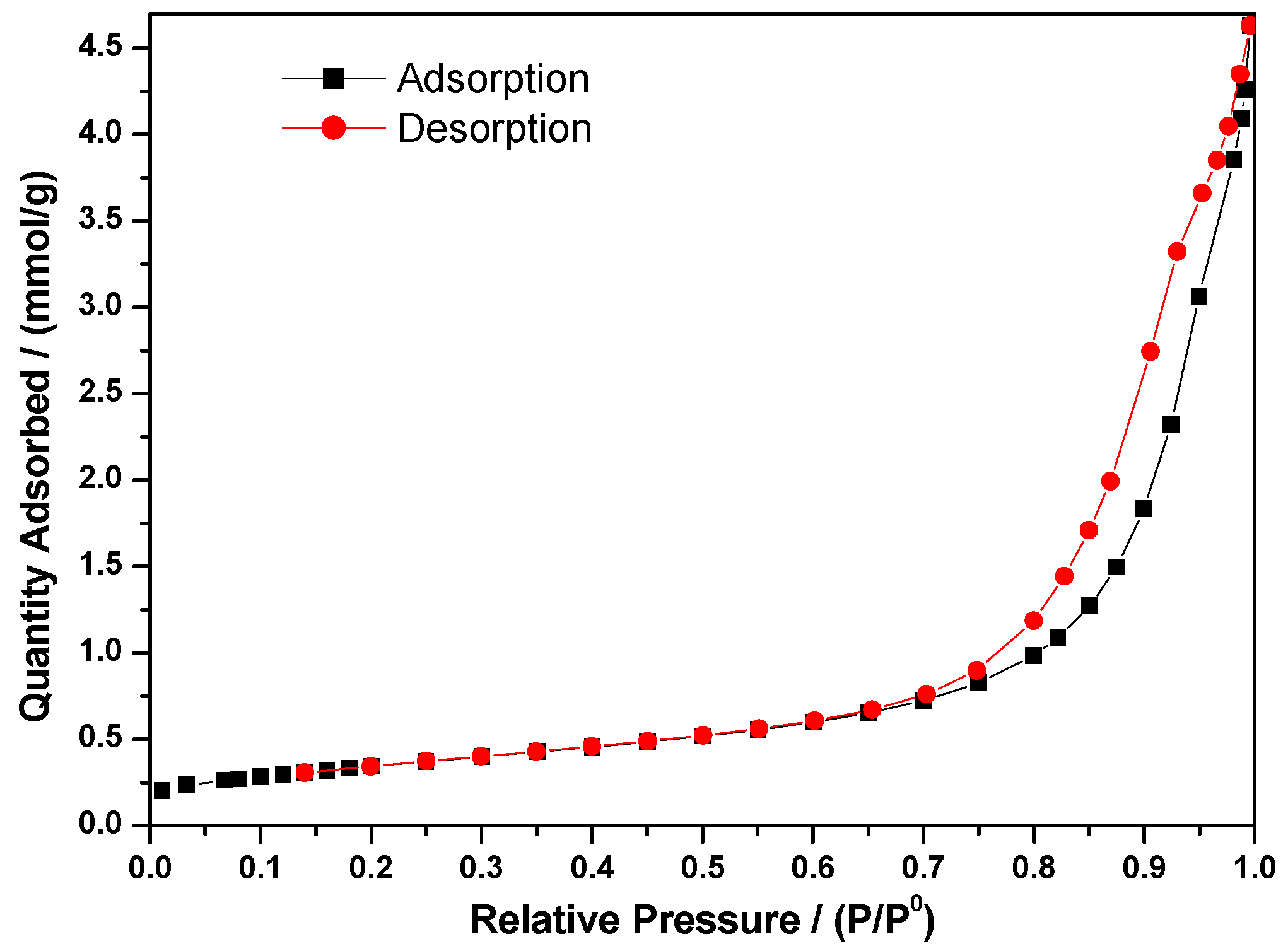
3.2.2. Specific Surface Area Measurement
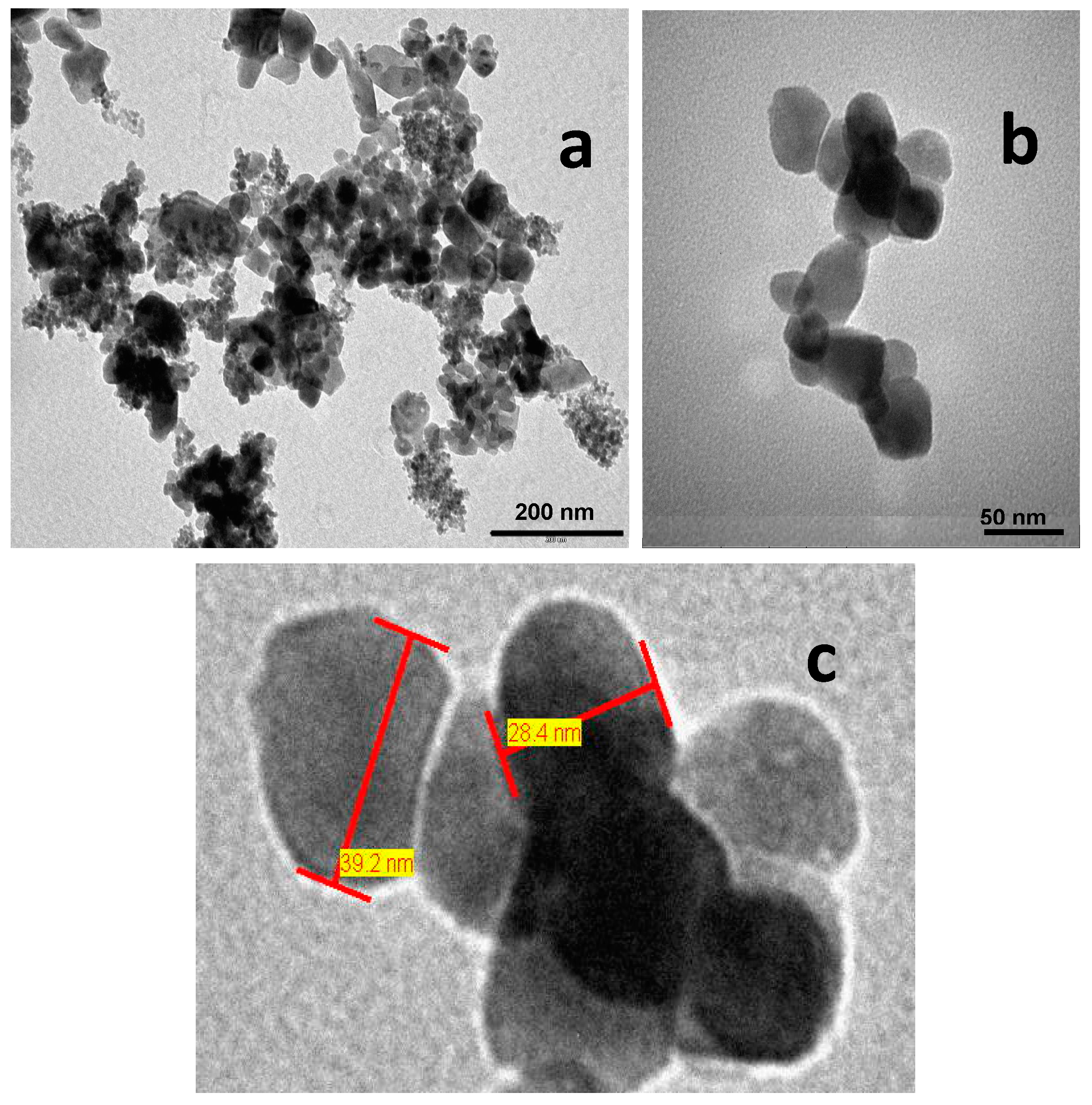
3.2.3. Transmission Electron Microscopy
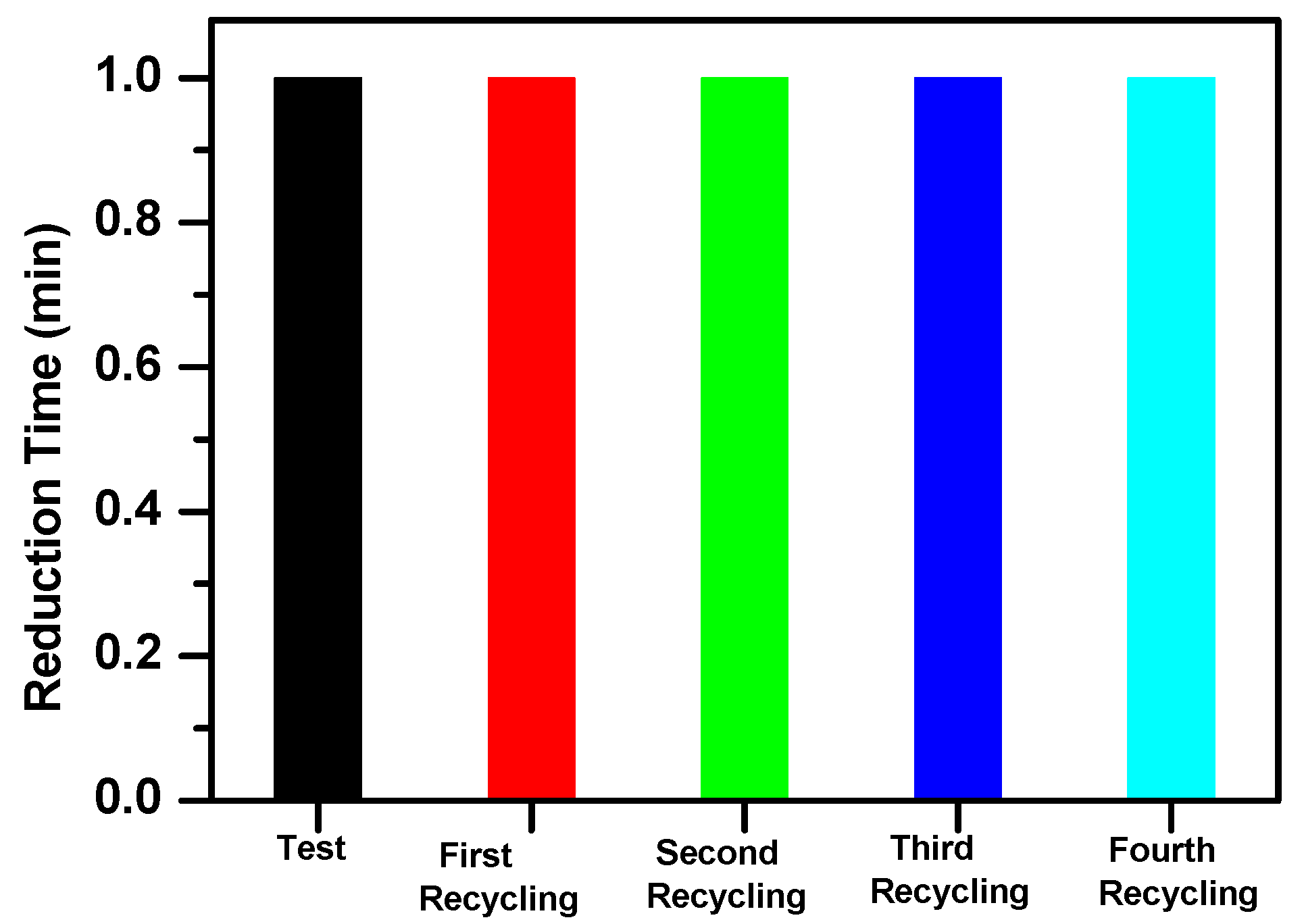
3.3. Reduction of Nitrophenol Isomers
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yang, P.; Xua, A.; Xiab, J.; Hea, J.; Xinga, H.; Zhanga, X.; Weia, S.; Wanga, N. Facile synthesis of highly catalytic activity Ni-Co-Pd-P composite for reduction of the p-Nitrophenol. Appl. Catal. A-Gen. 2014, 470, 89–96. [Google Scholar] [CrossRef]
- Laksmia, B.; Shivanandaa, K.N.; Mahendraa, K.N.; Gowdab, N.M.; Jagadeesha, R.V. An efficient platinum-catalyzed oxidation process and mechanism for the facile conversion of benzoxazoles to aminophenols. Chem. Eng. J. 2010, 163, 403–412. [Google Scholar] [CrossRef]
- Rico, J.L.; Avalos-Borja, M.; Barrera, A.; Hargreaves, J.S.J. Template-free synthesis of CoMoO4 rods and their characterization. Mater. Res. Bull. 2013, 48, 4614–4617. [Google Scholar] [CrossRef]
- Rosić, M.; Zarubica, A.; Šaponjić, A.; Babić, B.; Zagorac, J.; Jordanov, D.; Matović, B. Structural and photocatalytic examination of CoMoO4 nanopowders synthesized by GNP method. Mater. Res. Bull. 2018, 98, 111–120. [Google Scholar] [CrossRef]
- Shaoqing, Y.; Jun, H.; Jianlong, W. Radiation-induced catalytic degradation of p-nitrophenol (PNP) in the presence of TiO2 nanoparticles. Radiat. Phys. Chem. 2010, 79, 1039–1046. [Google Scholar] [CrossRef]
- Rodriguez, J.A.; Hanson, J.C.; Chaturvedi, S.; Brito, J.L. Characterization of oxide catalysts using time-resolved XRD and XANES: Properties of pure and sulfided CoMoO4and NiMoO4. Stud. Surf. Sci. Catal. 2000, 130, 2795–2800. [Google Scholar]
- Zagorac, D.; Schon, J.C.; Rosic, M.; Zagorac, J.; Jordanov, D.; Lukovic, J.; Matovic, B. Theoretical and Experimental Study of Structural Phases in CoMoO4. Cryst. Res. Technol. 2017, 52, 1700069. [Google Scholar] [CrossRef]
- Unnarkat, A.P.; Sridhar, T.; Mahajani, S.M.; Suresh, A.K. Study of Cobalt Molybdenum Oxide Supported on Mesoporous Silica for Liquid Phase Cyclohexane Oxidation. Catal. Today 2017, in press. [Google Scholar] [CrossRef]
- Sen, A.; Pramanik, P. Low-temperature synthesis of nano-sized metal molybdate powders. Mater. Lett. 2001, 10, 287–294. [Google Scholar] [CrossRef]
- Brito, J.L.; Barbosa, A.L. Effect of Phase Composition of the Oxidic Precursor on the HDS Activity of the SulfidedMolybdates of Fe(II), Co(II), and Ni(II). J. Catal. 1997, 171, 467–475. [Google Scholar] [CrossRef]
- Veerasubramani, G.K.; Krishnamoorthy, K.; Radhakrishnan, S.; Kim, N.; Kim, S.J. Synthesis, characterization, and electrochemical properties of CoMoO4 nanostructures. Int. J. Hydrog. Energy 2014, 39, 5186–5193. [Google Scholar] [CrossRef]
- Edrissi, M.; Samadanian-Isfahani, S.; Soleymani, M. Preparation of cobalt molybdate nanoparticles; Taguchi optimization and photocatalytic oxidation of Reactive Black 8 dye. Powder Technol. 2013, 249, 378–385. [Google Scholar] [CrossRef]
- Fan, Y.; Ma, W.; He, J.; Du, Y. CoMoO4 as a novel heterogeneous catalyst of peroxymonosulfate activation for the degradation of organic dyes. RSC Adv. 2017, 7, 36193–36200. [Google Scholar] [CrossRef]
- Koo Kim, J.; Hwa Kim, J.; Chan Kang, Y. Electrochemical Properties of Multicomponent Oxide and Selenide Microspheres Containing Co and Mo Components with Several Tens of Vacant Nanorooms Synthesized by Spray Pyrolysis. Chem. Eng. J. 2018, 333, 665–677. [Google Scholar] [CrossRef]
- Zhao, J.; Ren, X.; Ma, H.; Sun, X.; Zhang, Y.; Yan, T.; Wei, Q.; Wu, D. Synthesis of Self-supported Amorphous CoMoO4 Nanowire Array for Highly Efficient Hydrogen Evolution Reaction. Sustain. Chem. Eng. 2017, 5, 10093–10098. [Google Scholar] [CrossRef]
- Dam, D.T.; Huang, T.; Lee, J.M. Ultra-small and low crystalline CoMoO4 nanorods for electrochemical capacitors. Sustain. Energy Fuels 2017, 1, 324–335. [Google Scholar] [CrossRef]
- Goyal, A.; Bansal, S.; Singhal, S. Facile reduction of nitrophenols: Comparative catalytic efficiency of MFe2O4 (M = Ni, Cu, Zn) nano ferrites. Int. J. Hydrogen Energy 2014, 39, 4895–4908. [Google Scholar] [CrossRef]
- Nandanwar, S.U.; Chakraborty, M. Synthesis of Colloidal CuO/γ-Al2O3 by Microemulsion and Its Catalytic Reduction of Aromatic Nitro Compounds. Chin. J. Catal. 2012, 33, 1532–1541. [Google Scholar] [CrossRef]
- Xia, J.; He, G.; Zhang, L.; Sun, X.; Wang, X. Hydrogenation of nitrophenols catalyzed by carbon black-supported nickel nanoparticles under mild conditions. Appl. Catal. B-Environ. 2016, 180, 408–415. [Google Scholar] [CrossRef]
- Oudghiri-Hassani, H. Synthesis, characterization and catalytic performance of iron molybdate Fe2(MoO4)3 nanoparticles. Catal. Commun. 2015, 60, 19–22. [Google Scholar] [CrossRef]
- Ghorai, T.K.; Dhak, D.; Azizan, A.; Pramanik, P. Investigation of phase formation temperature of nano-sized solid solution of copper/cobalt molybdate and chromium-phosphate (M1xCr 1−x MoxP1−xO4) [M1 = Co, Cu]. Mater. Sci. Eng. B 2005, 121, 216–223. [Google Scholar] [CrossRef]
- Kadiri, N.; Ben Ali, A.; Abboudi, M.; Moran, E. A new synthesis method to produce oxalate complexes: Cases of copper and lanthanum oxalates. Phys. Chem. News 2008, 44, 11–14. [Google Scholar]
- Abboudi, M.; Messali, M.; Kadiri, N.; Ben Ali, A.; Moran, E. Synthesis of CuO, La2O3, and La2CuO4 by the Thermal-Decomposition of Oxalates Precursors Using a New Method. Synth. React. Inorg. Met.-Org. Nano 2011, 41, 683–688. [Google Scholar] [CrossRef]
- Messali, M.; Al Wadaani, F.; Oudghiri-Hassani, H.; Rakass, S.; Al Amri, S.; Benaissa, M.; Abboudi, M. Preparation, characterization and photocatalytic activity of hexagonal ZnO nanoparticles. Mater. Lett. 2014, 128, 187–190. [Google Scholar] [CrossRef]
- Gabal, M.A.; El-Bellihi, A.A.; El-Bahnasawy, H.H. Effect of calcination temperature on Co(II) oxalate dehydrate-iron(II) oxalate dihydrate mixture: DTA–TG, XRD, Mössbauer, FT-IR and SEM studies (Part II). Mater. Chem. Phys. 2003, 81, 84–92. [Google Scholar] [CrossRef]
- Angermann, A.; Topfer, J. Synthesis of nanocrystalline Mn-Zn ferrite powders through thermolysis of mixed oxalates. Ceram. Int. 2011, 37, 995–1002. [Google Scholar] [CrossRef]
- Ouyang, J.M.; Deng, S.P.; Zhou, N.; Tieke, B. Effect of tartrates with various counterions on the precipitation of calcium oxalate in vesicle solutions. Colloids Surf. A 2005, 256, 21–27. [Google Scholar] [CrossRef]
- Ng, K.Y.S.; Zhou, X.; Gulari, E. Spectroscopic characterization of molybdenum oxalate in solution and on alumina. J. Phys. Chem. 1985, 89, 2477–2481. [Google Scholar] [CrossRef]
- Mindru, I.; Gingasu, D.; Marinescu, G.; Patron, L.; Calderon-Moreno, J.M.; Bartha, C.; Andronescu, C.; Crisan, A. Cobalt chromite obtained by thermal decomposition of oxalate coordination compounds. Ceram. Int. 2014, 40, 15249–15258. [Google Scholar] [CrossRef]
- Ramis, G.; Yi, L.; Busca, G. Ammonia activation over catalysts for the selective catalytic reduction of NOx and the selective catalytic oxidation of NH3. An FT-IR study. Catal. Today 1996, 28, 373–380. [Google Scholar] [CrossRef]
- Oudghiri-Hassani, H.; Rakass, S.; Al Wadaani, F.; Al Ghamdi, K.; Omer, A.; Messali, M.; Abboudi, M. Synthesis, characterization and photocatalytic activity of α-Bi2O3 nanoparticles. J. Taibah Univ. Sci. 2015, 9, 508–512. [Google Scholar] [CrossRef]
- Rivenet, M.; Roussel, P.; Abraham, F. One-dimensional inorganic arrangement in the bismuth oxalate hydroxide Bi(C2O4)OH. J. Solid State Chem. 2008, 181, 2586–2590. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting Physisorption Data for Gas/Solid Systems with Special Reference to the Determination of Surface Area and Porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Anbia, M.; MooaviFard, S.E. Improving humidity sensing properties of nanoporous TiO2–10 mol % SnO2 thin film by co-doping with La3+ and K+. Sens. Actuator B-Chem. 2011, 160, 215–221. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds cobalt molybdate (β-CoMoO4) are available from the authors. |
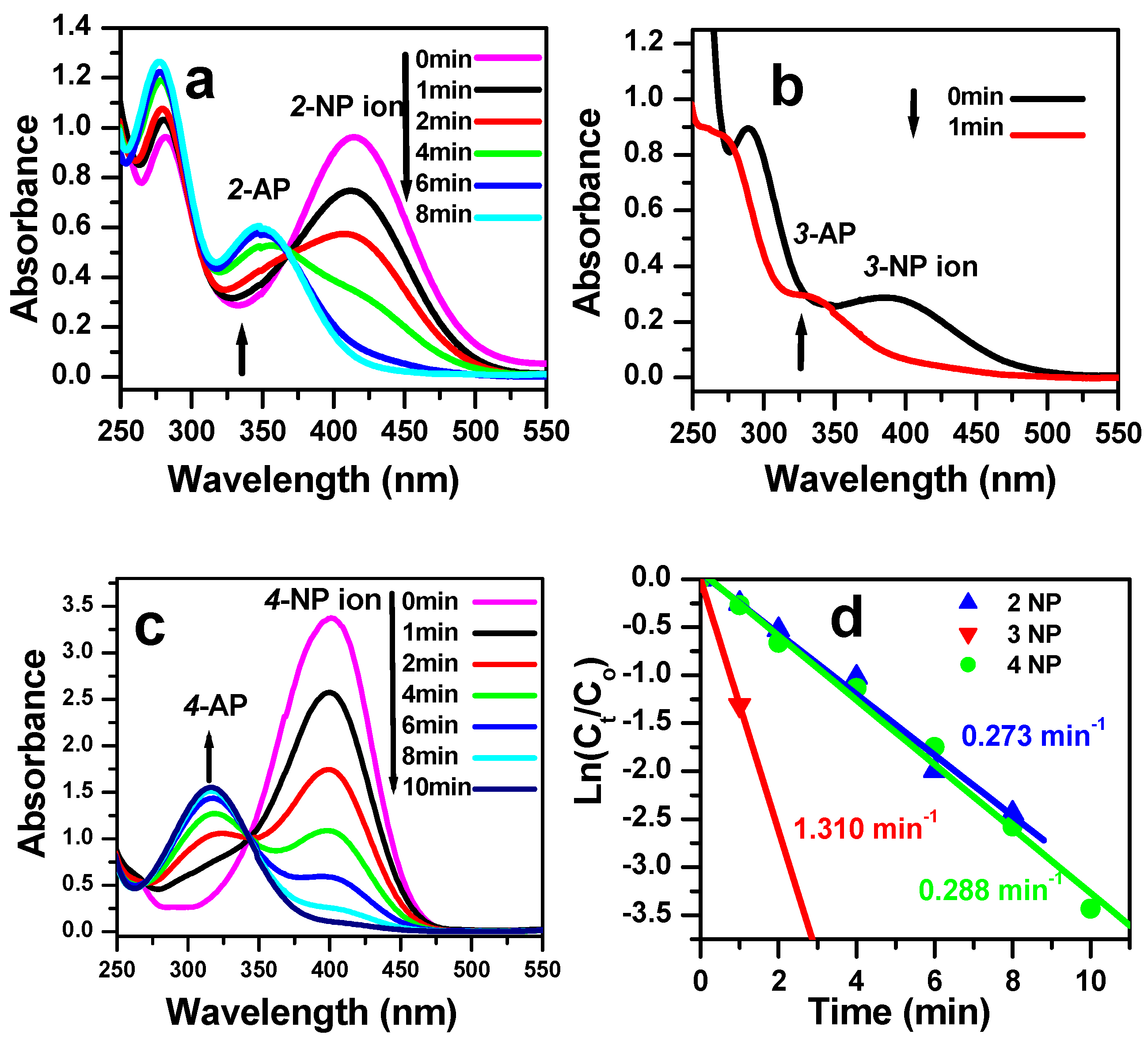
| Catalyst | Type | Concentration of NP (mol/L) | Reaction Time (min) | Rate Constant (min−1) | References |
|---|---|---|---|---|---|
| CoMoO4 | Nanoparticles | 2 × 10−4 | 8 | 0.273 for 2-NP | This work |
| 1 | 1.310 for 3-NP | ||||
| 8 | 0.288 for 4-NP | ||||
| CuFe2O4 | Nanoparticles | 3.6 × 10−5 | 3 | 3.676 for 2-NP | [17] |
| 1.5 | 0.983 for 3-NP | ||||
| 3 | 0.846 for 4-NP | ||||
| NiFe2O4 | Nanoparticles | 3.6 × 10−5 | 20 | 0.327 for 2-NP | [17] |
| 12 | 0.062 for 3-NP | ||||
| 16 | 0.118 for 4-NP | ||||
| CuO/γAl2O3 | Nanocomposites | 2.9 × 10−5 | 15 | ---- for 2-NP | [18] |
| 20 | ---- for 3-NP | ||||
| 12 | 0.174 for 4-NP | ||||
| Ni/C black | Nanocomposites | 5.0 × 10−4 | 15 | 0.594 for 2-NP | [19] |
| 15 | 0.594 for 3-NP | ||||
| 15 | 0.5970 for 4-NP | ||||
| Fe2(MoO4)3 | Nanoparticles | 2 × 10−4 | 12 | 0.160 for 2-NP | [20] |
| 4 | 0.427 for 3-NP | ||||
| 9 | 0.323 for 4-NP |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Wadaani, F.; Omer, A.; Abboudi, M.; Oudghiri Hassani, H.; Rakass, S.; Messali, M.; Benaissa, M. High Catalytic Efficiency of Nanostructured β-CoMoO4 in the Reduction of the Ortho-, Meta- and Para-Nitrophenol Isomers. Molecules 2018, 23, 364. https://doi.org/10.3390/molecules23020364
Al-Wadaani F, Omer A, Abboudi M, Oudghiri Hassani H, Rakass S, Messali M, Benaissa M. High Catalytic Efficiency of Nanostructured β-CoMoO4 in the Reduction of the Ortho-, Meta- and Para-Nitrophenol Isomers. Molecules. 2018; 23(2):364. https://doi.org/10.3390/molecules23020364
Chicago/Turabian StyleAl-Wadaani, Fahd, Ahmed Omer, Mostafa Abboudi, Hicham Oudghiri Hassani, Souad Rakass, Mouslim Messali, and Mohammed Benaissa. 2018. "High Catalytic Efficiency of Nanostructured β-CoMoO4 in the Reduction of the Ortho-, Meta- and Para-Nitrophenol Isomers" Molecules 23, no. 2: 364. https://doi.org/10.3390/molecules23020364
APA StyleAl-Wadaani, F., Omer, A., Abboudi, M., Oudghiri Hassani, H., Rakass, S., Messali, M., & Benaissa, M. (2018). High Catalytic Efficiency of Nanostructured β-CoMoO4 in the Reduction of the Ortho-, Meta- and Para-Nitrophenol Isomers. Molecules, 23(2), 364. https://doi.org/10.3390/molecules23020364