Chloro- and Dichloro-methylsulfonyl Nitrenes: Spectroscopic Characterization, Photoisomerization, and Thermal Decomposition
Abstract
:1. Introduction
2. Results and Discussion
2.1. Photolysis of CH2ClS(O)2N3
2.2. Flash Vacuum Pyrolysis of CH2ClS(O)2N3
2.3. Photolysis of CHCl2S(O)2N3
2.4. Flash Vacuum Pyrolysis of CHCl2S(O)2N3
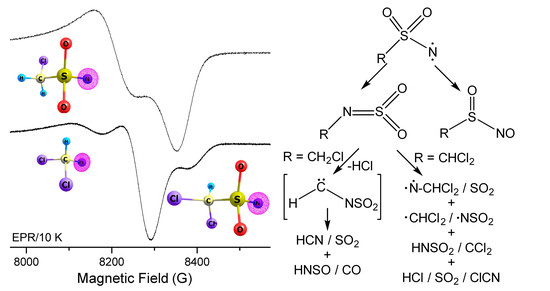
2.5. EPR Spectroscopy
2.6. Quantum Chemical Calculations
3. Materials and Methods
3.1. Sample Preparation
3.2. Spectroscopy
3.3. Matrix IR Spectroscopy
3.4. Computational Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Gritsan, N.P. Properties of Carbonyl Nitrenes and Related Acyl Nitrenes. Nitrenes and Nitrenium Ions, 1st ed.; Falvey, D.E., Gudmundsdottir, A.D., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; Volume 6, pp. 481–548. ISBN 978-0-470-39059-7. [Google Scholar]
- Jiang, H.L.; Lang, K.; Lu, H.J.; Wojtas, L.; Zhang, X.P. Intramolecular radical aziridination of allylic sulfamoyl azides by cobalt(II)-based metalloradical catalysis: Effective construction of strained heterobicyclic structures. Angew. Chem. Int. Ed. 2016, 55, 11604–11608. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.J.; Li, C.Q.; Jiang, H.L.; Lizardi, C.L.; Zhang, X.P. Chemoselective amination of propargylic C(sp3)‒H bonds by cobalt(II)-based metalloradical catalysis. Angew. Chem. Int. Ed. 2014, 126, 7148–7152. [Google Scholar] [CrossRef]
- Liu, L.-H.; Yan, M. Perfluorophenyl azides: New applications in surface functionalization and nanomaterial synthesis. Acc. Chem. Res. 2010, 43, 1434–1443. [Google Scholar] [CrossRef] [PubMed]
- Lwowski, W. Nitrenes and the decomposition of carbonylazides. Angew. Chem. Int. Ed. 1967, 6, 897–1012. [Google Scholar] [CrossRef]
- Kundu, S.; Miceli, E.; Farquhar, E.; Pfaff, F.F.; Kuhlmann, U.; Hildebrandt, P.; Braun, B.; Greco, C.; Ray, K. Lewis acid trapping of an elusive copper−tosylnitrene intermediate using scandium triflate. J. Am. Chem. Soc. 2012, 134, 14710–14713. [Google Scholar] [CrossRef] [PubMed]
- Gritsan, N.P.; Likhotvorik, I.; Tsao, M.-L.; Celebi, N.; Platz, M.S.; Karney, W.L.; Kemnitz, C.R.; Borden, W.T. Ring-expansion reaction of cyano-substituted singlet phenyl nitrenes: Theoretical predictions and kinetic results from laser flash photolysis and chemical trapping experiments. J. Am. Chem. Soc. 2001, 123, 1425–1433. [Google Scholar] [CrossRef]
- Kubicki, J.; Luk, H.L.; Zhang, Y.; Vyas, S.; Peng, H.-L.; Hadad, C.M.; Platz, M.S. Direct observation of a sulfonyl azide excited state and its decay processes by ultrafast time-resolved IR Spectroscopy. J. Am. Chem. Soc. 2012, 134, 7036–7044. [Google Scholar] [CrossRef] [PubMed]
- Kuzmin, A.V.; Neumann, C.; van Wilderen, L.J.G.W.; Shainyanb, B.A.; Bredenbeck, J. Exploring photochemistry of p-bromophenylsulfonyl, p-toylsulfonyl and methylsulfonyl azides by ultrafast UV-Pump-IR-Probe spectroscopy and computations. Phys. Chem. Chem. Phys. 2016, 18, 8662–8672. [Google Scholar] [CrossRef] [PubMed]
- Kubicki, J.; Zhang, Y.; Xue, J.; Luk, H.L.; Platz, M.S. Ultrafast time resolved studies of the photochemistry of acyl and sulfonyl azides. Phys. Chem. Chem. Phys. 2012, 14, 10377–10390. [Google Scholar] [CrossRef]
- Zeng, X.Q.; Beckers, H.; Willner, H. Thermally persistent fluorosulfonyl nitrene and unexpected formation of the fluorosulfonyl radical. J. Am. Chem. Soc. 2013, 135, 2096–2099. [Google Scholar] [CrossRef]
- Zeng, X.Q.; Beckers, H.; Willner, H.; Neuhaus, P.; Grote, D.; Sander, W. Photochemistry of matrix isolated (trifluoromethyl)sulfonyl azide, CF3SO2N3. J. Phys. Chem. A 2015, 119, 2281–2288. [Google Scholar] [CrossRef]
- Zeng, X.Q.; Beckers, H.; Neuhaus, P.; Grote, D.; Sander, W. Elusive fluoro sulfinyl nitrite, FS(O)NO, produced by photolysis of matrix-isolated FS(O)2N. Z. Anorg. Allg. Chem. 2012, 638, 526–533. [Google Scholar] [CrossRef]
- Obenhuber, A.H.; Gianetti, T.L.; Berrebi, X.; Bergman, R.G.; Arnold, J. Reaction of (bisimido)niobium(V) complexes with organic azides: [3+2] cycloaddition and reversible cleavage of β-diketiminato ligands involving nitrene transfer. J. Am. Chem. Soc. 2014, 136, 2994–2997. [Google Scholar] [CrossRef] [PubMed]
- Klima, R.F.; Gudmundsdottir, A.D. Intermolecular triplet-sensitized photolysis of alkyl azides trapping of triplet alkyl nitrenes. J. Photochem. Photobiol. A. 2004, 162, 239–247. [Google Scholar] [CrossRef]
- Hayes, J.C.; Sheridan, R.S. Infrared spectrum of triplet phenylnitrene. On the origin of didehydroazepine in low-temperature matrices. J. Am. Chem. Soc. 1990, 112, 5879–5881. [Google Scholar] [CrossRef]
- Leyva, E.; Platz, M.S.; Persy, G.; Wirz, J. Photochemistry of phenyl azide: The role of singlet and triplet phenylnitrene as transient intermediates. J. Am. Chem. Soc. 1986, 108, 3783–3790. [Google Scholar] [CrossRef]
- Kubicki, J.; Zhang, Y.; Vyas, S.; Burdzinski, G.; Luk, H.L.; Wang, J.; Xue, J.; Peng, H.-L.; Pritchina, E.A.; Sliwa, M.; et al. Photochemistry of 2-naphthoyl azide. An ultrafast time-resolved UV-vis and IR spectroscopic and computational Study. J. Am. Chem. Soc. 2011, 133, 9751–9761. [Google Scholar] [CrossRef] [PubMed]
- Vyas, S.; Kubicki, J.; Luk, H.L.; Zhang, Y.; Gritsan, N.P.; Hadad, C.M.; Platz, M.S. An ultrafast time-resolved infrared and UV-vis spectroscopic and computational study of the photochemistry of acyl azides. J. Phys. Org. Chem. 2012, 25, 693–703. [Google Scholar] [CrossRef]
- Zeng, X.Q.; Beckers, H.; Willner, H.; Grote, D.; Sander, W. The missing link: Triplet fluorocarbonyl nitrene FC(O)N. Chem. Eur. J. 2011, 17, 3977–3984. [Google Scholar] [CrossRef]
- Chuprakov, S.; Worrell, B.T.; Selander, N.; Sit, R.K.; Fokin, V.V. Stereoselective 1,3-insertions of rhodium(II) azavinyl carbenes. J. Am. Chem. Soc. 2014, 136, 195–202. [Google Scholar] [CrossRef]
- McIntosh, J.A.; Coelho, P.S.; Farwell, C.C.; Wang, Z.J.; Lewis, J.C.; Brown, T.R.; Arnold, F.H. Enantioselective intramolecular C‒H amination catalyzed by engineered cytochrome P450 enzymes in vitro and in vivo. Angew. Chem. Int. Ed. 2013, 125, 9479–9482. [Google Scholar] [CrossRef]
- Lu, H.J.; Jiang, H.L.; Wojtas, L.; Zhang, X.P. Selective intramolecular C‒H amination through the metalloradical activation of azides: Synthesis of 1,3-diamines under neutral and nonoxidative conditions. Angew. Chem. Int. Ed. 2010, 122, 10390–10394. [Google Scholar] [CrossRef]
- Lwowski, W.; Scheiffele, E. Curtius and lossen rearrangements. I. The benzenesulfonyl system. J. Am. Chem. Soc. 1965, 87, 4359–4365. [Google Scholar] [CrossRef]
- Sheridan, R.S.; Rempala, P. Books of Abstracts. In Proceedings of the 217th ACS National Meeting, Anaheim, CA, USA, 21–25 March 1999; American Chemical Society: Washington, DC, USA, 1999. [Google Scholar]
- Deng, G.H.; Wu, Z.; Li, D.Q.; Linguerri, R.; Francisco, J.S.; Zeng, X.Q. Simplest N-sulfonylamine HNSO2. J. Am. Chem. Soc. 2016, 138, 11509–11512. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.Q.; Beckers, H.; Willner, W. The iminyl radical O2SN. Angew. Chem. Int. Ed. 2013, 52, 7981–7984. [Google Scholar] [CrossRef] [PubMed]
- Deng, G.H.; Dong, X.L.; Liu, Q.F.; Li, D.Q.; Li, H.M.; Sun, Q.; Zeng, X.Q. The decomposition of benzenesulfonyl azide: A matrix isolation and computational study. Phys. Chem. Chem. Phys. 2017, 19, 3792–3799. [Google Scholar] [CrossRef] [PubMed]
- Weidner, K.; Giroult, A.; Panchaud, P.; Renaud, P. Efficient carboazidation of alkenes using a radical desulfonylative azide transfer process. J. Am. Chem. Soc. 2010, 132, 17511–17515. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Wan, H.B.; Xu, J.; Lu, B.; Lu, Y.; Eckhardt, A.K.; Schreiner, P.R.; Xie, C.J.; Guo, H.; Zeng, X.Q. The near-UV absorber OSSO and its isomers. Chem. Commun. 2018, 54, 4517–4520. [Google Scholar] [CrossRef] [PubMed]
- Deng, G.H.; Li, D.Q.; Wu, Z.; Li, H.M.; Bernhardt, E.; Zeng, X.Q. Methanesulfonyl azide: Molecular structure and photolysis in solid noble gas matrices. J. Phys. Chem. A 2016, 120, 5590–5597. [Google Scholar] [CrossRef]
- Lu, Y.; Li, H.M.; Wan, H.B.; Liu, Q.; Deng, G.H.; Zeng, X.Q. Flash vacuum pyrolysis of sulfamoyl azides and chlorides: Facile gas-hhase generation of transient N-sulfonylamines. J. Anal. Appl. Pyrolysis. 2018, 134, 476–483. [Google Scholar] [CrossRef]
- Schriver-Mazzuoli, L.; Chaabouni, H.; Schriver, A. Infrared spectra of SO2 and SO2: H2O ices at low temperature. J. Mol. Struct. 2003, 644, 151–164. [Google Scholar] [CrossRef]
- Allavena, M.; Rysnik, R.; White, D.; Calder, V.; Mann, D.E. Infrared spectra and geometry of SO2 isotopes in solid krypton matrices. J. Chem. Phys. 1969, 50, 3399–3410. [Google Scholar] [CrossRef]
- Hooper, N.; Beeching, L.J.; Dyke, J.M.; Morris, A.; Ogden, J.S. A study of the thermal decomposition of 2-azidoethanol and 2-azidoethyl acetate by ultraviolet photoelectron spectroscopy and matrix isolation infrared spectroscopy. J. Phys. Chem. A 2002, 106, 9968–9975. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, Q.F.; Li, D.Q.; Feng, R.J.; Zeng, X.Q. Flash vacuum pyrolysis of methoxysulfinyl azide: Stepwise decomposition via methoxysulfinyl nitrene. J. Anal. Appl. Pyrolysis. 2017, 124, 610–617. [Google Scholar] [CrossRef]
- Zeng, X.Q.; Bernhardt, E.; Beckers, H.; Banert, K.; Hagedorn, M.; Liu, H.L. Formyl azide: Properties and solid-state structure. Angew. Chem. Int. Ed. 2013, 52, 3503–3506. [Google Scholar] [CrossRef] [PubMed]
- d’Hendecourt, L.B.; Grim, R.J.A. Time-dependent chemistry in dense molecular clouds. Astron. Astrophys. 1986, 167, 161–165. [Google Scholar]
- Nonella, M.; Huber, J.R.; Ha, T.K. Photolytic preparation and isomerization of HNSO, HOSN, HSNO, and HONS in an argon matrix. An experimental and theoretical study. J. Phys. Chem. 1987, 91, 5203–5209. [Google Scholar] [CrossRef]
- Tobón, Y.A.; Nieto, L.I.; Romano, R.M.; Della Védova, C.O.; Downs, A.J. Photochemical reaction channels of OCS with Cl2, ICl, or IBr isolated together in an argon matrix: Isolation of syn-iodocarbonylsulfenyl bromide. J. Phys. Chem. A 2006, 110, 2674–2681. [Google Scholar] [CrossRef]
- Ramos, L.A.; Zeng, X.Q.; Ulic, S.E.; Beckers, H.; Willner, H.; Della Védova, C.O. Chlorodifluoroacetyl azide, ClF2CC(O)N3: Preparation, Properties, and Decomposition. J. Org. Chem. 2012, 77, 6456–6462. [Google Scholar] [CrossRef]
- Fridgen, T.D.; Zhang, X.K.; Parnis, J.M.; March, R.E. Isomerization and fragmentation products of CH2Cl2 and other dihalomethanes in rare-gas matrices: An electron bombardment matrix-isolation FTIR spectroscopic study. J. Phys. Chem. A 2000, 104, 3487–3497. [Google Scholar] [CrossRef]
- Tsang, W. Mechanisms for the formation and destruction of chlorinated organic products of incomplete combustion. Combust. Sci. Technol. 1990, 74, 99–116. [Google Scholar] [CrossRef]
- Yang, S.X.; Hou, G.Y.; Dai, J.H.; Chang, C.H.; Chang, B.C. Spectroscopic investigation of the multiphoton photolysis reactions of bromomethanes (CHBr3, CHBr2Cl, CHBrCl2, and CH2Br2) at near-ultraviolet wavelengths. J. Phys. Chem. A 2010, 114, 4785–4790. [Google Scholar] [CrossRef] [PubMed]
- Carver, T.G.; Andrews, L. Matrix infrared spectrum and bonding in the dichloromethyl radical. J. Chem. Phys. 1969, 50, 4235–4245. [Google Scholar] [CrossRef]
- Lu, Y.; Li, H.M.; Abe, M.; Bégué, D.; Wan, H.B.; Deng, G.H.; Xu, J.; Liu, K.; Zeng, X.Q. Sulfamoyl nitrenes: Singlet or triplet ground state? Chem. Commun. 2018, 54, 6136–6139. [Google Scholar] [CrossRef] [PubMed]
- Wentrup, C. Carbenes and nitrenes: Recent developments in fundamental chemistry. Angew. Chem. Int. Ed. 2018, 57, 11508–11521. [Google Scholar] [CrossRef] [PubMed]
- Wentrup, C. Flash vacuum pyrolysis of azides, triazoles, and tetrazoles. Chem. Rev. 2017, 117, 4562–4623. [Google Scholar] [CrossRef] [PubMed]
- Wasylenko, W.A.; Kebede, N.; Showalter, B.M.; Matsunaga, N.; Miceli, A.P.; Liu, Y.; Ryzhkov, L.R.; Hadad, C.M.; Toscano, J.P. Generation of oxynitrenes and confirmation of their triplet ground states. J. Am. Chem. Soc. 2006, 128, 13142–13150. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.L.; Zhu, B.F.; Wu, Z.; Zeng, X.Q.; Beckers, H.; Jenks, W.S. Thermally persistent carbonyl nitrene: FC(O)N. J. Org. Chem. 2015, 80, 2006–2009. [Google Scholar] [CrossRef]
- Li, H.M.; Wu, Z.; Li, D.Q.; Wan, H.B.; Xu, J.; Abe, M.; Zeng, X.Q. Direct observation of methoxycarbonylnitrene. Chem. Commun. 2017, 53, 4783–4786. [Google Scholar] [CrossRef]
- Feng, R.J.; Lu, Y.; Deng, G.H.; Xu, J.; Wu, Z.; Li, H.M.; Liu, Q.; Kadowaki, N.; Abe, M.; Zeng, X.Q. Magnetically bistable nitrenes: Matrix isolation of furoylnitrenes in both singlet and triplet states and triplet 3-furylnitrene. J. Am. Chem. Soc. 2018, 140, 10–13. [Google Scholar] [CrossRef]
- Yeh, P.-S.; Leu, G.-H.; Lee, Y.-P.; Chen, I.-C. Photodissociation of HNO3 at 193 nm: Near-infrared emission of NO detected by time-resolved fourier transform spectroscopy. J. Chem. Phys. 1995, 103, 4879–4886. [Google Scholar] [CrossRef]
- Tsao, M.-L.; Hadad, C.M.; Platz, M.S. A computational study of cyclopropylnitrene. Tetrahedron Lett. 2002, 43, 745–748. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Stratmann, R.E.; Scuseria, G.E.; Frisch, M.J. An efficient implementation of time-dependent density-functional theory for the calculation of excitation energies of large molecules. J. Chem. Phys. 1998, 109, 8218–8224. [Google Scholar] [CrossRef]
- Foresman, J.B.; Head-Gordon, M.; Pople, J.A.; Frisch, M.J. Toward a systematic molecular orbital theory for excited states. J. Phys. Chem. 1992, 96, 135–149. [Google Scholar] [CrossRef]
- Fukui, K. The path of chemical reactions – The IRC approach. Acc. Chem. Res. 1981, 4, 363–368. [Google Scholar] [CrossRef]
- Hratchian, H.P.; Schlegel, H.B. Using hessian updating to increase the efficiency of a hessian based predictor-corrector reaction path following method. J. Chem. Theory Comput. 2005, 1, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian, 09; Gaussian, Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
Sample Availability: Samples of the compounds are available from the authors. |







| Calculated a | Observed b | Assignment c | ||||
|---|---|---|---|---|---|---|
| Singlet | Triplet | N2-matrix | ||||
| ν | Δν | ν | Δν | ν | Δν | |
| 3183 (4) | 0.0 | 3176 (2) | 0.0 | 3023.0 (4) | <0.5 | νasym(CH2) |
| 3098 (5) | 0.0 | 3097 (3) | 0.0 | 2953.6 (7) | <0.5 | νsym(CH2) |
| 1431 (5) | 0.0 | 1435 (4) | 0.0 | 1398.3 (3) | <0.5 | δ(CH2) |
| 1400 (164) | 2.5 | 1357 (155) | 0.0 | 1354.1 (100) | <0.5 | νasym(SO2) |
| 1268 (13) | 0.1 | 1265 (10) | 0.0 | 1238.6 (6) | <0.5 | ω(CH2) |
| 1161 (1) | 0.0 | 1158 (83) | 0.0 | 1155.5 (40) | <0.5 | νsym(SO2) |
| 1053 (67) | 9.4 | 1149 (2) | 0.0 | 1128.2 (5) | <0.5 | τ(CH2) |
| 973 (12) | 11.5 | 875 (2) | 0.0 | 870.1 (8) | <0.5 | ρ(CH2) |
| 870 (<1) | 0.1 | 778 (6) | 0.3 | 748.8 (9) | <0.5 | ν(CCl) |
| 761 (21) | 0.5 | 699 (15) | 11.2 | 718.9 (6) | 11.1 | ν(SN) |
| 686 (16) | 0.9 | 656 (26) | 0.2 | 688.1 (7) | <0.5 | ν(SC) |
| 515 (83) | 3.0 | 497 (69) | 4.0 | 500.8 (42) | <0.5 | δ(SO2) |
| Calculated a | Observed (N2-matrix) b | Assignment c | ||
|---|---|---|---|---|
| ν | Δν | ν | Δν | |
| 3166 (<1) | 0.0 | νasym (CH2) | ||
| 3096 (8) | 0.0 | νsym (CH2) | ||
| 1497 (1) | 0.3 | 1470.3/1465.3 (2) | <0.5 | δ (CH2) |
| 1394 (198) | 0.3 | 1387.0/1381.6 (57) | <0.5 | νasym (SO2) |
| 1347 (26) | 4.2 | 1326.0/1324.7 (10) | 4.4 | ω (CH2) + ν (N=S) |
| 1287 (303) | 11.6 | 1283.7/1278.0 (100) | 10.4 | ν (N=S) |
| 1241 (52) | 0.8 | 1224.2/1219.7 (14) | <0.5 | τ (CH2) |
| 1125 (33) | 15.5 | 1114.6/1113.1 (13) | 15.5 | ν (CN) + νsym (SO2) |
| 985 (1) | 2.6 | 967.7/955.9 (12) | <0.5 | ρ (CH2) |
| 854 (20) | 9.1 | 849.4/846.7 (7) | 8.8 | ν (C–N–S) |
| 712 (81) | 0.8 | 706.4/699.0 (11) | 2.3 | ν (CCl) |
| 550 (4) | 6.0 | δ (S–O–N) | ||
| 502 (53) | 2.0 | 509.3/506.7 (8) | 2.2 | δ (SO2) |
| Calculated a | Observed b | Assignment c | ||||
|---|---|---|---|---|---|---|
| Singlet | Triplet | N2-matrix | ||||
| ν | Δν | ν | Δν | ν | Δν | |
| 3167 (8) | 0.0 | 3152 (6) | 0.0 | 3027.2 (4) | <0.5 | ν(CH) |
| 1409 (139) | 2.7 | 1367 (137) | 0.0 | 1366.5 (100) | <0.5 | νasym(SO2) |
| 1225 (15) | 0.0 | 1230 (11) | 0.0 | 1196.1 (7) | <0.5 | ρ(CH) |
| 1213 (5) | 0.1 | 1206 (9) | 0.0 | 1163.7 (4) | <0.5 | ω(CH) |
| 1048 (74) | 10.0 | 1157 (93) | 0.0 | 1153.2 (32) | <0.5 | νsym(SO2) + ω(CH) |
| 978 (13) | 11.1 | 777 (67) | 0.2 | 808.4 (35) | <0.5 | νasym(CCl2) |
| 762 (100) | 0.1 | 764 (24) | 0.4 | 747.7 (16) | <0.5 | νsym(CCl2) |
| 760 (17) | 0.6 | 701 (21) | 10.7 | 718.5 (7) | 9.6 | ν(SN) |
| 681 (13) | 0.4 | 654 (10) | 0.4 | 675.8 (5) | <0.5 | ν(SC) |
| 520 (101) | 2.7 | 507 (82) | 1.3 | 512.6 (50) | <0.5 | δ(SO2) |
| Calculated a | Observed b | Assignment c | ||||
|---|---|---|---|---|---|---|
| Singlet-I | Singlet-II | N2-matrix | ||||
| ν | Δν | ν | Δν | ν | Δν | |
| 3151 (2) | 0.0 | 3175 (1) | 0.0 | ν(CH) | ||
| 1403 (190) | 0.2 | 1409 (163) | 0.0 | 1410.2/1401.7 (100) | <0.5 | νasym(SO2) |
| 1340 (7) | 1.7 | 1348 (97) | 9.1 | 1335.0/1323.8 (22) | <0.5 | ρ(CH) |
| 1299 (431) | 15.0 | 1278 (326) | 7.8 | 1280.5/1262.7 (98) | 16.0 | ν(SN) + δ(SO2) |
| 1252 (27) | 0.0 | 1243 (25) | 0.9 | 1230.2/1227.1 (7) | <0.5 | ω(CH) |
| 1129 (32) | 15.3 | 1152 (52) | 14.3 | 1125.4/1117.0 (35) | 11.8 | ν(CN) + νsym(SO2) |
| 890 (44) | 12.1 | 845 (47) | 8.1 | 846.2/834.4 (33) | 9.0 | ν(CN) |
| 758 (58) | 1.4 | 721 (201) | 1.0 | 783.9/776.9 (56) | <0.5 | νsym(CCl2) |
| 745 (169) | 0.9 | 714 (18) | 2.5 | 767.2/759.7 (40) | <0.5 | νasym(CCl2) |
| 526 (70) | 1.8 | 597 (13) | 8.5 | 532.5 (13) | 2.2 | δ(SO2) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Chu, X.; Lu, Y.; Abe, M.; Zeng, X. Chloro- and Dichloro-methylsulfonyl Nitrenes: Spectroscopic Characterization, Photoisomerization, and Thermal Decomposition. Molecules 2018, 23, 3312. https://doi.org/10.3390/molecules23123312
Yang Y, Chu X, Lu Y, Abe M, Zeng X. Chloro- and Dichloro-methylsulfonyl Nitrenes: Spectroscopic Characterization, Photoisomerization, and Thermal Decomposition. Molecules. 2018; 23(12):3312. https://doi.org/10.3390/molecules23123312
Chicago/Turabian StyleYang, Yang, Xianxu Chu, Yan Lu, Manabu Abe, and Xiaoqing Zeng. 2018. "Chloro- and Dichloro-methylsulfonyl Nitrenes: Spectroscopic Characterization, Photoisomerization, and Thermal Decomposition" Molecules 23, no. 12: 3312. https://doi.org/10.3390/molecules23123312