Spectroscopic Methods in the Evaluation of Modified Vegetable Base Oils from Crambe abyssinica
Abstract
:1. Introduction
2. Results and Discussion
2.1. Oxidative Modification of Raw Crambe Oil
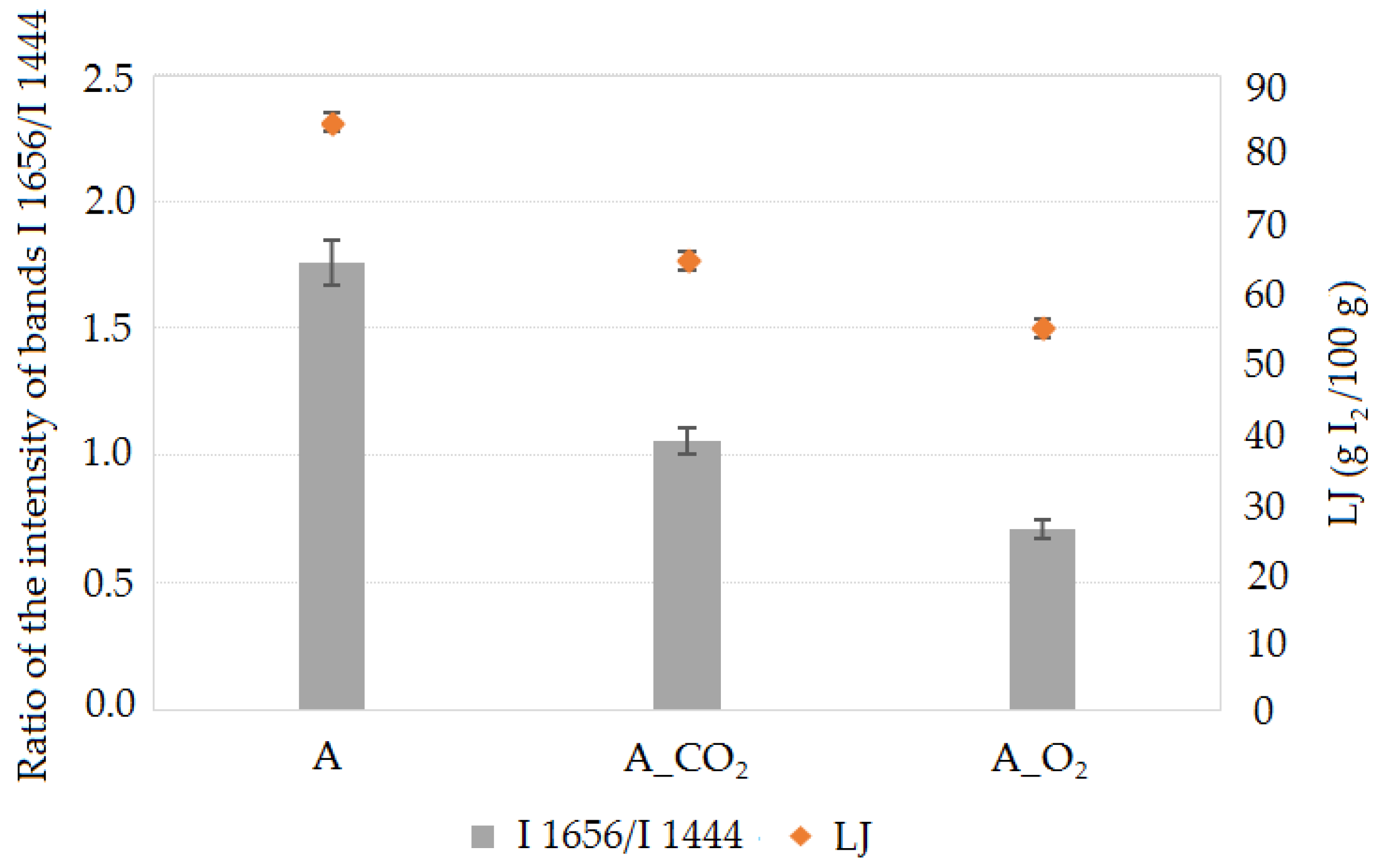
2.2. Oxidative Stability Tests of the Modified Oils
2.3. Spectroscopic Methods
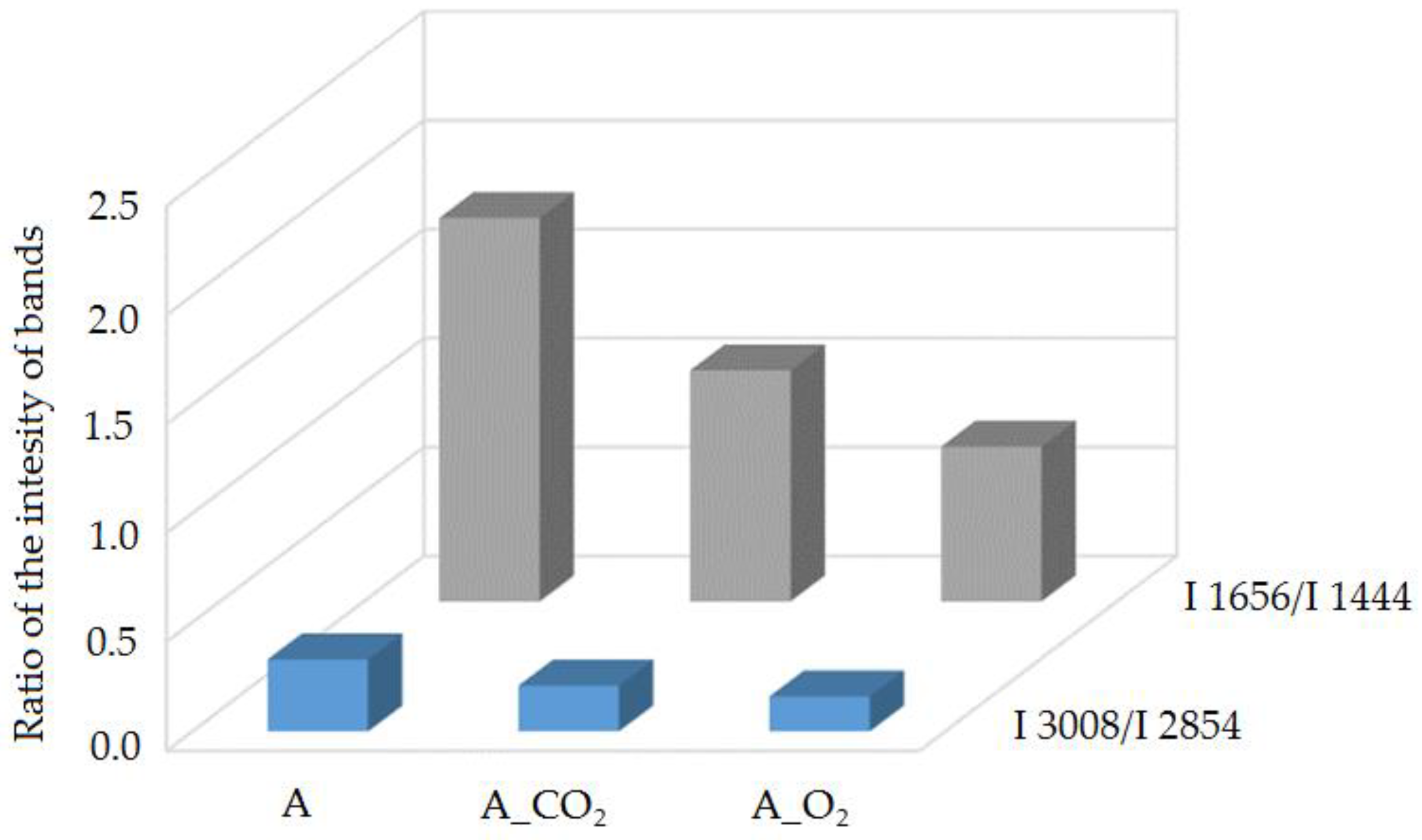
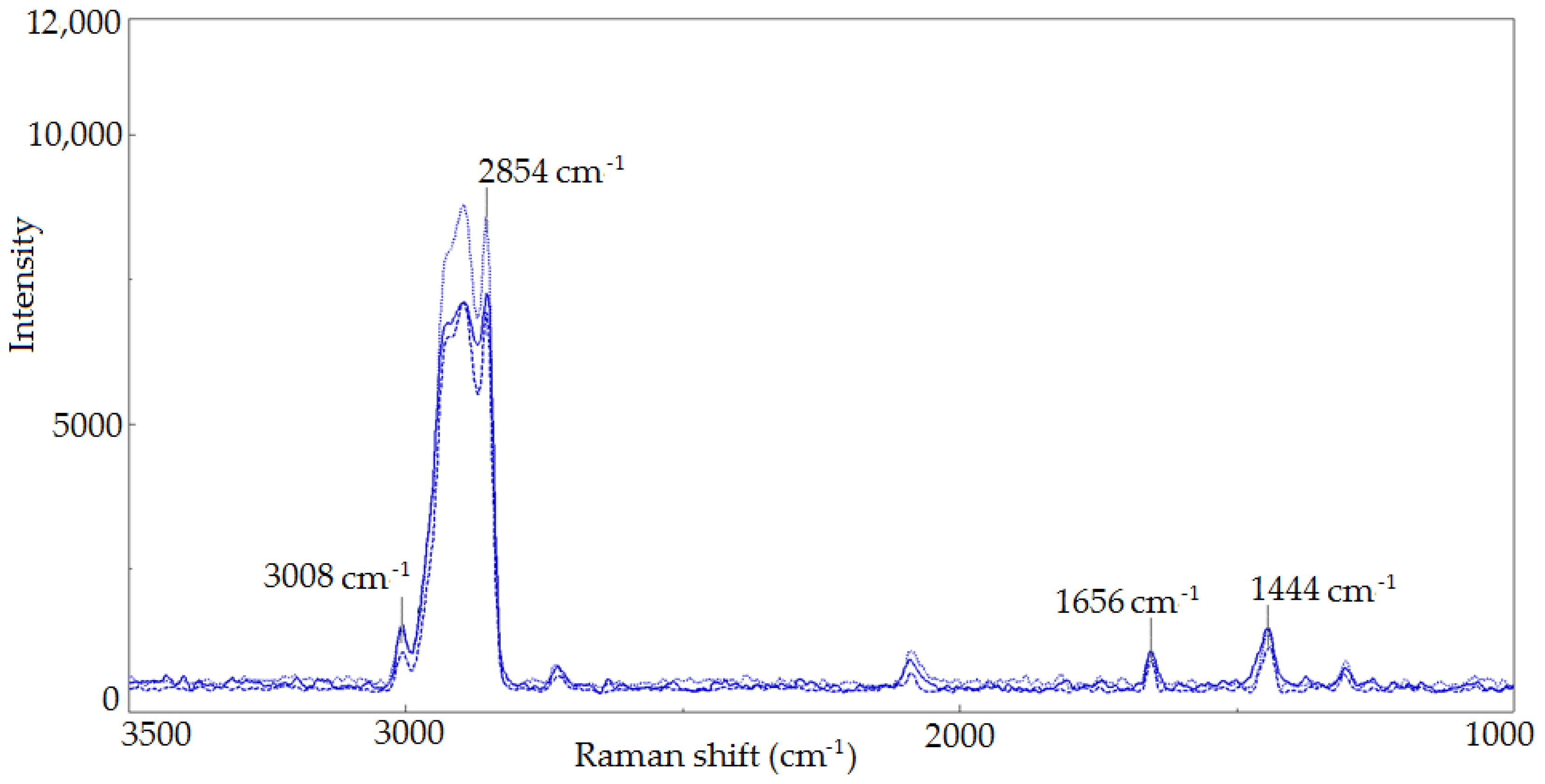
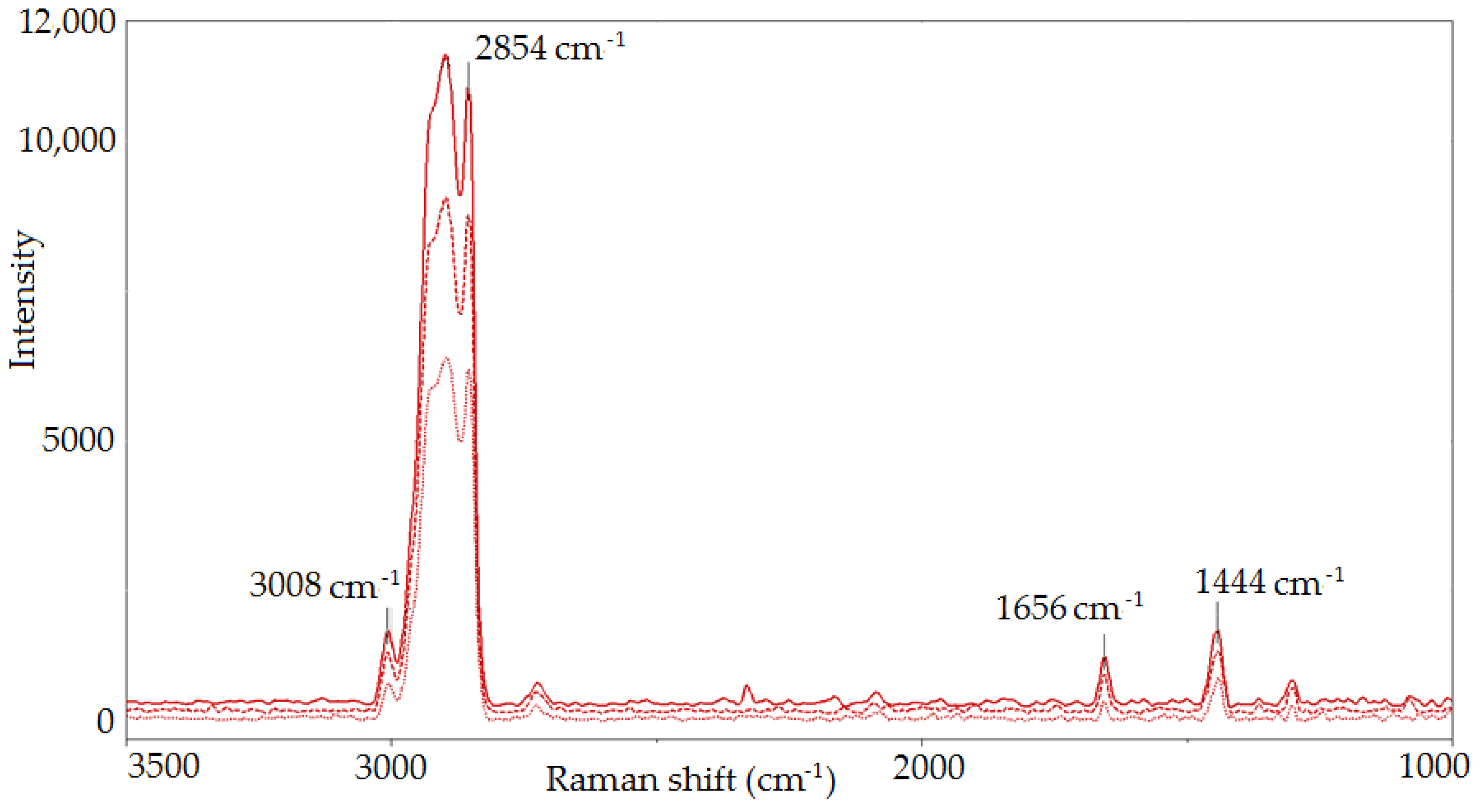
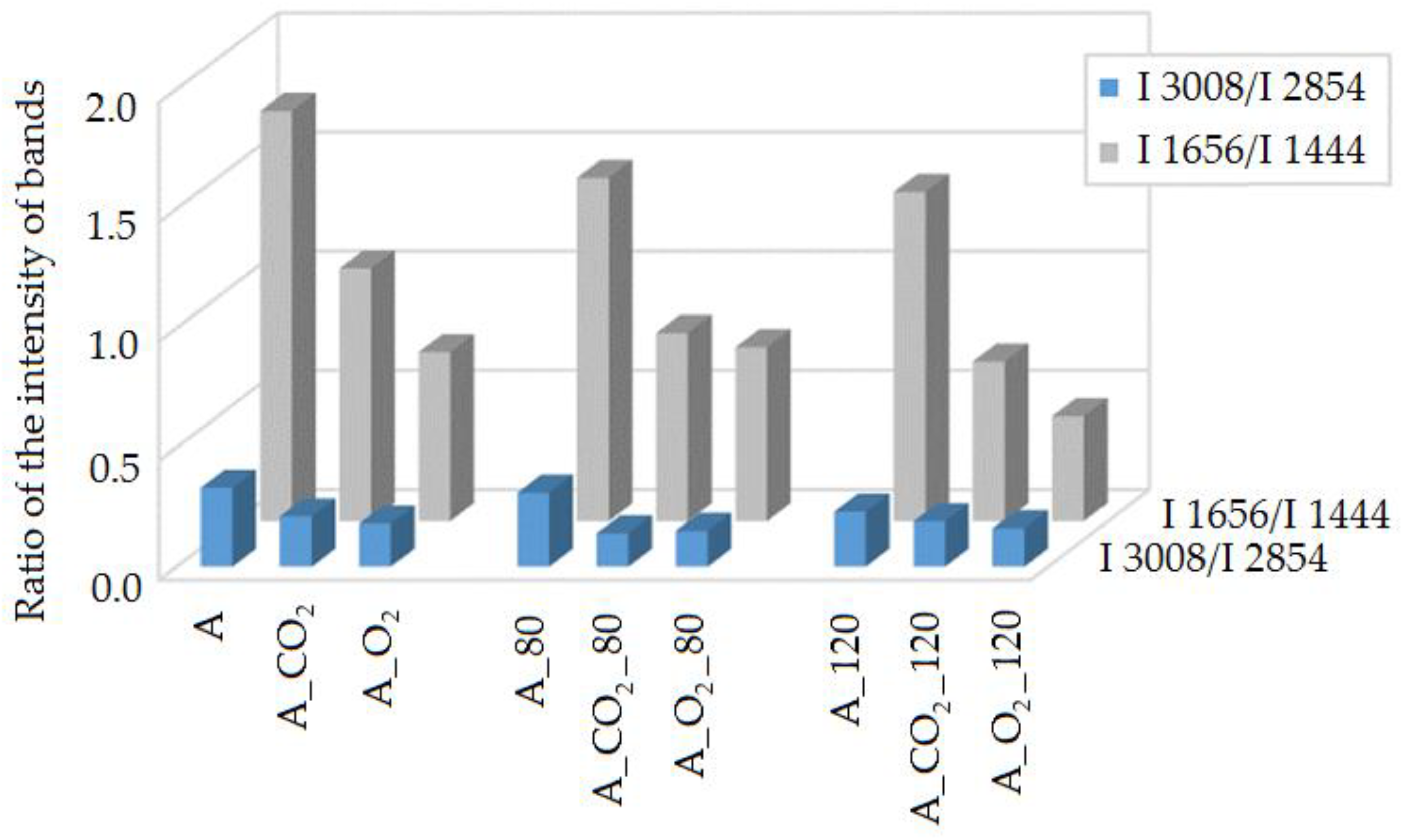
2.3.1. Raman Spectroscopy
2.3.2. UV-VIS Spectroscopy
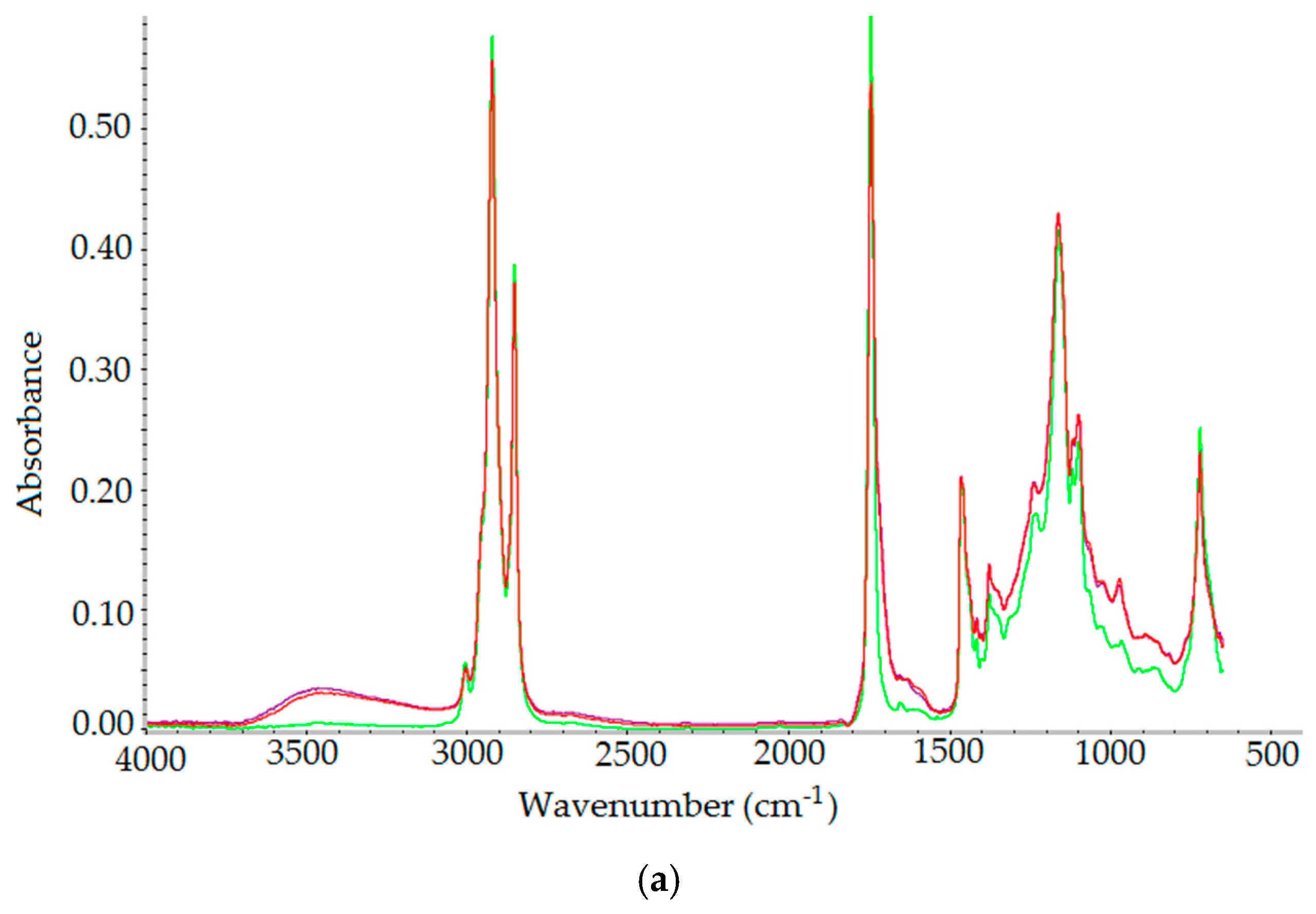
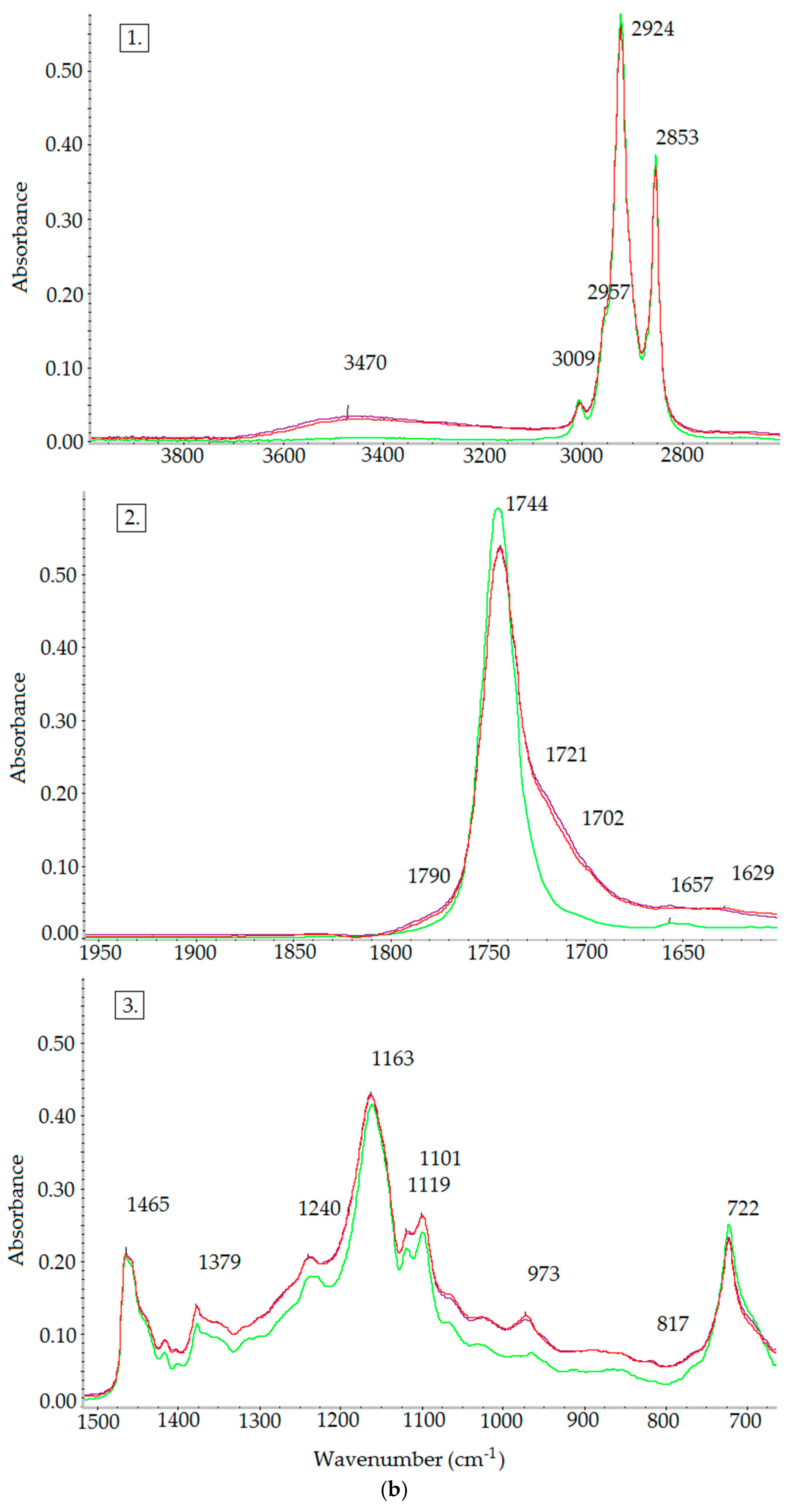
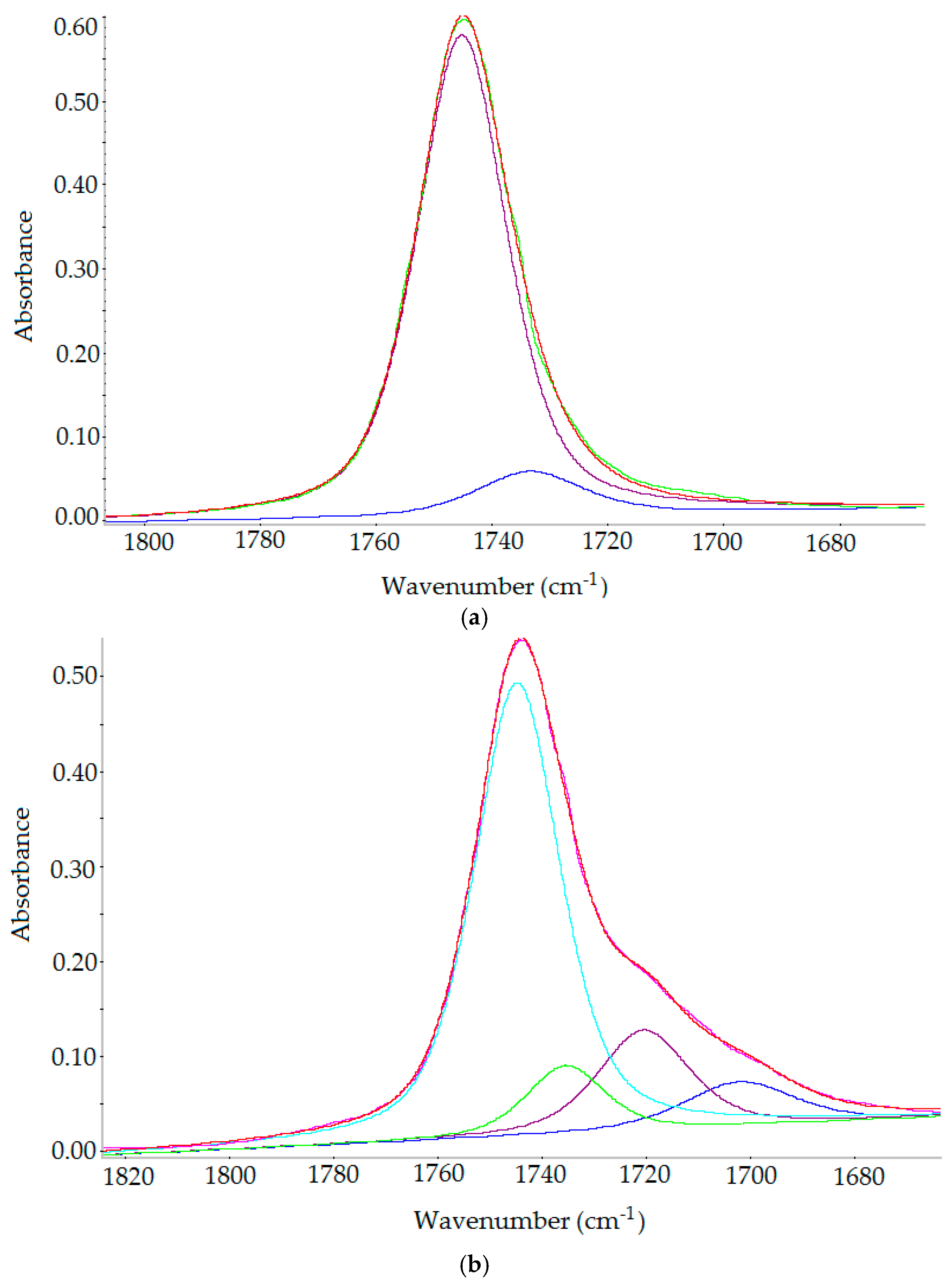
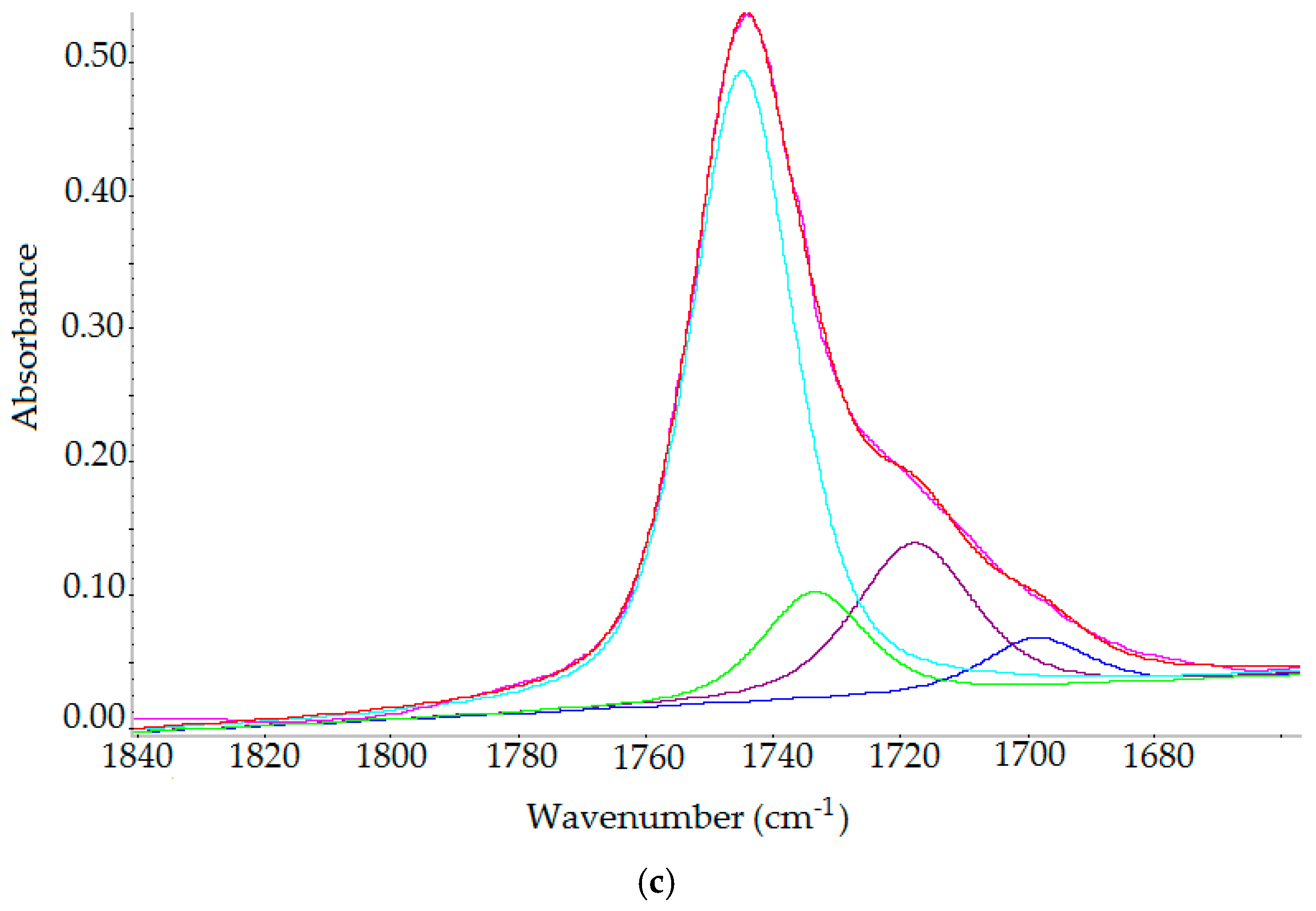
2.3.3. FT-IR Spectroscopy
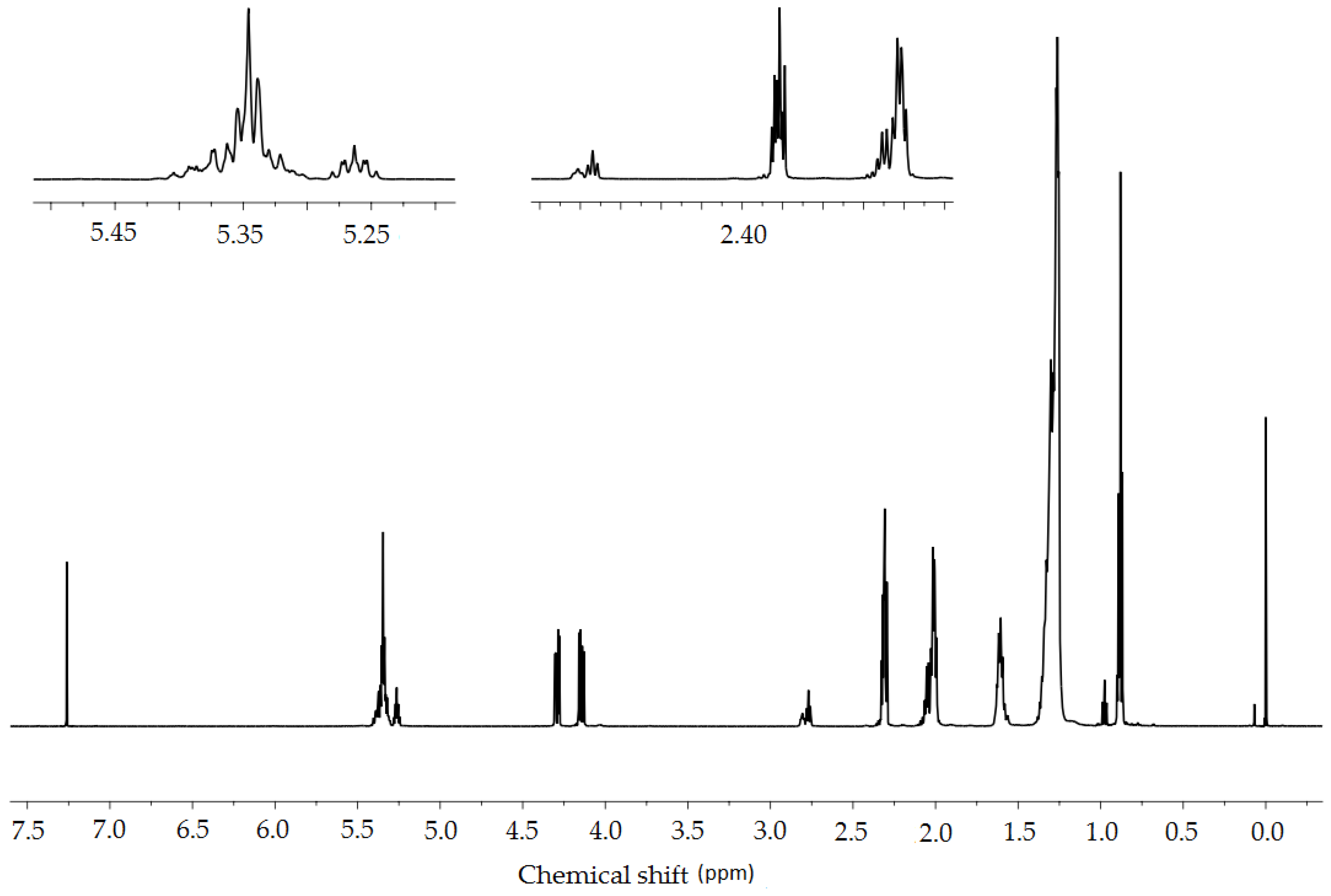
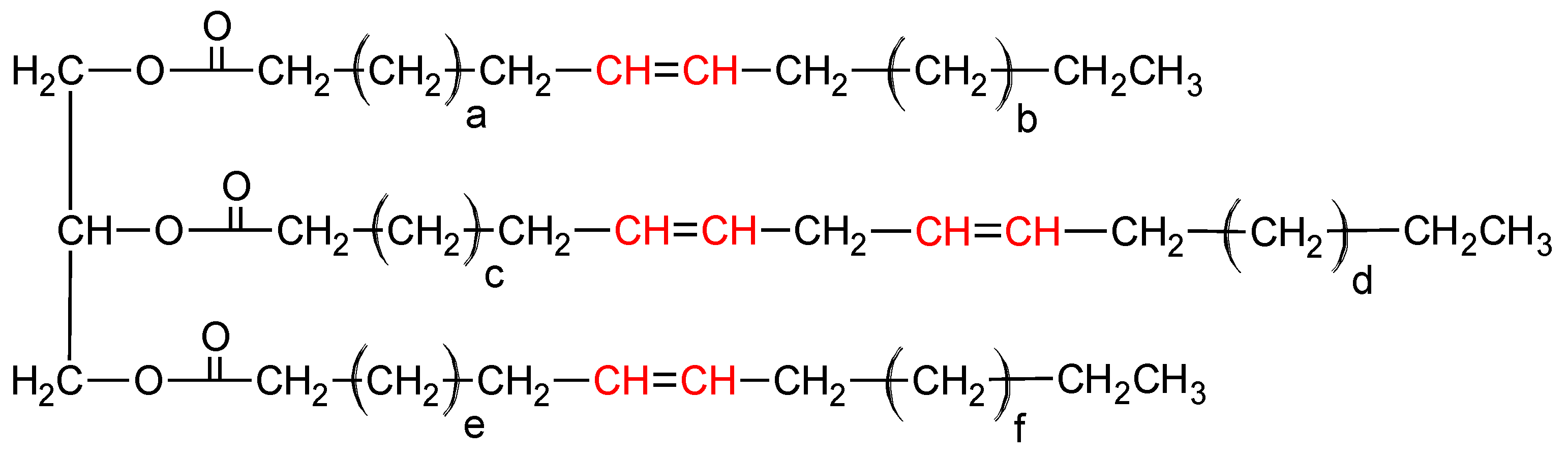
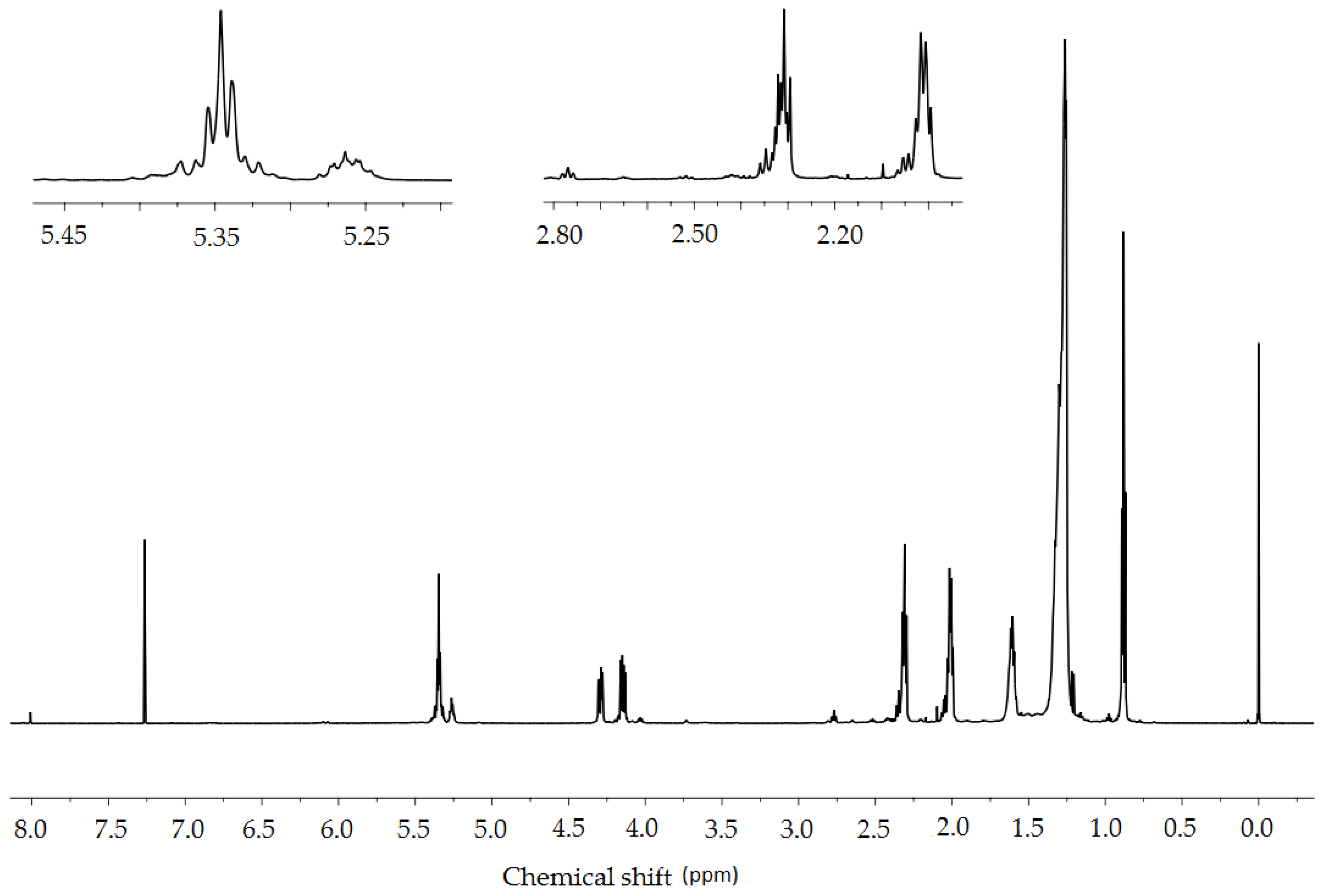
2.3.4. NMR Spectroscopy
3. Materials and Methods
3.1. Abyssinian Oil Modification
3.2. Raman Spectroscopy
3.3. UV-VIS Spectroscopy
3.4. FT-IR Specrocsopy
3.5. NMR Spectroscopy
3.6. Determination of Modified Oils Properties
4. Conclusions
5. Patents
Author Contributions
Funding
Conflicts of Interest
References
- Lankmayr, E.; Mocak, J.; Serdt, K.; Balla, B.; Wenzl, T.; Bandoniene, D.; Gfrerer, M.; Wagner, S. Chemometrical classification of pumpkin seed oils using UV-Vis, NIR and FTIR spectra. J. Biochem. Biophys. Methods 2004, 61, 95–106. [Google Scholar] [CrossRef]
- Gromadzka, J.; Wardencki, W. Trends in edible vegetable oils analysis. part b. application of different analytical techniques. Pol. J. Food Nutr. Sci. 2011, 61, 89–99. [Google Scholar] [CrossRef]
- Aluyor, E.O.; Ozigagu, C.E.; Oboh, O.I.; Aluyor, P. Chromatographic analysis of vegetable oils: A review. Sci. Res. Essays 2009, 4, 191–197. [Google Scholar]
- Nawrocka, A.; Lamorska, J. Determination of food quality by using spectroscopic methods. Adv. Agrophys. Res. 2013, 14. [Google Scholar] [CrossRef]
- Yasushi, E. Analytical methods to evaluate the quality of edible fats and oils: The JOCS standard methods for analysis of fats, oils and related materials (2013) and advanced methods. J. Oleo Sci. 2018, 67, 1–10. [Google Scholar] [CrossRef]
- Kruzlcova, D.; Mocak, J.; Katsoyannos, E.; Lankmayr, E. Classification and characterization of olive oils by UV-Vis absorption spectrometry and sensorial analysis. J. Food Nutr. Res. 2008, 47, 181–188. [Google Scholar]
- Gonçalves, R.P.; Março, P.H.; Valderrama, P. Thermal edible oil evaluation by UV–Vis spectroscopy and chemometrics. Food Chem. 2014, 163, 83–86. [Google Scholar] [CrossRef]
- Leder, P.J.S.; Porcu, O.M. The importance of UV-Vis spectroscopy: Application in food products characterization. Scholar J. Food Nutr. 2018, 1. [Google Scholar] [CrossRef]
- Vlachos, N.; Skopelitis, Y.; Psaroudaki, M.; Konstantinidou, V.; Chatzilazarou, A.; Tegou, E. Applications of fourier transform-infrared spectroscopy to edible oils. Anal. Chim. Acta 2006, 573–574, 459–465. [Google Scholar] [CrossRef]
- Alexa, E.; Dragomirescu, A.; Pop, G.; Jianu, C.; Drago, D. The use of FT-IR spectroscopy in the identification of vegetable oils adulteration. J. Food Agric. Environ. 2009, 7, 20–24. [Google Scholar]
- Nigri, S.; Oumeddour, R. Fourier transform infrared and fluorescence spectroscopy for analysis of vegetable oils. MATEC Web Conf. 2013, 5, 04028. [Google Scholar] [CrossRef] [Green Version]
- Rohman, A. Infrared spectroscopy for quantitative analysis and oil parameters of olive oil and virgin coconut oil: A review. Int. J. Food Prop. 2017, 20, 1447–1456. [Google Scholar] [CrossRef]
- Karim, M.M.; Karim, S.F.E.; Rana, A.A.; Masum, S.M.; Mondol, A.; Israt, S.S. ATR-FTIR spectroscopy and chemometric techniques for the identification of edible vegetable oils. Bangladesh J. Sci. Ind. Res. 2015, 50, 233–240. [Google Scholar] [CrossRef]
- Siddiqui, N.; Ahmad, A. Infrared spectroscopic studies on edible and medicinal oils. Int. J. Sci. Environ. Tech. 2013, 2, 1297–1306. [Google Scholar]
- Jafari, M.; Kadivar, M.; Keramat, J. Detection of adulteration in iranian olive oils using instrumental (GC, NMR, DSC) methods. J. Am. Oil Chem. Soc. 2009, 86, 103–110. [Google Scholar] [CrossRef]
- Popescu, R.; Costinel, D.; Dinca, O.R.; Marinescu, A.; Stefanescu, I.; Ionete, R.E. Discrimination of vegetable oils using NMR spectroscopy and chemometrics. Food Control. 2015, 48, 84–90. [Google Scholar] [CrossRef]
- Almoselhy, R.I.M.; Allam, M.H.; Kalyoubi, M.H.E.; Sharkawya, A.A.E. 1H-NMR spectral analysis as a new aspect to evaluate the stability of some edible oils. Ann. Agric. Sci. 2014, 59, 201–206. [Google Scholar] [CrossRef]
- Vaskova, H.; Buckova, M. Thermal degradation of vegetable oils: Spectroscopic measurement and analysis. Procedia Eng. 2015, 100, 630–635. [Google Scholar] [CrossRef]
- Jentzsch, P.V.; Ciobotă, V. Raman spectroscopy as an analytical tool for analysis of vegetable and essential oils. Flavour Fragr. J. 2014, 29, 287–295. [Google Scholar] [CrossRef]
- Alvarenga, B.R., Jr.; Xavier, F.A.N.; Soares, F.L.F.; Carneiro, R.L. Thermal stability assessment of vegetable oils by Raman spectroscopy and chemometrics. Food Anal. Methods 2018, 11, 1969–1976. [Google Scholar] [CrossRef]
- Jiménez-Sanchidrián, C.; Ruiz, J.R. Use of Raman spectroscopy for analyzing edible vegetable oils. Appl. Spectrosc. Rev. 2016, 51, 417–430. [Google Scholar] [CrossRef]
- Adhvaryu, A.; Perez, J.M.; Duda, L.J. Quantitative NMR spectroscopy for the prediction of base oil properties. J. Tribol. Trans. 2008, 43. [Google Scholar] [CrossRef]
- Adhvaryu, A.; Perez, J.M.; Singh, I.D.; Tyagi, O.S. Spectroscopic studies of oxidative degradation of base oils. Energy Fuels 1998, 12, 1369–1374. [Google Scholar] [CrossRef]
- Sarpal, A.S.; Kapur, G.S.; Mukherjee, S.; Jain, S.K. Characterization by 13C n.m.r. spectroscopy of base oils produced by different processes. Fuel 1997, 76, 931–937. [Google Scholar] [CrossRef]
- Papke, B.L.; Tutunjian, P.N. Applications of 13C-NMR to predict low temperature viscosity performance of base oils. Tribotest 2006, 12, 211–222. [Google Scholar] [CrossRef]
- Krasodomski, W.; Krasodomski, M. GC/MS application in the structural group analysis of basic lubricant oils. Part I—State of knowledge. NAFTA-GAZ 2010, 8, 711–718. [Google Scholar]
- Wooton, D.L. Applications of spectroscopy in the fuels and lubrication industry. Appl. Spectrosc. Rev. 2001, 36, 315–332. [Google Scholar] [CrossRef]
- Patty, D.J.; Lokollo, R.R. FTIR spectrum interpretation of lubricants with treatment of variation mileage. Adv. Phys. Theor. Appl. 2016, 52, 13–20. [Google Scholar]
- Pinchuk, D.; Akochi-Koblé, E.; Cocciardi, R.A.; Pinchuk, J.; van de Voort, F.R.; Sedman, J. Demystifying and Understanding Your Lubricants Using ft-ir Spectroscopic Analysis. Article of Thermal Lube company. Available online: http://thermal-lube.com/wp-content/uploads/2014/02/DEMYSTIFYING-AND-UNDERSTANDING-YOUR-LUBRICANTS.pdf (accessed on 12 October 2018).
- Drabik, J.; Iłowska, J.; Gniady, J.; Kozupa, M.; Szmatoła, M.; Semeniuk, I. Wpływ bazy olejowej i składu zagęszczacza na właściwości użytkowe smarów plastycznych. Przem. Chem. 2012, 91, 1922–1926. (In Polish) [Google Scholar]
- Drabik, J.; Iłowska, J.; Semeniuk, I.; Bereska, B. Wykorzystanie spektroskopii w podczerwieni do oceny procesu wytwarzania smarów plastycznych. Przem. Chem. 2012, 91, 1957–1962. (In Polish) [Google Scholar]
- Drabik, J.; Iłowska, J. Non-toxic lubricant for the food industry. Probl. Eksploat. 2015, 2, 5–13. [Google Scholar]
- Iłowska, J.; Chrobak, J.; Grabowski, R.; Szmatoła, M.; Woch, J.; Szwach, I.; Drabik, J.; Trzos, M.; Kozdrach, R.; Wrona, M. Designing lubricating properties of vegetable base oils. Molecules 2018, 23. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L. Crambe (Crambe abyssinica). In Industrial Oil Crops; McKeon, T.A., Hayes, D.G., et al., Eds.; Academic Press and AOCS Press: New York, NY, USA, 2016; pp. 195–205. [Google Scholar]
- Ferrie, A.M.R.; Caswell, K.L. Applications of doubled haploidy for improving industrial oilseeds. Ind. Oil Crops 2016, 359–378. [Google Scholar] [CrossRef]
- Lalas, S.; Gortzi, O.; Athanasiadis, V.; Dourtoglou, E.; Dourtoglou, V. Full characterisation of crambe abyssinica hochst. seed Oil. J. Am. Oil. Chem. Soc. 2012, 89, 2253–2258. [Google Scholar] [CrossRef]
- Mattson, F.H.; Volpenhein, R.A. The Specific Distribution of Fatty Acids in the Glycerides of Vegetable Fats. J. Biol. Chem. 1961, 236, 1891–1894. [Google Scholar] [PubMed]
- Muik, B.; Lendl, B.; Molina-Diaz, A.; Valcarcel, M.J.; Ayora-Canada, M.J. Two-dimensional correlation spectroscopy and multivariate curve resolution for the study of lipid oxidation in edible oils monitored by FTIR and FT-Raman spectroscopy. Anal. Chim. Acta 2007, 593, 54–67. [Google Scholar] [CrossRef] [PubMed]
- Brambilla, L.; Riedo, C.; Baraldi, C.; Nevin, A.; Gamberini, M.C.; D’Andrea, C.; Chiantore, O.; Goidanich, S.; Toniolo, L. Characterization of fresh and aged natural ingredients used in historical ointments by molecular spectroscopic techniques: IR, Raman and fluorescence. Anal. Bioanal. Chem. 2011, 401, 1827–1837. [Google Scholar] [CrossRef] [PubMed]
- El-Abassy, R.M.; Donfack, P.; Materny, A. Rapid determination of free fatty acid in extra virgin olive oil by Raman spectroscopy and multivariate analysis. J. Am. Oil Chem. Soc. 2009, 86, 507–511. [Google Scholar] [CrossRef]
- El-Abassy, R.M.; Donfack, P.; Materny, A. Assessment of conventional and microwave heating induced degradation of carotenoids in olive oil by VIS Raman spectroscopy and classical methods. Food Res. Int. 2010, 43, 694–700. [Google Scholar] [CrossRef]
- Muik, B.; Lendl, B.; Molina-Diaz, A.; Valcarcel, M.J.; Ayora-Canada, M.J. Direct monitoring of lipid oxidation in edible oils by Fourier transform Raman spectroscopy. Chem. Phys. Lipids 2005, 134, 173–182. [Google Scholar] [CrossRef]
- Drabik, J.; Kozdrach, R.; Wolszczak, M.; Wrona, M. The proecological base oils of highly specialized lubricants. Przem. Chem. 2018, 97, 538–541. [Google Scholar] [CrossRef]
- Raba, D.N.; Poiana, M.-A.; Borozan, A.B.; Stef, M.; Radu, F.; Popa, M.-V. Investigation on crude and high-temperature heated coffee oil by ATR-FTIR spectroscopy along with antioxidant and antimicrobial properties. PLoS ONE 2015, 10, e0138080. [Google Scholar] [CrossRef] [PubMed]
- Poiana, M.; Alexa, E.; Munteanu, M. Use of ATR-FTIR spectroscopy to detect the changes in extra virgin olive oil by adulteration with soybean oil and high temperature heat treatment. Open Chem. 2015, 13. [Google Scholar] [CrossRef] [Green Version]
- Dlugogorski, B.Z.; Kennedy, E.M.; Mackie, J.C. Low temperature oxidation of linseed oil: A review. Fire Sci. Rev. 2012, 1, 3. [Google Scholar] [CrossRef]
- Xia, W.; Budge, S.M.; Lumsden, M.D. 1H-NMR Characterization of Epoxides Derived from Polyunsaturated Fatty Acids. J. Am. Oil Chem. Soc. 2016, 93, 467–478. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |




| Oil | A | A_CO2 | A_O2 | |||
|---|---|---|---|---|---|---|
| Mean Value | Standard Deviation | Mean Value | Standard Deviation | Mean Value | Standard Deviation | |
| Kinematic viscosity at 40 °C, mm2/s | 48.20 | ±0.35 | 143.40 | ±0.54 | 156.90 | ±0.26 |
| Kinematic viscosity at 100 °C, mm2/s | 10.10 | ±0.05 | 16.10 | ±0.07 | 17.20 | ±0.08 |
| LN, meq O2/kg | 2.80 | ±0.09 | 13.10 | ±0.31 | 15.16 | ±0.54 |
| LJ, g I2/100 g | 83.48 | ±0.65 | 63.70 | ±0.57 | 54.10 | ±0.48 |
| LZ, mg KOH/g | 170.50 | ±0.76 | 248.70 | ±0.91 | 214.23 | ±0.67 |
| LK, mg KOH/g | 0.34 | ±0.04 | 28.05 | ±0.04 | 25.70 | ±0.02 |
| Oil | A | A_CO2 | A_O2 | |||
|---|---|---|---|---|---|---|
| Mean Value | Standard Deviation | Mean Value | Standard Deviation | Mean Value | Standard Deviation | |
| VG viscosity class, ISO 3448 | 46 | - | 150 | - | 150 | - |
| LJ, g I2/100 g | 83.48 | ±0.65 | 63.70 | ±0.57 | 54.10 | ±0.48 |
| Oxidation induction time at 80 °C, h | 32.80 | ±2.62 | 2.71 | ±0.22 | 2.38 | ±0.19 |
| Oxidation induction time at 120 °C, h | 2.55 | ±0.20 | 0.66 | ±0.05 | 0.67 | ±0.05 |
| Raman Shift, cm−1 | Bond | Group | Vibrational Mode |
|---|---|---|---|
| 3100–2800 | =C-H, C-H | -CH3, -CH2 | stretching |
| 1656 | C=C | cis RCH=CHR | stretching |
| 1444 | C-H | -CH2 | stretching |
| 1300 | −C-H | -CH2 | scissoring |
| 1266 | =C-H | -CH2 | twisting |
| 1087 | C-C | -(CH2)n | stretching |
| Proton | δ [ppm] | Integral | ||
|---|---|---|---|---|
| Crambe abyssinica Oil | A_O2 | A_CO2 | ||
| CH2CH3 | 0.88 | 7.08 | 7.81 | 7.75 |
| (CH2)n | 1.35–1.20 | 60.23 | 62.36 | 62.13 |
| CH2CH3 | 1.65–1.55 | 5.96 | 7.38 | 7.09 |
| CH2-CH=CH-CH2- | 2.10–1.97 | 9.37 | 6.94 | 7.21 |
| CH2COO | 2.34–2.28 | 5.34 | 6.12 | 6.08 |
| CH=CH-CH2-CH=CH | 2.83–2.75 | 1.34 | 0.35 | 0.38 |
| OCH2CH(O)CH2O | 4.32–4.12 | 3.65 | 3.83 | 3.74 |
| OCH2CH(O)CH2O | 5.28–5.20 | 0.94 | 0.93 | 0.88 |
| CH=CH | 5.43–5.28 | 5.54 | 3.42 | 3.58 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szmatoła, M.; Chrobak, J.; Grabowski, R.; Iłowska, J.; Woch, J.; Szwach, I.; Semeniuk, I.; Drabik, J.; Wrona, M.; Kozdrach, R.; et al. Spectroscopic Methods in the Evaluation of Modified Vegetable Base Oils from Crambe abyssinica. Molecules 2018, 23, 3243. https://doi.org/10.3390/molecules23123243
Szmatoła M, Chrobak J, Grabowski R, Iłowska J, Woch J, Szwach I, Semeniuk I, Drabik J, Wrona M, Kozdrach R, et al. Spectroscopic Methods in the Evaluation of Modified Vegetable Base Oils from Crambe abyssinica. Molecules. 2018; 23(12):3243. https://doi.org/10.3390/molecules23123243
Chicago/Turabian StyleSzmatoła, Michał, Justyna Chrobak, Rafał Grabowski, Jolanta Iłowska, Julia Woch, Iwona Szwach, Izabela Semeniuk, Jolanta Drabik, Małgorzata Wrona, Rafał Kozdrach, and et al. 2018. "Spectroscopic Methods in the Evaluation of Modified Vegetable Base Oils from Crambe abyssinica" Molecules 23, no. 12: 3243. https://doi.org/10.3390/molecules23123243





