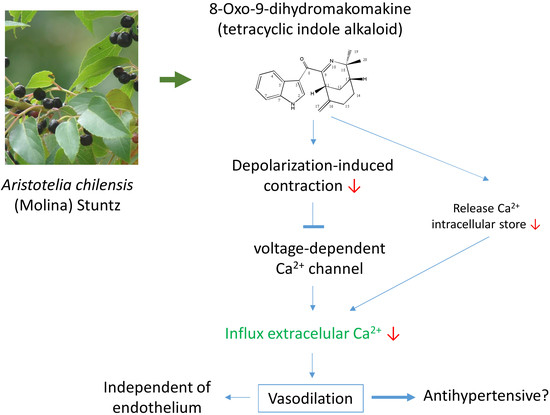
8-Oxo-9-Dihydromakomakine Isolated from Aristotelia chilensis Induces Vasodilation in Rat Aorta: Role of the Extracellular Calcium Influx
Abstract
:1. Introduction
2. Results
2.1. 8-Oxo-9-Dihydromakomakine Induced Vasodilation in Rat Aorta
2.2. 8-Oxo-9-Dihydromakomakine Reduced the Contractile Response to KCl and PE
2.3. Role of Potassium Channels in the Vascular Response to 8-Oxo-9-Dihydromakomakine
2.4. Role of Na,K-ATPase in the Vascular Response to 8-Oxo-9-Dihydromakomakine
2.5. Role of Extracellular Calcium in the Vascular Response to 8-Oxo-9-Dihydromakomakine
2.6. Chemical Characterization of 8-Oxo-9-Dihydromakomakine
3. Discussion
4. Materials and Methods
4.1. Reagents and Chemicals
4.2. Plant Material
4.3. Apparatus
4.4. Isolation of 8-Oxo-9-Dihydromakomakine from Aristotelia chilensis
4.5. Animals
4.6. Isolation of Aortic Rings
4.7. Vascular Reactivity Experiments
4.8. Assessment of the Effects of 8-Oxo-9-Dihydromakomakine on the Vasodilation in Isolated Aortic Rings Pre-Contracted with PE, with and without Endothelium
4.9. Assessment of the Effects of 8-Oxo-9-Dihydromakomakine on Endothelial Nitric Oxide Synthase (eNOS)
4.10. Effect Calcium Dependence Extracellular Calcium Ionic and Effect of Barium chloride, Tetraethylammonium (TEA), and Bay K6844
4.11. Effect Accumulative KCl and Phenylephrine Modulated by 8-Oxo-9-Dihydromakomakine
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cespedes, C.L.; Balbontin, C.; Avila, J.G.; Dominguez, M.; Alarcon, J.; Paz, C.; Burgos, V.; Ortiz, L.; Penaloza-Castro, I.; Seigler, D.S.; et al. Inhibition on cholinesterase and tyrosinase by alkaloids and phenolics from Aristotelia chilensis leaves. Food Chem. Toxicol. 2017, 109, 984–995. [Google Scholar] [CrossRef] [PubMed]
- Cespedes, C.L.; El-Hafidi, M.; Pavon, N.; Alarcon, J. Antioxidant and cardioprotective activities of phenolic extracts from fruits of Chilean blackberry Aristotelia chilensis (Elaeocarpaceae), Maqui. Food Chem. 2008, 107, 820–829. [Google Scholar] [CrossRef]
- Fredes, C.; Becerra, C.; Parada, J.; Robert, P. The microencapsulation of Maqui (Aristotelia chilensis (Mol.) Stuntz) juice by spray-drying and freeze-drying produces powders with similar anthocyanin stability and bioaccessibility. Molecules 2018, 23, 15. [Google Scholar] [CrossRef] [PubMed]
- Halcox, J.P.; Quyyumi, A.A. Coronary vascular endothelial function and myocardial ischemia: Why should we worry about endothelial dysfunction? Coron. Artery Dis. 2001, 12, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Paz, C.; Becerra, J.; Silva, M.; Cabrera-Pardo, J.; Burgos, V.; Heydenreich, M.; Schmidt, B. (-)-8-Oxohobartine a new indole alkaloid from Aristotelia chilensis (Mol.) Stuntz. Rec. Nat. Prod. 2016, 10, 68–73. [Google Scholar]
- Paz, C.; Becerra, J.; Silva, M.; Freire, E.; Baggio, R. A polymorphic form of 4,4-dimethyl-8-methylene-3-azabicyclo 3.3.1 non-2-en-2-yl 3-indolyl ketone, an indole alkaloid extracted from Aristotelia chilensis (maqui). Acta Crystallogr. C Struct. Chem. 2013, 69, 1509. [Google Scholar] [CrossRef] [PubMed]
- Fusi, F.; Spiga, O.; Trezza, A.; Sgaragli, G.; Saponara, S. The surge of flavonoids as novel, fine regulators of cardiovascular Ca. Eur. J. Pharmacol. 2017, 796, 158–174. [Google Scholar] [CrossRef] [PubMed]
- Luna-Vazquez, F.J.; Ibarra-Alvarado, C.; Rojas-Molina, A.; Rojas-Molina, I.; Zavala-Sanchez, M.A. Vasodilator compounds derived from plants and their mechanisms of action. Molecules 2013, 18, 5814–5857. [Google Scholar] [CrossRef] [PubMed]
- Godfraind, T.; Miller, R.; Wibo, M. Calcium antagonism and calcium entry blockade. Pharmacol. Rev. 1986, 38, 321–416. [Google Scholar] [PubMed]
- Cifuentes, F.; Palacios, J.; Nwokocha, C.R. Synchronization in the Heart Rate and the Vasomotion in Rat Aorta: Effect of Arsenic Trioxide. Cardiovasc. Toxicol. 2016, 16, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Palacios, J.; Vega, J.L.; Paredes, A.; Cifuentes, F. Effect of phenylephrine and endothelium on vasomotion in rat aorta involves potassium uptake. J. Physiol. Sci. 2013, 63, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Palacios, J.; Marusic, E.T.; Lopez, N.C.; Gonzalez, M.; Michea, L. Estradiol-induced expression of N(+)-K(+)-ATPase catalytic isoforms in rat arteries: Gender differences in activity mediated by nitric oxide donors. Am. J. Physiol. Heart Circ. Physiol. 2004, 286, H1793–H1800. [Google Scholar] [CrossRef] [PubMed]
- Cicala, C.; Morello, S.; Iorio, C.; Capasso, R.; Borrelli, F.; Mascolo, N. Vascular effects of caffeic acid phenethyl ester (CAPE) on isolated rat thoracic aorta. Life Sci. 2003, 73, 73–80. [Google Scholar] [CrossRef]
- Karaki, H.; Weiss, G.B. Calcium release in smooth muscle. Life Sci. 1988, 42, 111–122. [Google Scholar] [CrossRef]
- Lee, C.H.; Poburko, D.; Sahota, P.; Sandhu, J.; Ruehlmann, D.O.; van Breemen, C. The mechanism of phenylephrine-mediated [Ca2+]i oscillations underlying tonic contraction in the rabbit inferior vena cava. J. Physiol. 2001, 534, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Missiaen, L.; Parys, J.B.; De Smedt, H.; Himpens, B.; Casteels, R. Inhibition of inositol trisphosphate-induced calcium release by caffeine is prevented by ATP. Biochem. J. 1994, 300, 81–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, C.; Dacquet, C.; Mironneau, C.; Mironneau, J. Caffeine-induced inhibition of calcium channel current in cultured smooth cells from pregnant rat myometrium. Br. J. Pharmacol. 1989, 98, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Echeverri, D.; Montes, F.R.; Cabrera, M.; Galán, A.; Prieto, A. Caffeine’s vascular mechanisms of action. Int. J. Vasc. Med. 2010, 2010, 834060. [Google Scholar] [CrossRef] [PubMed]
- Iturriaga-Vásquez, P.; Miquel, R.; Ivorra, M.D.; D’Ocon, M.P.; Cassels, B.K. Simplified tetrandrine congeners as possible antihypertensive agents with a dual mechanism of action. J. Nat. Prod. 2003, 66, 954–957. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, M.R.; Achike, F.I. Dicentrine is preferentially antagonistic to rat aortic than splenic alpha 1-adrenoceptor stimulation. Acta Pharmacol. Sin. 2000, 21, 1165–1168. [Google Scholar] [PubMed]
- Wang, K.; Zhou, X.Y.; Wang, Y.Y.; Li, M.M.; Li, Y.S.; Peng, L.Y.; Cheng, X.; Li, Y.; Wang, Y.P.; Zhao, Q.S. Macrophyllionium and macrophyllines A. and B., oxindole alkaloids from Uncaria macrophylla. J. Nat. Prod. 2011, 74, 12–15. [Google Scholar] [CrossRef] [PubMed]
- Orallo, F.; Alzueta, A.F. Preliminary study of the vasorelaxant effects of (+)-nantenine, an alkaloid isolated from Platycapnos spicata, in rat aorta. Planta Med. 2001, 67, 800–806. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Choi, B.K.; Choi, J.Y.; Ryu, T.; Roh, W.S.; Song, S.Y. Role of calcium channels responsible for phenylephrine-induced contraction in rat aorta 3 days after acute myocardial infarction. Korean J. Anesthesiol. 2014, 66, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Maione, F.; Cicala, C.; Musciacco, G.; De Feo, V.; Amat, A.G.; Ialenti, A.; Mascolo, N. Phenols, Alkaloids and terpenes from medicinal plants with antihypertensive and vasorelaxant activities. A review of natural products as leads to potential therapeutic agents. Nat. Prod. Commun. 2013, 8, 539–544. [Google Scholar] [PubMed]
- Ok, S.H.; Byon, H.J.; Kwon, S.C.; Park, J.; Lee, Y.; Hwang, Y.; Baik, J.; Choi, M.J.; Sohn, J.T. Lipid emulsion inhibits vasodilation induced by a toxic dose of bupivacaine via attenuated dephosphorylation of myosin phosphatase target subunit 1 in isolated rat aorta. Int. J. Med. Sci. 2015, 12, 958–967. [Google Scholar] [CrossRef] [PubMed]
- Rameshrad, M.; Babaei, H.; Azarmi, Y.; Fouladia, D.F. Rat aorta as a pharmacological tool for in vitro and in vivo studies. Life Sci. 2016, 145, 190–204. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of 8-oxo-9-dihydromakomakine is available from the corresponding author. |






© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cifuentes, F.; Palacios, J.; Paredes, A.; Nwokocha, C.R.; Paz, C. 8-Oxo-9-Dihydromakomakine Isolated from Aristotelia chilensis Induces Vasodilation in Rat Aorta: Role of the Extracellular Calcium Influx. Molecules 2018, 23, 3050. https://doi.org/10.3390/molecules23113050
Cifuentes F, Palacios J, Paredes A, Nwokocha CR, Paz C. 8-Oxo-9-Dihydromakomakine Isolated from Aristotelia chilensis Induces Vasodilation in Rat Aorta: Role of the Extracellular Calcium Influx. Molecules. 2018; 23(11):3050. https://doi.org/10.3390/molecules23113050
Chicago/Turabian StyleCifuentes, Fredi, Javier Palacios, Adrián Paredes, Chukwuemeka R. Nwokocha, and Cristian Paz. 2018. "8-Oxo-9-Dihydromakomakine Isolated from Aristotelia chilensis Induces Vasodilation in Rat Aorta: Role of the Extracellular Calcium Influx" Molecules 23, no. 11: 3050. https://doi.org/10.3390/molecules23113050