Synthesis and Characterizations of Zinc Oxide on Reduced Graphene Oxide for High Performance Electrocatalytic Reduction of Oxygen
Abstract
:1. Introduction
2. Results and Discussion
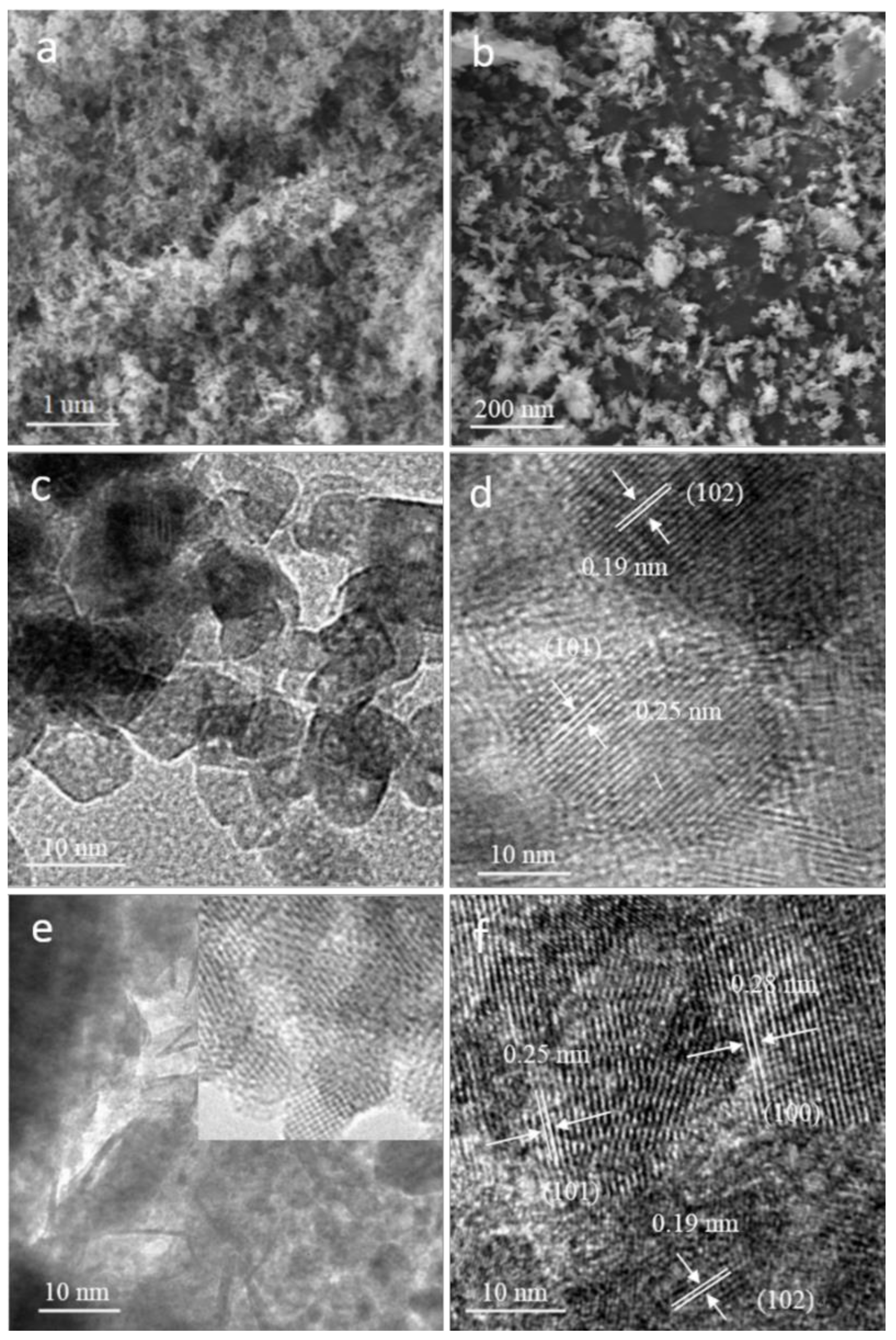
2.1. Structural Characterization
2.2. Electrocatalytic Activity towards Oxygen Reduction Reaction
3. Materials and Methods
Sample Preparation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Pendashteh, A.; Palma, J.; Anderson, M.; Marcilla, R. NiCoMnO4 nanoparticles on N-doped graphene: Highly efficient bifunctional electrocatalyst for oxygen reduction/evolution reactions. Appl. Catal. B: Environ. 2017, 201, 241–252. [Google Scholar] [CrossRef]
- Chatterjee, K.; Ashokkumar, M.; Gullapalli, H.; Gong, Y.; Vajtai, R.; Thanikaivelan, P.; Ajayan, P.M. Nitrogen-rich carbon nano-onions for oxygen reduction reaction. Carbon 2018, 130, 645–651. [Google Scholar] [CrossRef]
- Debe, M.K. Electrocatalyst approaches and challenges for automotive fuel cells. Nature 2012, 486, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Gewirth, A.A.; Thorum, M.S. Electroreduction of dioxygen for fuel-cell applications: Materials and challenges. ChemInform 2010, 49, 3557–3566. [Google Scholar] [CrossRef] [PubMed]
- Jia, Q.; Zhao, Z.; Cao, L.; Li, J.; Ghoshal, S.; Davies, V.; Stavitski, E.; Attenkofer, K.; Liu, Z.; Li, M.; et al. Roles of mo surface dopants in enhancing the ORR performance of octahedral PtNi nanoparticles. Nano Lett. 2018, 18, 798–804. [Google Scholar] [CrossRef] [PubMed]
- Geng, Z.; Wang, H.; Wang, R.; Zhang, P.; Lang, J.; Wang, C. Facile synthesis of hierarchical porous carbon for supercapacitor with enhanced electrochemical performance. Mater. Lett. 2016, 182, 1–5. [Google Scholar] [CrossRef]
- Xu, J.J.; Yang, J. Nanostructured amorphous manganese oxide cryogel as a high-rate lithium intercalation host. Electrochem. Commun. 2003, 5, 230–235. [Google Scholar] [CrossRef]
- Cheng, F.; Su, Y.; Liang, J.; Tao, Z.; Chen, J. MnO2-Based Nanostructures as Catalysts for Electrochemical Oxygen Reduction in Alkaline Media. Chem. Mater. 2010, 22, 898–905. [Google Scholar] [CrossRef]
- Feng, X.; Chen, N.; Zhang, Y.; Yan, Z.; Liu, X.; Ma, Y.; Shen, Q.; Wang, L.; Huang, W. The self-assembly of shape controlled functionalized graphene-MnO2 composites for application as supercapacitors. J. Mater. Chem. A 2014, 2, 9178–9184. [Google Scholar] [CrossRef]
- Hossen, M.M.; Artyushkova, K.; Atanassov, P.; Serov, A. Synthesis and characterization of high performing Fe-N-C catalyst for oxygen reduction reaction (ORR) in alkaline exchange membrane fuel cells. J. Power Sources 2018, 375, 214–221. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, H.; Zhou, J.; Li, Y.; Jian, W.; Regier, T.; Dai, H. Covalent hybrid of spinel manganese-cobalt oxide and graphene as advanced oxygen reduction electrocatalysts. J. Am. Chem. Soc. 2012, 134, 3517–3523. [Google Scholar] [CrossRef]
- Kannan, M.V.; Gnana Kumar, G. Current status, key challenges and its solutions in the design and development of graphene based ORR catalysts for the microbial fuel cell applications. Biosens. Bioelectron. 2016, 77, 1208–1220. [Google Scholar] [CrossRef]
- Bassetto, V.C.; Xiao, J.; Oveisi, E.; Amstutz, V.; Liu, B.; Girault, H.H.; Lesch, A. Rapid inkjet printing of high catalytic activity Co3O4/N-rGO layers for oxygen reduction reaction. Appl. Catal. A General 2018, 563, 9–17. [Google Scholar] [CrossRef]
- Li, J.; Wang, Q.; Liu, K.; Jiang, J.; Qian, D.; Li, J.; Chen, Z. An extremely facile route to Co@CoO/N-RGO/AB with an enhanced electrocatalytic activity for ORR. Mater. Lett. 2017, 186, 189–192. [Google Scholar] [CrossRef]
- Yao, T.; Guo, X.; Qin, S.; Xia, F.; Li, Q.; Li, Y.; Chen, Q.; Li, J.; He, D. Effect of rGO coating on interconnected Co3O4 nanosheets and improved supercapacitive behavior of Co3O4/rGO/NF architecture. Nano-Micro Lett. 2017, 9, 38. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Zhou, X.; Lu, Y.; Jv, R.; Liu, Y.; Li, N.; Chen, S. CoMn2O4 doped reduced graphene oxide as an effective cathodic electrocatalyst for ORR in microbial fuel cells. Electrochim. Acta 2019, 296, 214–223. [Google Scholar] [CrossRef]
- Yu, J.; Liu, Z.; Zhai, L.; Huang, T.; Han, J. Reduced graphene oxide supported TiO2 as high performance catalysts for oxygen reduction reaction. Int. J. Hydrogen Energy 2016, 41, 3436–3445. [Google Scholar] [CrossRef]
- Guo, D.; Dou, S.; Li, X.; Xu, J.; Wang, S.; Lai, L.; Liu, H.K.; Ma, J.; Dou, S.X. Hierarchical MnO2/rGO hybrid nanosheets as an efficient electrocatalyst for the oxygen reduction reaction. Int. J. Hydrogen Energy 2016, 41, 5260–5268. [Google Scholar] [CrossRef]
- Yan, Z.; Qi, H.; Bai, X.; Huang, K.; Chen, Y.-R.; Wang, Q. Mn doping of cobalt oxynitride coupled with N-rGO nanosheets hybrid as a highly efficient electrocatalyst for oxygen reduction and oxygen evolution reaction. Electrochim. Acta 2018, 283, 548–559. [Google Scholar] [CrossRef]
- Nasser, R.; Othmen, W.B.H.; Elhouichet, H.; Férid, M. Preparation, characterization of Sb-doped ZnO nanocrystals and their excellent solar light driven photocatalytic activity. Appl. Surf. Sci. 2017, 393, 486–495. [Google Scholar] [CrossRef]
- Sun, Y.; Shen, Z.; Xin, S.; Ma, L.; Xiao, C.; Ding, S.; Li, F.; Gao, G. Ultrafine Co-doped ZnO nanoparticles on reduced graphene oxide as an efficient electrocatalyst for oxygen reduction reaction. Electrochim. Acta 2017, 224, 561–570. [Google Scholar] [CrossRef]
- Yang, J.; Jia, H.; Lv, X.; Wang, Y. Facile preparation of urchin-like ZnO nanostructures and their photocatalytic performance. Ceram. Int. 2016, 42, 12409–12413. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, T.; Zhou, X.; Pei, Q.; Hao, T.; Zhang, W.; Wu, S.; Mao, H.; Song, X.-M. Facile preparation of one dimension ZnO/chalcogenide semiconductor heterostructure for efficient photoelectrochemical water splitting. J. Alloy. Compd. 2016, 685, 581–586. [Google Scholar] [CrossRef]
- Zhang, Z.; Song, X.; Chen, Y.; She, J.; Deng, S.; Xu, N.; Chen, J. Controllable preparation of 1-D and dendritic ZnO nanowires and their large area field-emission properties. J. Alloy. Compd. 2017, 690, 304–314. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Zhou, G.-Q.; Guo, J.; Liu, T.-Q. Controllable preparation of porous ZnO microspheres with a niosome soft template and their photocatalytic properties. Ceram. Int. 2016, 42, 12467–12474. [Google Scholar] [CrossRef]
- Zhou, J.; Xiao, H.; Zhou, B.; Huang, F.; Zhou, S.; Xiao, W.; Wang, D. Hierarchical MoS2-rGO nanosheets with high MoS2 loading with enhanced electro-catalytic performance. Appl. Surf. Sci. 2015, 358, 152–158. [Google Scholar] [CrossRef]
- Zuo, L.-X.; Wang, W.-J.; Song, R.-B.; Lv, J.-J.; Jiang, L.-P.; Zhu, J.-J. NaCl crystal tuning nitrogen self-doped porous graphitic carbon nanosheets for efficient oxygen reduction. ACS Sustain Chem. Eng. 2017, 5, 10275–10282. [Google Scholar] [CrossRef]
- Motaung, D.E.; Mhlongo, G.H.; Nkosi, S.S.; Malgas, G.F.; Mwakikunga, B.W.; Coetsee, E.; Swart, H.C.; Abdallah, H.M.; Moyo, T.; Ray, S.S. Shape-selective dependence of room temperature ferromagnetism induced by hierarchical ZnO nanostructures. ACS Appl. Mater. Interfaces 2014, 6, 8981–8995. [Google Scholar] [CrossRef]
- Park, S.M.; Ikegami, T.; Ebihara, K. Effects of substrate temperature on the properties of Ga-doped ZnO by pulsed laser deposition. Thin Solid Films 2006, 513, 90–94. [Google Scholar] [CrossRef]
- Chandraboss, V.L.; Kamalakkannan, J.; Prabha, S.; Senthilvelan, S. An efficient removal of methyl violet from aqueous solution by an AC-Bi/ZnO nanocomposite material. RSC Adv. 2015, 5, 479–490. [Google Scholar] [CrossRef]
- Hong, Y.; Tian, C.; Jiang, B.; Wu, A.; Zhang, Q.; Tian, G.; Fu, H. Facile synthesis of sheet-like ZnO assembly composed of small ZnO particles for highly efficient photocatalysis. J. Mater. Chem. A 2013, 1, 5700–5708. [Google Scholar] [CrossRef]
- Khandelwal, M.; Chandrasekaran, S.; Hur, S.H.; Chung, J.S. Chemically controlled in-situ growth of cobalt oxide microspheres on N,S-co-doped reduced graphene oxide as an efficient electrocatalyst for oxygen reduction reaction. J. Power Sources 2018, 407, 70–83. [Google Scholar] [CrossRef]
- Yan, Z.; Dai, C.; Lv, X.; Zhang, M.; Zhao, X.; Xie, J. Iron promoted nitrogen doped porous graphite for efficient oxygen reduction reaction in alkaline and acidic media. J. Alloy. Compd. 2019, 773, 819–827. [Google Scholar] [CrossRef]
- Luo, Q.; Yang, X.; Zhao, X.; Wang, D.; Yin, R.; Li, X.; An, J. Facile preparation of well-dispersed ZnO/cyclized polyacrylonitrile nanocomposites with highly enhanced visible-light photocatalytic activity. Appl. Catal. B Environ. 2017, 204, 304–315. [Google Scholar] [CrossRef]
- Bian, W.; Yang, Z.; Strasser, P.; Yang, R. A CoFe2O4/graphene nanohybrid as an efficient bi-functional electrocatalyst for oxygen reduction and oxygen evolution. J. Power Sources 2014, 250, 196–203. [Google Scholar] [CrossRef]
- Camacho, B.R.; Morais, C.; Valenzuela, M.A.; Alonso-Vante, N. Enhancing oxygen reduction reaction activity and stability of platinum via oxide-carbon composites. Catal. Today 2013, 202, 36–43. [Google Scholar] [CrossRef]
- Hung, T.F.; Bei, W.; Tsai, C.W.; Tu, M.H.; Wang, G.X.; Liu, R.S.; Tsai, D.P.; Lo, M.Y.; Shy, D.S.; Xing, X.K. Sulfonation of graphene nanosheet-supported platinum via a simple thermal-treatment toward its oxygen reduction activity in acid medium. Int. J. Hydrogen Energy 2012, 37, 14205–14210. [Google Scholar] [CrossRef]
- He, C.; Tao, J.; Shen, P.K. Solid synthesis of ultrathin palladium and its alloys’ nanosheets on RGO with high catalytic activity for oxygen reduction reaction. ACS Catal. 2018, 8, 910–919. [Google Scholar] [CrossRef]
- Wang, S.; Yu, D.; Dai, L. Polyelectrolyte functionalized carbon nanotubes as efficient metal-free electrocatalysts for oxygen reduction. J. Am. Chem. Soc. 2011, 133, 5182–5185. [Google Scholar] [CrossRef]
- Kakaei, K.; Hasanpour, K. Synthesis of graphene oxide nanosheets by electrochemical exfoliation of graphite in cetyltrimethylammonium bromide and its application for oxygen reduction. J. Mater. Chem. A 2014, 2, 15428–15436. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, D.; Kishore, B.; Ranganatha, S.; Munichandraiah, N.; Venkataramanan, N.S. Electrochemical investigations of Co3Fe-RGO as a bifunctional catalyst for oxygen reduction and evolution reactions in alkaline media. Appl. Surf. Sci. 2017, 418, 79–86. [Google Scholar] [CrossRef]
- Hibino, T.; Kobayashi, K.; Heo, P. Oxygen reduction reaction over nitrogen-doped graphene oxide cathodes in acid and alkaline fuel cells at intermediate temperatures. Electrochim. Acta 2013, 112, 82–89. [Google Scholar] [CrossRef]
- Ahmed, M.S.; Lee, D.W.; Kim, Y.B. Graphene supported silver nanocrystals preparation for efficient oxygen reduction in alkaline fuel cells. J. Electrochem. Soc. 2016, 163, F1169–F1176. [Google Scholar] [CrossRef]
- Wang, G.; Shen, X.; Wang, B.; Yao, J.; Park, J. Synthesis and characterisation of hydrophilic and organophilic graphene nanosheets. Carbon 2009, 47, 1359–1364. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, F. Self-assembled fabrication and flame-retardant properties of reduced graphene oxide/waterborne polyurethane nanocomposites. J. Therm. Anal. Calorim. 2014, 118, 1561–1568. [Google Scholar] [CrossRef]
Sample Availability: Samples are available from the authors. |





| Sample | ZnO | ZnO-rGO |
|---|---|---|
| i0 (mA/cm2) | 6.67 × 10−7 | 9.21 × 10−5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J.; Huang, T.; Jiang, Z.; Sun, M.; Tang, C. Synthesis and Characterizations of Zinc Oxide on Reduced Graphene Oxide for High Performance Electrocatalytic Reduction of Oxygen. Molecules 2018, 23, 3227. https://doi.org/10.3390/molecules23123227
Yu J, Huang T, Jiang Z, Sun M, Tang C. Synthesis and Characterizations of Zinc Oxide on Reduced Graphene Oxide for High Performance Electrocatalytic Reduction of Oxygen. Molecules. 2018; 23(12):3227. https://doi.org/10.3390/molecules23123227
Chicago/Turabian StyleYu, Jiemei, Taizhong Huang, Zhankun Jiang, Min Sun, and Chengchun Tang. 2018. "Synthesis and Characterizations of Zinc Oxide on Reduced Graphene Oxide for High Performance Electrocatalytic Reduction of Oxygen" Molecules 23, no. 12: 3227. https://doi.org/10.3390/molecules23123227






