Preparation, Characterization and Catalytic Activity of Nickel Molybdate (NiMoO4) Nanoparticles
Abstract
:1. Introduction
2. Experimental
2.1. Catalyst Preparation
2.2. Characterization
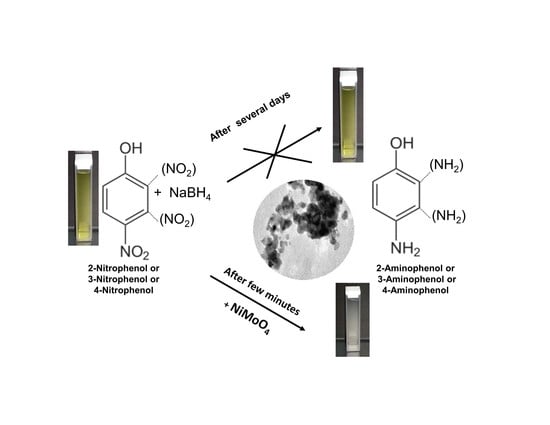
2.3. Test of Nitrophenol Isomers Reduction
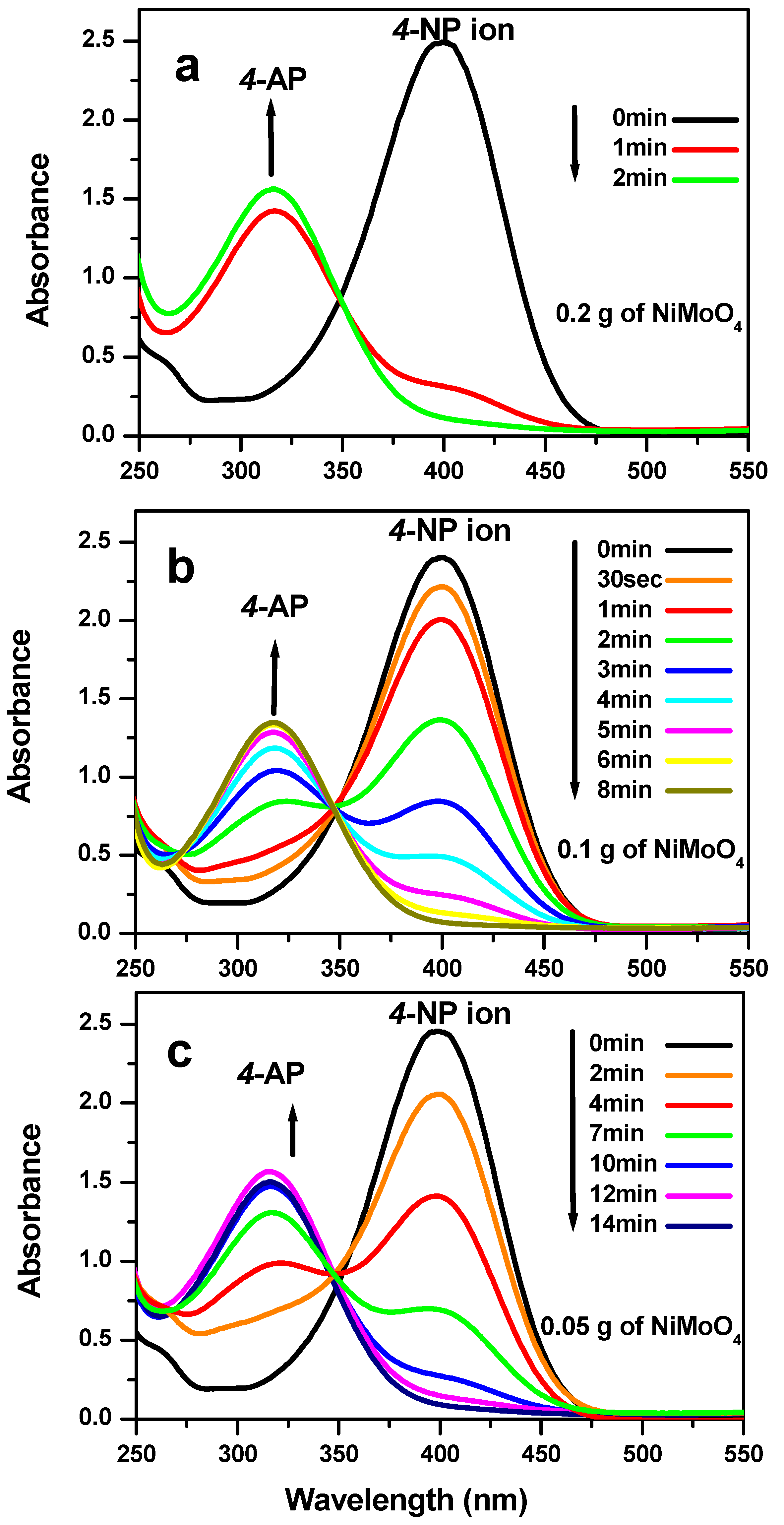
3. Results and Discussion
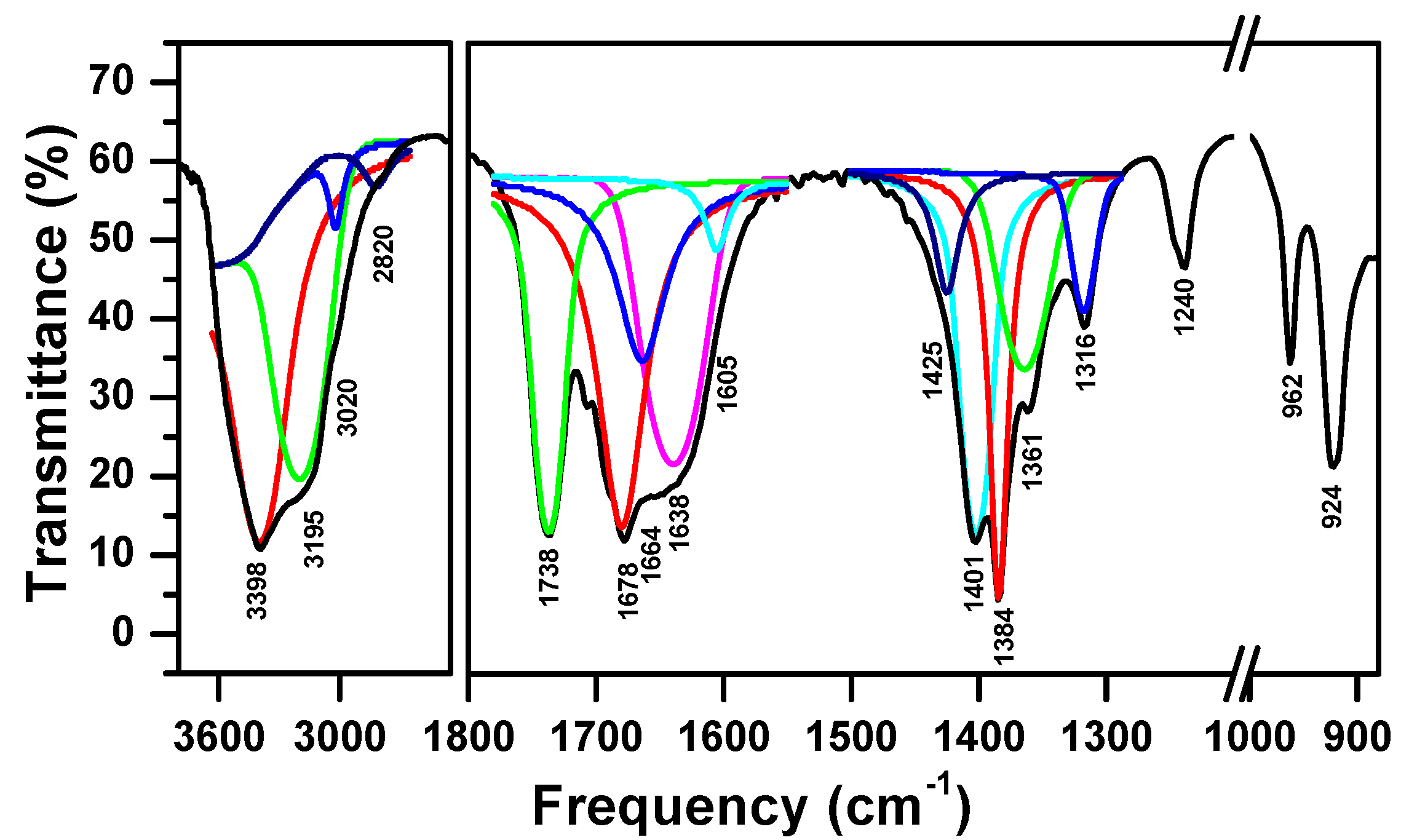
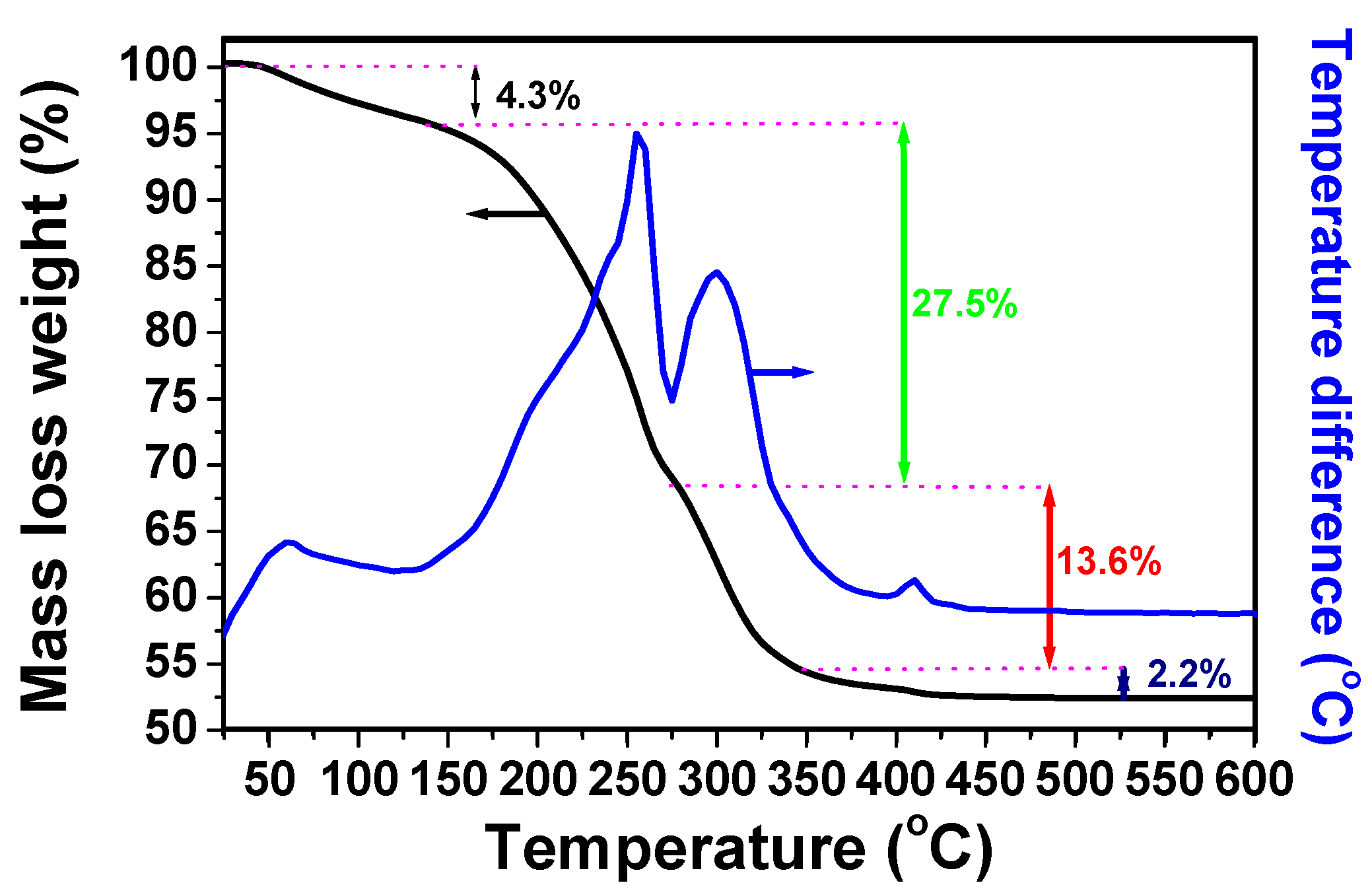
3.1. Characterizations of the Complex
3.2. Nickel Molybdate Characterization
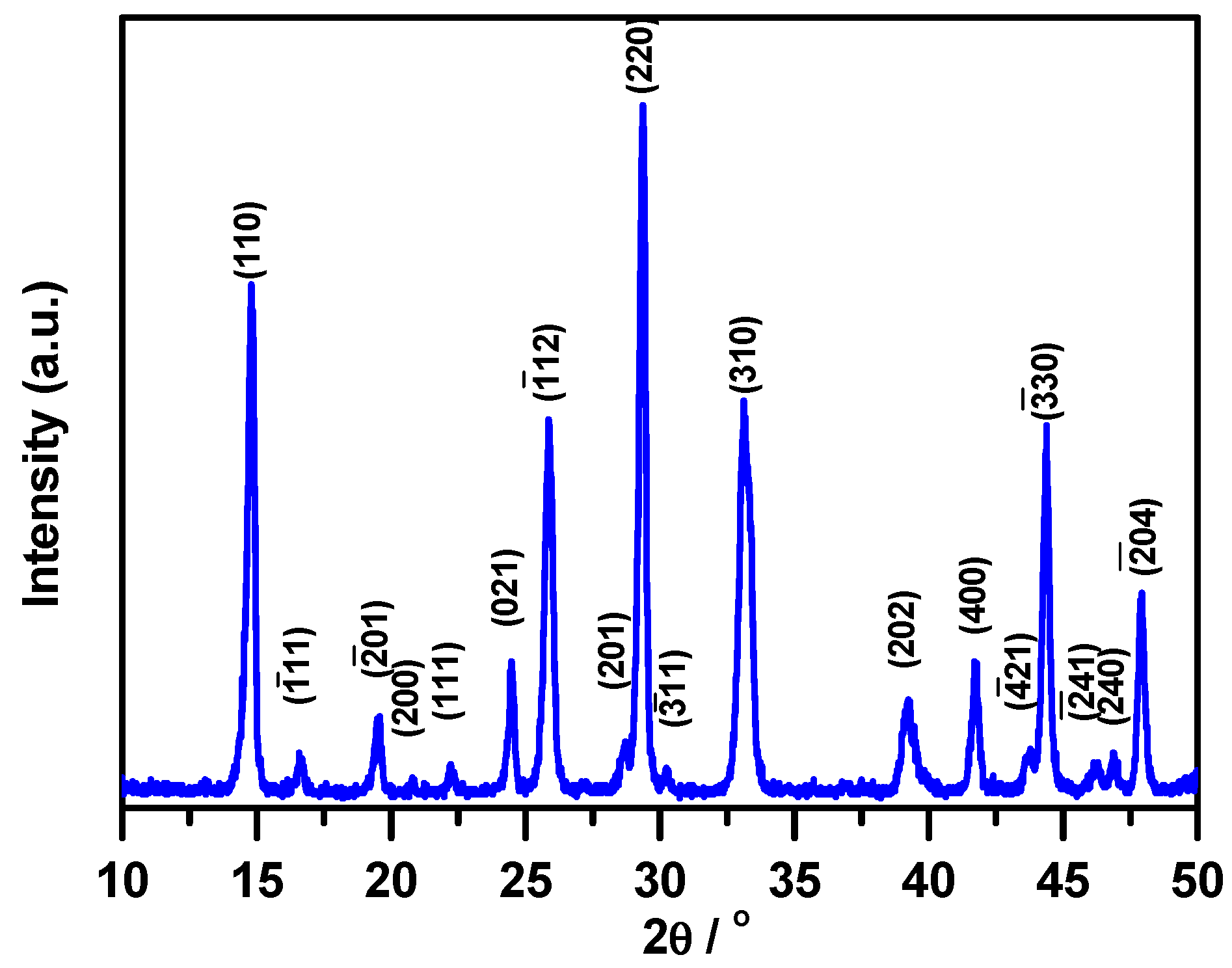
3.2.1. X-ray Diffraction
3.2.2. Specific Surface Area Determination
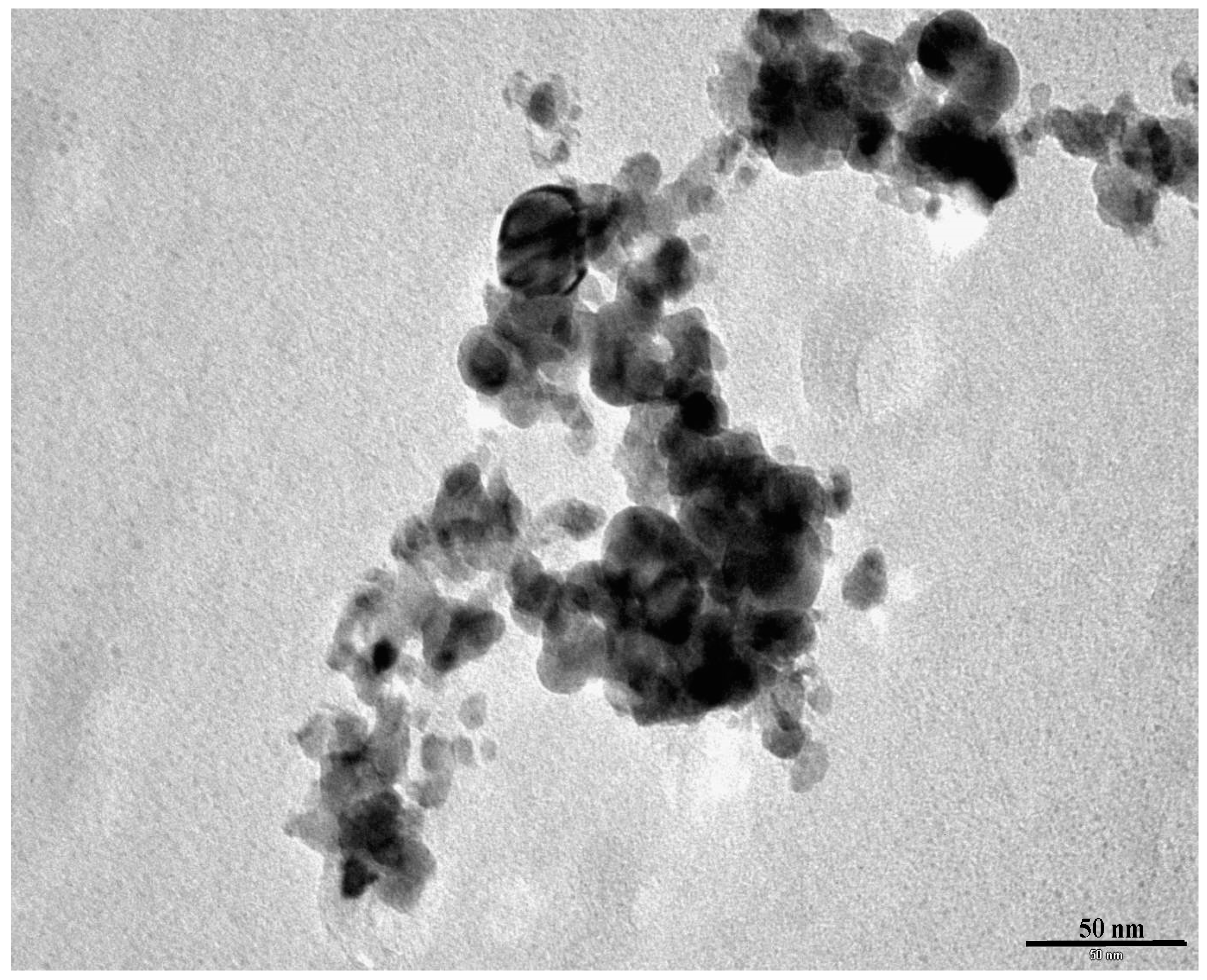
3.2.3. Transmission Electron Microscopy
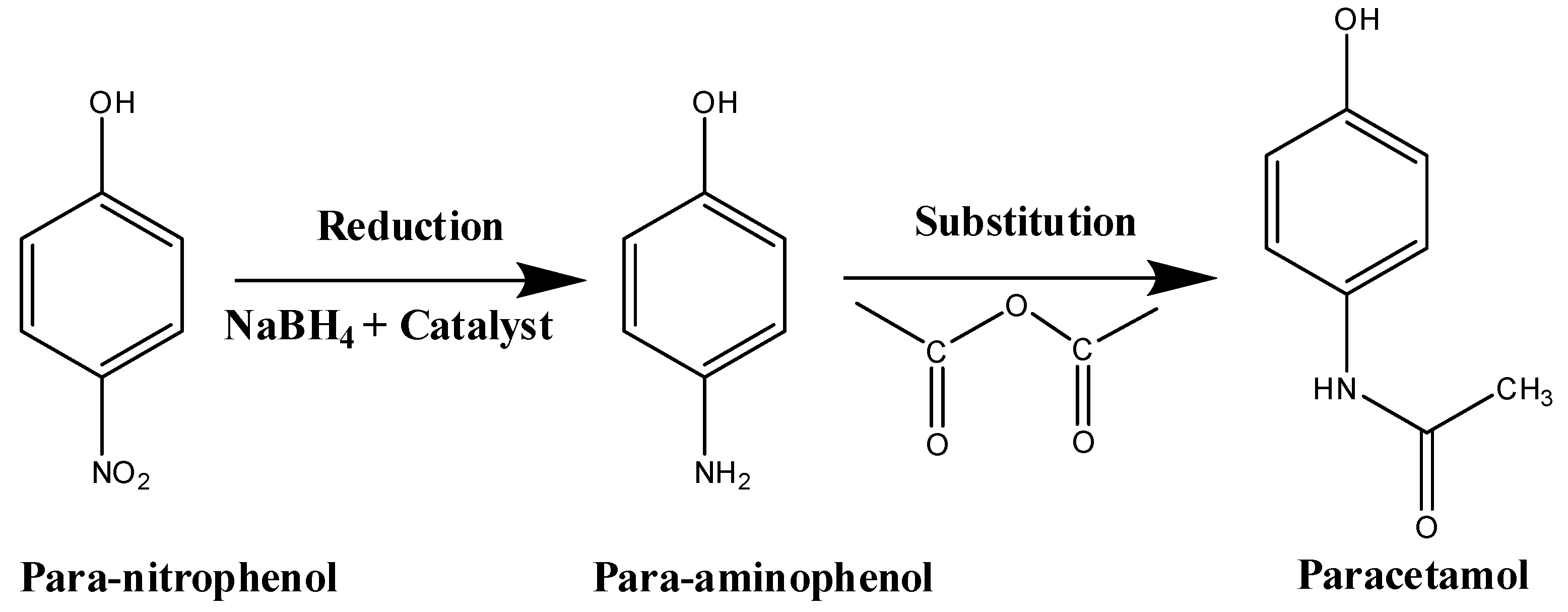
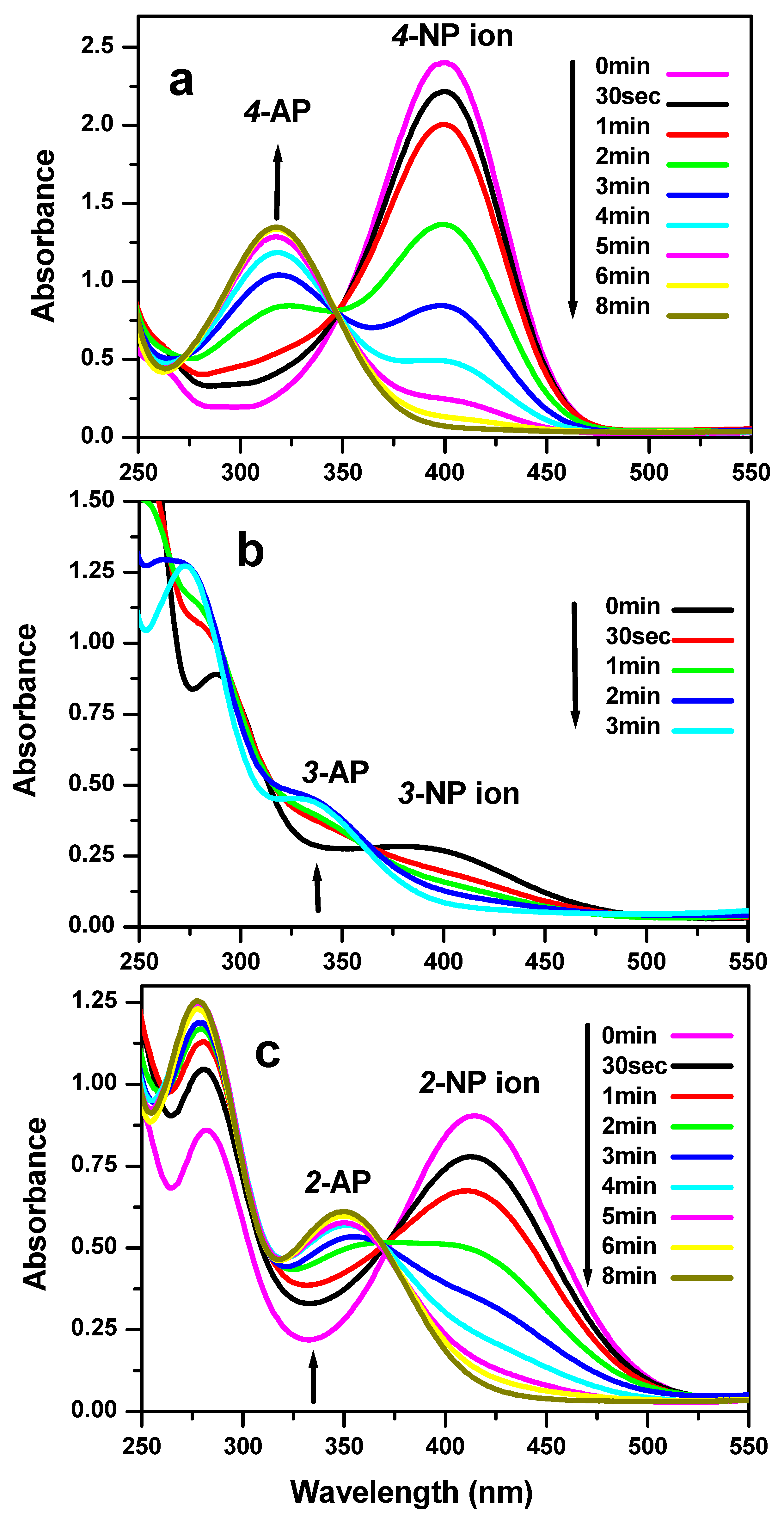
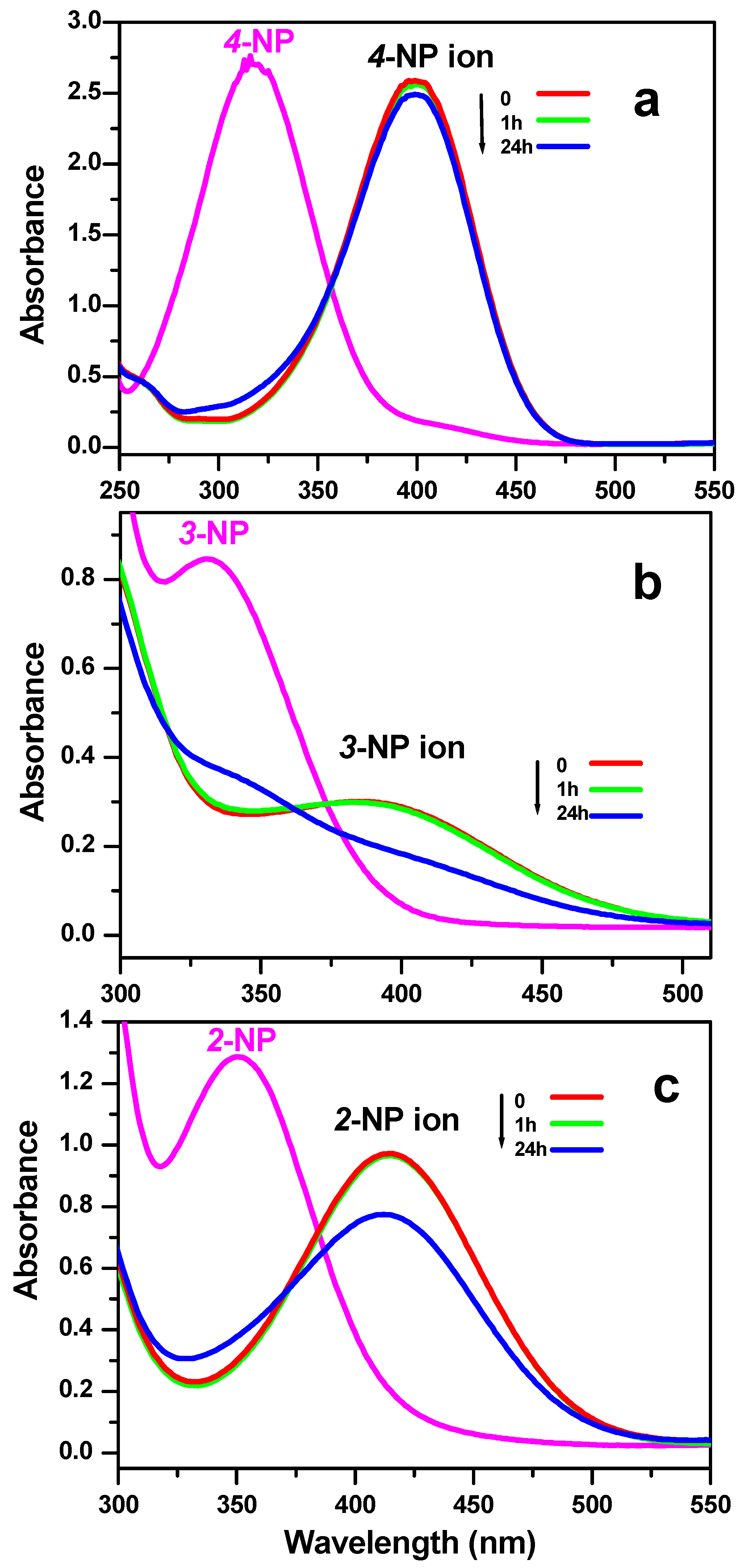
3.3. Reduction Test of Nitrophenol Isomers
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Shimazu, M.; Mulchandani, A.; Che, W. Simultaneous degradation of organophosphorus pesticides and p-nitrophenol by a genetically engineered Moraxella sp. With surface-expressed organophosphorus hydrolase. Biotechnol. Bioeng. 2001, 76, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Espinosa Bosch, M.; Ruiz Sánchez, A.J.; Sánchez Rojas, F.; Bosch Ojeda, C. Determination of paracetamol: Historical evolution. J. Pharm. Biomed. 2006, 42, 291–321. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Xua, A.; Xiab, J.; Hea, J.; Xinga, H.; Zhanga, X.; Weia, S.; Wanga, N. Facile synthesis of highly catalytic activity Ni–Co–Pd–P composite for reduction of the p-Nitrophenol. Appl. Catal. A-Gen. 2014, 470, 89–96. [Google Scholar] [CrossRef]
- Laksmia, B.; Shivanandaa, K.; Puttaswamya; Mahendraa, K.N.; Gowdab, N.M.; Jagadeesha, V.R. An efficient platinum-catalyzed oxidation process and mechanism for the facile conversion of benzoxazoles to aminophenols. Chem. Eng. J. 2010, 163, 403–412. [Google Scholar] [CrossRef]
- Brito, J.L.; Barbosa, A.L.; Albornoz, A.; Severino, F.; Laine, J. Nickel molybdate as precursor of HDS catalysts: Effect of phase composition. Catal. Lett. 1994, 26, 329–337. [Google Scholar] [CrossRef]
- Kaddouri, A.; Anouchinsky, R.; Mazzocchia, C.; Madeira, L.M.; Portela, M.F. Oxidative dehydrogenation of ethane on the α and β phases of NiMoO4. Catal. Today 1998, 40, 201–206. [Google Scholar] [CrossRef]
- Pillay, B.; Mathebula, M.R.; Friedrich, H.B. The oxidative dehydrogenation of n-hexane over Ni–Mo–O catalysts. Appl. Catal. A 2009, 361, 57–64. [Google Scholar] [CrossRef]
- Rodriguez, J.A.; Chaturvedi, S.; Hanson, J.C.; Brito, J.L. Reaction of H2 and H2S with CoMoO4 and NiMoO4: TPR, XANES, Time-Resolved XRD, and Molecular-Orbital Studies. J. Phys. Chem. 1999, 103, 770–781. [Google Scholar] [CrossRef]
- Sundaram, R.; Nagaraja, K.S. Solid state electrical conductivity and humidity sensing studies on metal molybdate–molybdenum trioxide composites (M = Ni2+, Cu2+ and Pb2+). Sens. Actuators B Chem. 2004, 101, 353–360. [Google Scholar] [CrossRef]
- Mi, Y.; Huang, Z.; Hu, F.; Jiang, J.; Li, Y. Controlled synthesis and growth mechanism of alpha nickel molybate microhombohedron. Mater. Lett. 2010, 64, 695–697. [Google Scholar] [CrossRef]
- Baoyi, S.; Aiju, X.; Jiang, W. The impact of preparation methods on the structure and catalytic performance of NiMoO4 for oxidative dehydrogenation of propane. Integr. Ferroelectr. 2016, 171, 16–22. [Google Scholar] [CrossRef]
- Bettahar, M.M.; Costentin, G.; Savary, L.; Lavalley, J.C. On the partial oxidation of propane and propylene on mixed metal oxide catalysts. Appl. Catal. A-Gen. 1996, 145, 1–48. [Google Scholar] [CrossRef]
- Senthilkumar, B.; Vijaya Sankar, K.; Selvan, R.K.; Danielle, M.; Manickam, M. Nano α-NiMoO4 as a new electrode for electrochemical supercapacitors. RSC Adv. 2013, 3, 352–357. [Google Scholar] [CrossRef]
- Liu, M.; Kong, L.; Lu, C.; Li, X.; Luo, Y.; Kang, L. Waste paper based activated carbon monolith as electrode materials for high performance electric double-layer capacitors. RSC Adv. 2012, 2, 1890–1896. [Google Scholar] [CrossRef]
- Liu, P.; Deng, Y.; Zhang, Q.; Hu, Z.; Xu, Z.; Liu, Y.; Yao, M.; Ai, Z. Facile synthesis and characterization of high-performance NiMoO4·xH2O nanorods electrode material for supercapacitors. Ionics 2015, 21, 2797–2804. [Google Scholar] [CrossRef]
- Amin Alborzi, A.; Khademolhoseini, S. Nickel molybdate nanoparticles: Synthesis, characterization, optical and photocatalytic properties. J. Mater. Sci. Mater. Electron. 2016, 27, 3963–3967. [Google Scholar] [CrossRef]
- Mosleh, M. Facile approach to synthesize nanocristalline NiMoO4 in the presence of amino acids as capping agent. J. Mater. Sci. Mater. Electron. 2017, 28, 6788–6793. [Google Scholar] [CrossRef]
- Yang, L.; Wang, J.; Wan, Y.; Li, Y.; Xie, H.; Cheng, H.; Seo, H.J. Structure and effective visible-light-driven photocatalytic activity of α-NiMoO4 for degradation of methylene blue dye. J. Alloys Compd. 2016, 664, 756–763. [Google Scholar] [CrossRef]
- Rodriguez, J.A.; Hanson, J.C.; Chaturvedi, S.; Maiti, A.; Brito, J.L. Phase transformations and electronic properties in mixed-metal oxides: Experimental and theoretical studies on the behavior of NiMoO4 and MgMoO4. J. Chem. Phys. 2000, 112, 935–945. [Google Scholar] [CrossRef]
- Cherian, C.T.; Reddy, M.V.; Haur, S.C.; Chowdari, B.V.R. Interconnected Network of CoMoO4 Submicrometer Particles as High Capacity Anode Material for Lithium Ion Batteries. ACS Appl. Mater. Interfaces 2013, 5, 918–923. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Yu, S.H.; Liu, C.; Zang, Z.A. 3D Architectures of Iron Molybdate: Phase Selective Synthesis, Growth Mechanism, and Magnetic Properties. Chem. Eur. J. 2007, 13, 746–753. [Google Scholar] [CrossRef] [PubMed]
- Moreno, B.; Chinarro, E.; Colomer, M.; Jurado, J. Combustion synthesis and electrical behavior of nanometric β-NiMoO4. J. Phys. Chem. C 2010, 114, 4251–4257. [Google Scholar] [CrossRef]
- Liu, M.; Kang, L.; Kong, L.; Lu, C.; Ma, X.; Li, X.; Luo, Y. Facile synthesis of NiMoO4·xH2O nanorods as a positive electrode material for supercapacitors. RSC Adv. 2013, 3, 6472–6478. [Google Scholar] [CrossRef]
- Wan, H.; Jiang, J.; Ji, X.; Miao, L.; Zhang, L.; Xu, K.; Chen, H.; Ruan, Y. Rapid microwave-assisted synthesis NiMoO4·H2O nanoclusters for supercapacitors. Mater. Lett. 2013, 108, 164–167. [Google Scholar] [CrossRef]
- Cai, D.; Liu, B.; Wang, D.; Liu, Y.; Wang, L.; Li, H.; Wang, Y.; Wang, C.; Li, Q.; Wang, T. Facile hydrothermal synthesis of hierarchical ultrathin mesoporous NiMoO4 nanosheets for high performance supercapacitors. Electrochim. Acta 2014, 115, 358–363. [Google Scholar] [CrossRef]
- Masteri, F.; Mahdavi, S.; Rafizadeh, M. Microemulsion mediated synthesis and characterization of monodispersed nickel molybdate nanocrystals. Ceram. Int. 2013, 39, 4619–4625. [Google Scholar] [CrossRef]
- Ray, S.K.; Dipesh, D.; Yuwaraj, K.K.; Soo, W.L. Cu-α-NiMoO4 photocatalyst for degradation of Methylene blue with pathways and antibacterial performance. J. Photochem. Photobiol. A 2017, 348, 18–32. [Google Scholar] [CrossRef]
- Ramachandran, S.P.; Ravi, G.; Ganesh, V.; Sakunthala, A.; Yuvakkumar, R. Morphology dependent electrochemical capacitor performance of NiMoO4 nanoparticles. Mater. Lett. 2017, 209, 1–4. [Google Scholar]
- Cai, D.; Liu, B.; Wang, D.; Liu, Y.; Wang, L.; Li, H.; Wang, Y.; Wang, C.; Li, Q.; Wang, T. Enhanced performance of supercapacitors with ultrathin mesoporous NiMoO4 nanosheets. Electrochim. Acta 2014, 125, 294–301. [Google Scholar] [CrossRef]
- Gentil, S.; Lalaoui, N.; Dutta, A.; Nedellec, Y.; Cosnier, S.; Shaw, W.J.; Artero, V.; Le Goff, A. Carbon-Nanotube-Supported Bio-Inspired Nickel Catalyst and Its Integration in Hybrid Hydrogen/Air Fuel Cells. Angew. Chem. Int. Ed. 2017, 56, 1845–1849. [Google Scholar] [CrossRef] [PubMed]
- Tahir, M.; Pan, L.; Zhang, R.; Wang, Y.C.; Shen, G.; Aslam, I.; Qadeer, M.A.; Mahmood, N.; Xu, W.; Wang, L.; et al. High-Valence-State NiO/Co3O4 Nanoparticles on Nitrogen-Doped Carbon for Oxygen Evolution at Low Overpotential. ACS Energy Lett. 2017, 2, 2177–2182. [Google Scholar] [CrossRef]
- Oudghiri-Hassani, H. Synthesis, characterization and catalytic performance of iron molybdate Fe2(MoO4)3 nanoparticles. Catal. Commun. 2015, 60, 19–22. [Google Scholar] [CrossRef]
- Abboudi, M.; Messali, M.; Kadiri, N.; Ben Ali, A.; Moran, E. Synthesis of CuO, La2O3, and La2CuO4 by the Thermal-Decomposition of Oxalates Precursors Using a New Method. Synth. React. Inorg. Met.-Org. Chem. 2011, 41, 683–688. [Google Scholar] [CrossRef]
- Messali, M.; Al Wadaani, F.; Oudghiri-Hassani, H.; Rakass, S.; Al Amri, S.; Benaissa, M.; Abboudi, M. Preparation, characterization and photocatalytic activity of hexagonal ZnO nanoparticles. Mater. Lett. 2014, 128, 187–190. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds Part B: Applications in Coordination, Organometallic, and Bioinorganic Chemistry, 6th ed.; Wiley: New York, NY, USA, 2009; pp. 152–165. ISBN 978-0-471-74493-1. [Google Scholar]
- Ng, K.Y.S.; Zhou, X.; Gulari, E. Spectroscopic characterization of molybdenum oxalate in solution and on alumina. J. Phys. Chem. 1985, 89, 2477–2481. [Google Scholar] [CrossRef]
- Ramis, G.; Yi, L.; Busca, G. Ammonia activation over catalysts for the selective catalytic reduction of NOx and the selective catalytic oxidation of NH3. An FT-IR study. Catal. Today 1996, 28, 373–380. [Google Scholar] [CrossRef]
- Wen, N.; Brooker, M.H. Ammonium Carbonate, Ammonium Bicarbonate, and Ammonium Carbamate Equilibria: A Raman Study. J. Phys. Chem. 1995, 99, 359–368. [Google Scholar] [CrossRef]
- Rivenet, M.; Roussel, P.; Abraham, F. One-dimensional inorganic arrangement in the bismuth oxalate hydroxide Bi(C2O4)OH. J. Solid State Chem. 2008, 181, 2586–2590. [Google Scholar] [CrossRef]
- Shaheen, W.M. Thermal behaviour of pure and binary basic nickel carbonate and ammonium molybdate systems. Mater. Lett. 2002, 52, 272–282. [Google Scholar] [CrossRef]
- Angermann, A.; Topfer, J. Synthesis of nanocrystalline Mn–Zn ferrite powders through thermolysis of mixed oxalates. Ceram. Int. 2011, 37, 995–1002. [Google Scholar] [CrossRef]
- Fagerlund, G. Determination of specific surface by BET method. Mater. Constr. 1973, 6, 239–245. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar]
- Goyal, A.; Bansal, S.; Singhal, S. Facile reduction of nitrophenols: Comparative catalytic efficiency of MFe2O4 (M = Ni, Cu, Zn) nano ferrites. Int. J. Hydrogen Energy 2014, 39, 4895–4908. [Google Scholar] [CrossRef]
- Nandanwar, S.U.; Chakraborty, M. Synthesis of Colloidal CuO/γ-Al2O3 by Microemulsion and Its Catalytic Reduction of Aromatic Nitro Compounds. Chin. J. Catal. 2012, 33, 1532–1541. [Google Scholar] [CrossRef]
- Xia, J.; He, G.; Zhang, L.; Sun, X.; Wang, X. Hydrogenation of nitrophenols catalyzed by carbon black-supported nickel nanoparticles under mild conditions. Appl. Catal. B-Environ. 2016, 180, 408–415. [Google Scholar] [CrossRef]
- Holbrook, K.A.; Twist, P.J. Hydrolysis of the Borohydride Ion catalysed by Metal-Boron Alloys. J. Chem. Soc. A Inorg. Phys. Theor. 1971, 890–894. [Google Scholar] [CrossRef]
- Pandey, S.; Mishra, S.B. Catalytic reduction of p-nitrophenol by using platinum nanoparticles stabilised by guar gum. Carbohydr. Polym. 2014, 113, 525–531. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds nickel molybdate (α-NiMOO4) are available from the authors. |
| Catalyst | Type | Concentration of NP (mol/L) | Reaction Time (min) | References |
|---|---|---|---|---|
| NiMoO4 | Nanoparticles | 2 × 10−4 | 8 for 4-NP 3 for 3-NP 8 for 2-NP | This work |
| CuFe2O4 | Nanoparticles | 3.6 × 10−5 | 4 for 4-NP 5 for 3-NP 3 for 2-NP | [44] |
| NiFe2O4 | Nanoparticles | 3.6 × 10−5 | 38 for 4-NP 36 for 3-NP 28 for 2-NP | [44] |
| CuO/γAl2O3 | Nanocomposites | 2.9 × 10−5 | 12 for 4-NP 20 for 3-NP 15 for 2-NP | [45] |
| Ni/C black | Nanocomposites | 5.0 × 10−4 | 15 for 4-NP 15 for 3-NP 15 for 2-NP | [46] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oudghiri-Hassani, H.; Al Wadaani, F. Preparation, Characterization and Catalytic Activity of Nickel Molybdate (NiMoO4) Nanoparticles. Molecules 2018, 23, 273. https://doi.org/10.3390/molecules23020273
Oudghiri-Hassani H, Al Wadaani F. Preparation, Characterization and Catalytic Activity of Nickel Molybdate (NiMoO4) Nanoparticles. Molecules. 2018; 23(2):273. https://doi.org/10.3390/molecules23020273
Chicago/Turabian StyleOudghiri-Hassani, Hicham, and Fahd Al Wadaani. 2018. "Preparation, Characterization and Catalytic Activity of Nickel Molybdate (NiMoO4) Nanoparticles" Molecules 23, no. 2: 273. https://doi.org/10.3390/molecules23020273
APA StyleOudghiri-Hassani, H., & Al Wadaani, F. (2018). Preparation, Characterization and Catalytic Activity of Nickel Molybdate (NiMoO4) Nanoparticles. Molecules, 23(2), 273. https://doi.org/10.3390/molecules23020273