Synthetic Methodologies to Gold Nanoshells: An Overview
Abstract
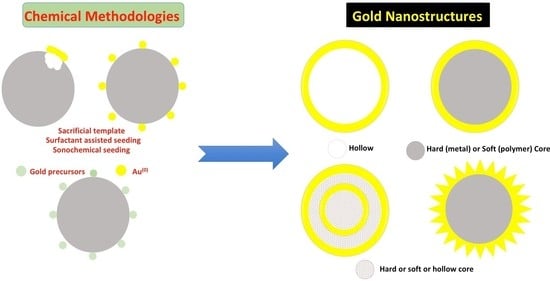
:1. Gold Nanoshells: Introduction
2. Gold Nanoshells with a Core or Hollow: Synthesis
2.1. Gold Nanoshells on SiO2 Core
(i) Surfactant Assisted Seeding Method
(ii) Single Sep Deposition-Precipitation (DP) Seeding Method
(iii) Sonochemical Gold Seeding Method
(iv) Sandwiched Gold Seeded Shell Synthesis
(v) One Pot Synthesis
Gold Nanoshells on a Polymer Core
(i) Solvent Assisted Approach
(ii) Combined Swelling-Heteroaggregation
(iii) Gold Colloid Seeding
(iv) Gold Ion Seeding Method
2.2. Hollow Gold Nanoshells
2.2.1. Sacrificial Template Method
(i) Effect of Growth Parameters on Hollow Gold Nanoshells Surface Plasmon Resonance Properties
2.2.2. Template Galvanic Replacement of Silver Method
2.2.3. Electrochemical Synthesis of Hollow Gold Nanoshells
2.2.4. Nonmetallic Structures for Hollow Gold Nanoshell Synthesis
(i) Emulsion Droplets
(ii) Biomaterial Templates
3. Other Types of Gold Nanoshells
3.1. Multilayer Gold Nanoshells
Synthesis of Multilayer Gold Nanoshells
3.2. Anisotropic and Spiky Gold Nanoshells
Synthesis of Anisotropic and Spiky Gold Nanoshells
4. Gold Nanoshell Stabilization
Gold Nanoshell Electrostatic and Steric Stabilization
Gold Nanoshell Stabilization: Surface Modification and Bioconjugation
5. Characterization of Gold Nanoshells
5.1. Imaging Techniques: Transmission Electron Microscopy (TEM) and Scanning Electron Microscopy
5.2. Dynamic Light Scattering (DLS)
5.3. Zeta Potential (ς)
5.4. Energy-Dispersive X-ray Spectroscopy (EDX), X-ray Diffraction (XRD) and X-ray Photoelectron Spectroscopy (XPS)
5.5. Thermogravimetric Analysis (TGA)
5.6. UV-Vis Spectroscopy (UV-Vis)
6. Conclusions
Funding
Conflicts of Interest
References
- Faraday, M.X. The Bakerian Lecture—Experimental relations of gold (and other metals) to light. Philos. Trans. R. Soc. Lond. 1857, 147, 145–181. [Google Scholar] [CrossRef]
- Mie, G. Beiträge zur Optik trüber Medien, speziell kolloidaler Metallösungen. Annalen der Physik 1908, 330, 377–445. [Google Scholar] [CrossRef] [Green Version]
- Petryayeva, E.; Krull, U.J. Localized surface plasmon resonance: Nanostructures, bioassays and biosensing—A review. Anal. Chim. Acta 2011, 706, 8–24. [Google Scholar] [CrossRef] [PubMed]
- Willets, K.A.; Duyne, R.P.V. Localized surface plasmon resonance spectroscopy and sensing. Annu. Rev. Phys. Chem. 2007, 58, 267–297. [Google Scholar] [CrossRef] [PubMed]
- Kelly, K.L.; Coronado, E.; Zhao, L.L.; Schatz, G.C. The optical properties of metal nanoparticles: The influence of size, shape, and dielectric environment. J. Phys. Chem. B 2003, 107, 668–677. [Google Scholar] [CrossRef]
- Lee, K.-S.; El-Sayed, M.A. Gold and silver nanoparticles in densing and imaging: Sensitivity of plasmon response to size, shape, and metal composition. J. Phys. Chem. B 2006, 110, 19220–19225. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.K.; Nath, S.; Kundu, S.; Esumi, K.; Pal, T. Solvent and ligand effects on the localized surface plasmon resonance (LSPR) of gold volloids. J. Phys. Chem. B 2004, 108, 13963–13971. [Google Scholar] [CrossRef]
- Underwood, S.; Mulvaney, P. Effect of the solution refractive index on the color of gold colloids. Langmuir 1994, 10, 3427–3430. [Google Scholar] [CrossRef]
- Mock, J.J.; Barbic, M.; Smith, D.R.; Schultz, D.A.; Schultz, S. Shape effects in plasmon resonance of individual colloidal silver nanoparticles. J. Phys. Chem. B 2002, 116, 6755–6759. [Google Scholar] [CrossRef]
- Halas, N. Playing with plasmons: Tuning the optical resonant properties of metallic nanoshells. MRS Bull. 2005, 30, 362–367. [Google Scholar] [CrossRef]
- Miller, M.M.; Lazarides, A.A. Sensitivity of metal nanoparticle surface plasmon resonance to the dielectric environment. J. Phys. Chem. B 2005, 109, 21556–21565. [Google Scholar] [CrossRef] [PubMed]
- Ng, V.W.; Berti, R.; Lesage, F.; Kakkar, A. Gold: A versatile tool for in vivo imaging. J. Mater. Chem. B 2013, 1, 9–25. [Google Scholar] [CrossRef]
- Agarwal, A.; Huang, S.W.; O’Donnell, M.; Day, K.C.; Day, M.; Kotov, N.; Ashkenazi, S. Targeted gold nanorod contrast agent for prostate cancer detection by photoacoustic imaging. J. Appl. Phys. 2007, 102, 064701. [Google Scholar] [CrossRef]
- Yang, X.; Skrabalak, S.E.; Li, Z.-Y.; Xia, Y.; Wang, L.V. Photoacoustic tomography of a rat cerebral cortex in vivo with Au nanocages as an optical contrast agent. Nano Lett. 2007, 7, 3798–3802. [Google Scholar] [CrossRef] [PubMed]
- Skrabalak, S.E.; Chen, J.; Sun, Y.; Lu, X.; Au, L.; Cobley, C.M.; Xia, Y. Gold nanocages: Synthesis, properties, and applications. Acc. Chem. Res. 2008, 41, 1587–1595. [Google Scholar] [CrossRef] [PubMed]
- Rouleau, L.; Berti, R.; Ng, V.W.K.; Matteau-Pelletier, C.; Lam, T.; Saboural, P.; Kakkar, A.; Lesage, F.; Rhéaume, E.; Tardif, J.-C. VCAM-1 targeting gold nanoshell probe for photoacoustiv imaging of atherosclerotic plaque in mice. Contrast Media Mol. Imaging 2013, 8, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Cho, E.C.; Chen, J.; Song, K.H.; Au, L.; Favazza, C.; Zhang, Q.; Cobley, C.M.; Gao, F.; Xia, Y.; et al. In vivo molecular photoacoustic tomography of melanomas targeted by bioconjugated gold nanocages. ACS Nano 2010, 4, 4559–4564. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Huang, Q.; Ku, G.; Wen, X.; Zhou, M.; Guzatov, D.; Brecht, P.; Su, R.; Oraevsky, A.; Wang, L.V.; et al. Photoacoustic imaging of living mouse brain vasculature using hollow gold nanospheres. Biomaterials 2010, 31, 2617–2626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Neal, D.P.; Hirsch, L.R.; Halas, N.J.; Payne, J.D.; West, J.L. Photo-thermal tumor ablation in mice using near infrared-absorbing nanoparticles. Cancer Lett. 2004, 209, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Melancon, M.P.; Lu, W.; Yang, Z.; Zhang, R.; Cheng, Z.; Elliot, A.M.; Stafford, J.; Olson, T.; Zhang, J.Z.; Li, C. In vitro and in vivo targeting of hollow gold nanoshells directed at epidermal growth factor receptors for photothermal ablation therapy. Mol. Cancer Ther. 2008, 7, 1730–1739. [Google Scholar] [CrossRef] [PubMed]
- Melancon, M.P.; Elliott, A.; Ji, X.; Shetty, A.; Yang, Z.; Tian, M.; Taylor, B.; Stafford, R.J.; Li, C. Theranostics with multifunctional magnetic gold nanoshells: Photothermal therapy and T2* magnetic resonance imaging. Investig. Radiol. 2011, 46, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Vankayala, R.; Lin, C.-C.; Kalluru, P.; Chiang, C.-S.; Hwang, K.C. Gold nanoshells-mediated bimodal photodynamic and photothermal cancer treatment using ultra-low doses of near infra-red light. Biomaterials 2014, 35, 5527–5538. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Sanz-Rodríguez, F.; Rivero, F.; Rodríguez, E.M.; Torres, R.A.; Ortgies, D.H.; Solé, J.G.; Alfonso, F.; Jaque, D. Gold nanoshells: Contrast agents for cell imaging by cardiovascular optical coherence tomography. Nano Res. 2018, 11, 676–685. [Google Scholar] [CrossRef]
- Adler, D.C.; Huang, S.-W.; Huber, R.; Fujimoto, J.G. Photothermal detection of gold nanoparticles using phase-sensitive optical coherence tomography. Opt. Express 2008, 16, 4376–4393. [Google Scholar] [CrossRef] [PubMed]
- Loo, C.; Lin, A.; Hirsch, L.; Lee, M.-H.; Barton, J.; Halas, N.; West, J.; Drezek, R. Nanoshell-enabled photonics-based imaging and therapy of cancer. Technol. Cancer Res. Treat. 2004, 3, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Hauck, T.S.; Chan, W.C. Gold nanoshells in cancer imaging and therapy: Towards clinical application. Nanomedicine 2007, 2, 735–738. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Xia, Y. Increased sensitivity of surface plasmon resonance of gold nanoshells compared to that of gold solid colloids in response to environmental changes. Anal. Chem. 2002, 74, 5297–5305. [Google Scholar] [CrossRef] [PubMed]
- Schwartzberg, A.M.; Olson, T.Y.; Talley, C.E.; Zhang, J.Z. Synthesis, characterization and tunable optical properties of hollow gold nanospheres. J. Phys. Chem. B 2006, 110, 19935–19944. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.S.; Honma, I.; Komiyama, H.; Haus, J.W. Controlled synthesis and quantum-size effect in gold-coated nanoparticles. Phys. Rev. B 1994, 50, 12052–12056. [Google Scholar] [CrossRef]
- Oldenburg, S.J.; Averitt, R.D.; Westcott, S.L.; Halas, N.J. Nanoengineering of optical resonances. Chem. Phys. Lett. 1998, 288, 243–247. [Google Scholar] [CrossRef]
- Wang, Y.; Qian, W.; Tan, Y.; Ding, S.; Zhang, H. Direct electrochemistry and electroanalysis of hemoglobin adsorbed in self-assembled films of gold nanoshells. Talanta 2007, 72, 1134–1140. [Google Scholar] [CrossRef] [PubMed]
- Bardhan, R.; Grady, N.K.; Halas, N.J. Nanoscale control of near-infrared fluorescence enhancement using Au nanoshells. Small 2008, 4, 1716–1722. [Google Scholar] [CrossRef] [PubMed]
- Khanadeev, V.A.; Khlebtsov, B.N.; Staroverov, S.A.; Vidyasheva, I.V.; Skaptsov, A.A.; Ileneva, E.S.; Bogatyrev, V.A.; Dykman, L.A.; Khlebtsov, N.G. Quantitative cell bioimaging using gold-nanoshell conjugates and phage antibodies. J. Biophotonics 2011, 4, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Carpin, L.B.; Bickford, L.R.; Agollah, G.; Yu, T.-K.; Schiff, R.; Li, Y.; Drezek, R.A. Immunoconjugated gold nanoshell-mediated photothermal ablation of trastuzumab-resistant breast cancer cells. Breast Cancer Res. Treat. 2011, 125, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Porter, R.; Shan, F.; Carter, J.D.; Guo, T. Synthesis of tubular gold and silver nanoshells using silica nanowire core templates. Langmuir 2006, 22, 6367–6374. [Google Scholar] [CrossRef] [PubMed]
- Duff, D.G.; Baiker, A. A new hydrosol of gold clusters. 1. Formation and particle size variation. Langmuir 1993, 9, 2301–2309. [Google Scholar] [CrossRef]
- Xie, Y.; Chen, T.; Cheng, Y.; Wang, H.; Qian, H.; Yao, W. SiO2@Au nanoshells-based SERS method for detection of sunset yellow and chrysoidine. Spectrochim. Acta Part A 2014, 132, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Ashayer, R.; Mannan, S.H.; Sajjadi, S. Synthesis and characterization of gold nanoshells using poly(diallyldimethyl ammonium chloride). Colloids Surf. A 2008, 329, 134–141. [Google Scholar] [CrossRef]
- Xue, J.; Wang, C.; Ma, Z. A facile method to prepare a series of SiO2@Au core/shell structured nanoparticles. Mater. Chem. Phys. 2007, 105, 419–425. [Google Scholar] [CrossRef]
- Prati, L.; Martra, G. New gold catalysts for liquid phase oxidation. Gold Bull. 1999, 32, 96–101. [Google Scholar] [CrossRef] [Green Version]
- Kung, H.H.; Kung, M.C.; Costello, C.K. Supported Au catalysts for low temperature CO oxidation. J. Catal. 2003, 216, 425–432. [Google Scholar] [CrossRef]
- Haruta, M. Nanoparticulate Gold catalysts for low-temperature CO oxidation. J. New Mater. Electrochem. Syst. 2004, 7, 9. [Google Scholar] [CrossRef]
- Moreau, F.; Bond, G.C.; Taylor, A.O. Gold on titania catalysts for the oxidation of carbon monoxide: Control of pH during preparation with various gold contents. J. Catal. 2005, 231, 105–114. [Google Scholar] [CrossRef]
- Ivanova, S.; Pitchon, V.; Petit, C.; Herschbach, H.; Dorsselaer, A.V.; Leize, E. Preparation of alumina supported gold catalysts: Gold complexes genesis, identification and speciation by mass spectrometry. Appl. Catal. A 2006, 298, 203–210. [Google Scholar] [CrossRef]
- Kah, J.C.Y.; Phonthammachai, N.; Wan, R.C.Y.; Song, J.; White, T.; Mhaisalkar, S.; Ahmadb, I.; Shepparda, C.; Olivoc, M. Synthesis of gold nanoshells based on the deposition-precipitation process. Gold Bull. 2008, 41, 23–36. [Google Scholar] [CrossRef]
- Pol, V.G.; Gedanken, A.; Calderon-Moreno, J. Deposition of gold nanoparticles on silica spheres: A sonochemical approach. Chem. Mater. 2003, 15, 1111–1118. [Google Scholar] [CrossRef]
- Suslick, K.S. Ultrasound: Its Chemical, Physical, and Biological Effects; VCH: Weinheim, Germany, 1988. [Google Scholar]
- Zhang, Q.; Ge, J.; Goebl, J.; Hu, Y.; Sun, Y.; Yin, Y. Tailored synthesis of superparamagnetic gold nanoshells with tunable optical properties. Adv. Mater. 2010, 22, 1905–1909. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Goebl, J.; Yin, Y. Seeded growth route to noble metal nanostructures. J. Mater. Chem. C 2013, 1, 3898–3909. [Google Scholar] [CrossRef]
- Graf, C.; Dembski, S.; Hofmann, A.; Rühl, E. A general method for the controlled embedding of nanoparticles in silica colloids. Langmuir 2006, 22, 5604–5610. [Google Scholar] [CrossRef] [PubMed]
- Graf, C.; Vossen, D.L.J.; Imhof, A.; van Blaaderen, A. A general Method to coat colloidal particles with silica. Langmuir 2003, 19, 6693–6700. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, T.; Ge, J.; Yin, Y. Permeable silica shell through surface-protected etching. Nano Lett. 2008, 8, 2867–2871. [Google Scholar] [CrossRef] [PubMed]
- Storti, B.; Elisei, F.; Abbruzzetti, S.; Viappiani, C.; Latterini, L. One-pot synthesis of gold nanoshells with high photon-to-heat conversion efficiency. J. Phys. Chem. C 2009, 113, 7516–7521. [Google Scholar] [CrossRef]
- Wang, X.; Lin, K.S.K.; Chan, J.C.C.; Cheng, S. Preparation of ordered large pore SBA-15 silica functionalized with aminopropyl groups through one-pot synthesis. Chem. Commun. 2004, 23, 2762–2763. [Google Scholar] [CrossRef] [PubMed]
- Allouche, J.; Soule, S.; Dupin, J.-C.; Masse, S.; Coradin, T.; Martinez, H. Design of gold nanoshells via a gelatin-mediated self-assembly of gold nanoparticles on silica cores. RSC Adv. 2014, 4, 63234–63237. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, J.; Wang, S.; Zhan, P.; Wang, Z.; Ming, N. Facile methods to coat polystyrene and silica colloids with metal. Adv. Funct. Mater. 2004, 14, 1089–1096. [Google Scholar] [CrossRef]
- Lee, J.-H.; Mahmoud, M.A.; Sitterle, V.B.; Sitterle, J.J.; Meredith, J.C. Highly scattering, surface-enhanced raman scattering-active, metal nanoparticle-coated polymers prepared via combined swelling−heteroaggregation. Chem. Mater. 2009, 21, 5654–5663. [Google Scholar] [CrossRef]
- Lee, J.-H.; Mahmoud, M.A.; Sitterle, V.; Sitterle, J.; Meredith, J.C. Facile preparation of highly-scattering metal nanoparticle-coated polymer microbeads and their surface plasmon resonance. J. Am. Chem. Soc. 2009, 131, 5048–5049. [Google Scholar] [CrossRef] [PubMed]
- Dokoutchaev, A.; James, J.T.; Koene, S.C.; Pathak, S.; Prakash, G.K.S.; Thompson, M.E. Colloidal metal deposition onto functionalized polystyrene microspheres. Chem. Mater. 1999, 11, 2389–2399. [Google Scholar] [CrossRef]
- Shi, W.; Sahoo, Y.; Swihart, M.T.; Prasad, P.N. Gold nanoshells on polystyrene cores for control of surface plasmon resonance. Langmuir 2005, 21, 1610–1617. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Gao, C.; Gu, J.; Jiang, Y.; Yang, X.; Li, S.; Gao, W.; An, T.; Duan, H.; Fu, J.; et al. Hyaluronic acid stabilized iodine-containing nanoparticles with Au nanoshell coating for X-ray CT imaging and photothermal therapy of Tumors. ACS Appl. Mater. Interfaces 2016, 8, 27622–27631. [Google Scholar] [CrossRef] [PubMed]
- Bao, H.; Bihr, T.; Smith, A.-S.; Klupp Taylor, R.N. Facile colloidal coating of polystyrene nanospheres with tunable gold dendritic patches. Nanoscale 2014, 6, 3954–3966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graf, C.; van Blaaderen, A. Metallodielectric colloidal core−shell particles for photonic applications. Langmuir 2002, 18, 524–534. [Google Scholar] [CrossRef]
- Liang, H.-P.; Wan, L.-J.; Bai, C.-L.; Jiang, L. Gold hollow nanospheres: Tunable surface plasmon resonance controlled by interior-cavity sizes. J. Phys. Chem. B 2005, 109, 7795–7800. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Horie, M.; Konno, M.; Rodríguez-González, B.; Liz-Marzán, L.M. Preparation and properties of silica-coated cobalt nanoparticles. J. Phys. Chem. B 2003, 107, 7420–7425. [Google Scholar] [CrossRef]
- Liang, H.-P.; Zhang, H.-M.; Hu, J.-S.; Guo, Y.-G.; Wan, L.-J.; Bai, C.-L. Pt Hollow nanospheres: Facile synthesis and enhanced electrocatalysts. Angew. Chem. 2004, 116, 1566–1569. [Google Scholar] [CrossRef]
- Glavee, G.N.; Klabunde, K.J.; Sorensen, C.M.; Hadjipanayis, G.C. Borohydride reduction of cobalt ions in water. Chemistry leading to nanoscale metal, boride, or borate particles. Langmuir 1993, 9, 162–169. [Google Scholar] [CrossRef]
- Sugimoto, T. The theory of the nucleation of monodisperse particles in open systems and its application to AgBr systems. J. Colloid Interface Sci. 1992, 150, 208–225. [Google Scholar] [CrossRef]
- Matijevic, E. Preparation and properties of uniform size colloids. Chem. Mater. 1993, 5, 412–426. [Google Scholar] [CrossRef]
- Adams, S.; Thai, D.; Mascona, X.; Schwartzberg, A.M.; Zhang, J.Z. Key factors affecting the reproducibility of synthesis and growth mechanism of near-infrared absorbing hollow gold nanospheres. Chem. Mater. 2014, 26, 6805–6810. [Google Scholar] [CrossRef]
- Lin, A.Y.; Ariel, J.K.Y.; Nixon, V.; Drezek, R.A. Encapsulated Fe3O4/Ag complexed cores in hollow gold nanoshells for enhanced theranostic magnetic resonance imaging and photothermal therapy. Small 2014, 10, 3246–3251. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Mayers, B.; Xia, Y. Metal nanostructures with hollow interiors. Adv. Mater. 2003, 15, 641–646. [Google Scholar] [CrossRef]
- Sun, Y.; Mayers, B.T.; Xia, Y. Template-engaged replacement reaction: A one-step approach to the large-scale synthesis of metal nanostructures with hollow interiors. Nano Lett. 2002, 2, 481–485. [Google Scholar] [CrossRef]
- Selvakannan, P.R.; Sastry, M. Hollow gold and platinum nanoparticles by a transmetallation reaction in an organic solution. Chem. Commun. 2005, 13, 1684–1686. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Lin, Y.; Yan, J.; Di, J. Synthesis of hollow gold nanoparticles on the surface of indium tin oxide glass and their application for plasmonic biosensor. Spectrochim. Acta Part A 2013, 110, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Hu, T.; Chen, N.; Zhang, W.; Di, J. Development of silver/gold nanocages onto indium tin oxide glass as a reagentless plasmonic mercury sensor. Anal. Chim. Acta 2014, 825, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Hu, T.; Guo, W.; Deng, A.; Di, J. A label-free immunosensor for determination of salbutamol based on localized surface plasmon resonance biosensing. Bioprocess. Biosyst. Eng. 2014, 37, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Wu, Y.; Di, J. Formation of substrate-based gold nanocage chains through dealloying with nitric acid. Beilstein J. Nanotechnol. 2015, 6, 1362–1368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, M.Z.H. Nanoparticles modified ITO based biosensor. J. Electron. Mater. 2017, 46, 2254–2268. [Google Scholar] [CrossRef]
- Christian, Z.; Claus, F.; Matthias, W.; Dagmar, G. Nanoscale gold hollow spheres through a microemulsion approach. Small 2007, 3, 1347–1349. [Google Scholar]
- Dominic, W.; Benedicte, L.; Stephen, M. Morphosynthesis of calcium carbonate (vaterite) microsponges. Adv. Mater. 1999, 11, 324–328. [Google Scholar]
- Bruinsma, P.J.; Kim, A.Y.; Liu, J.; Baskaran, S. Mesoporous silica synthesized by solvent evaporation: Spun fibers and spray-dried hollow spheres. Chem. Mater. 1997, 9, 2507–2512. [Google Scholar] [CrossRef]
- Wang, W.; Han, Y.; Tian, M.; Fan, Y.; Tang, Y.; Gao, M.; Wang, Y. Cationic gemini surfactant-assisted synthesis of hollow Au nanostructures by stepwise reductions. ACS Appl. Mater. Interfaces 2013, 5, 5709–5716. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Qi, L.; Ma, J.; Cheng, H. Synthesis of submicrometer-sized hollow silver spheres in mixed polymer–surfactant solutions. Adv. Mater. 2002, 14, 1499–1502. [Google Scholar] [CrossRef]
- Guan, Y.; Xue, Z.; Liang, J.; Huang, Z.; Yang, W. One-pot synthesis of size-tunable hollow gold nanoshells via APTES-in-water suspension. Colloids Surf. A 2016, 502, 6–12. [Google Scholar] [CrossRef]
- Wang, S.; Dai, Z.; Ke, H.; Qu, E.; Qi, X.; Zhang, K.; Wang, J. Contrast ultrasound-guided photothermal therapy using gold nanoshelled microcapsules in breast cancer. Eur. J. Radiol. 2014, 83, 117–122. [Google Scholar] [CrossRef] [PubMed]
- François, L.; Frédéric, L.; Jocelyne, B.; Gilles, L.; Chantal, A.; Guy, V.; Patrice, L.B.; Fernand, C.; Stephane, P. Gold hollow spheres obtained using an innovative emulsion process: Towards multifunctional Au nanoshells. Nanotechnology 2009, 20, 355603. [Google Scholar]
- Dujardin, E.; Peet, C.; Stubbs, G.; Culver, J.N.; Mann, S. Organization of metallic nanoparticles using tobacco mosaic virus templates. Nano Lett. 2003, 3, 413–417. [Google Scholar] [CrossRef]
- Behrens, S.; Rahn, K.; Habicht, W.; Bohm, K.-J.; Rosner, H.; Dinjus, E.; Unger, E. Nanoscale particle arrays induced by highly ordered protein assemblies. Adv. Mater. 2002, 14, 1621–1625. [Google Scholar] [CrossRef]
- Kobayashi, M.; Seki, M.; Tabata, H.; Watanabe, Y.; Yamashita, I. Fabrication of aligned magnetic nanoparticles using tobacco viruses. Nano Lett. 2010, 10, 773–776. [Google Scholar] [CrossRef] [PubMed]
- Ullah, M.W.; Shi, Z.; Shi, X.; Zeng, D.; Li, S.; Yang, G. Microbes as structural templates in biofabrication: Study of surface chemistry and applications. ACS Sustain. Chem. Eng. 2017, 5, 11163–11175. [Google Scholar] [CrossRef]
- Zhu, W.-K.; Cong, H.-P.; Guan, Q.-F.; Yao, W.-T.; Liang, H.-W.; Wang, W.; Yu, S.-H. Coupling microbial growth with nanoparticles: A universal strategy to produce functional fungal hyphae macrospheres. ACS Appl. Mater. Interfaces 2016, 8, 12693–12701. [Google Scholar] [CrossRef] [PubMed]
- Blum, A.S.; Soto, C.M.; Wilson, C.D.; Cole, J.D.; Kim, M.; Gnade, B.; Chatterji, A.; Ochoa, W.F.; Lin, T.; Johnson, J.E.; et al. Cowpea mosaic virus as a scaffold for 3-D patterning of gold nanoparticles. Nano Lett. 2004, 4, 867–870. [Google Scholar] [CrossRef]
- Gillitzer, E.; Willits, D.; Young, M.; Douglas, T. Chemical modification of a viral cage for multivalent presentation. Chem. Commun. 2002, 20, 2390–2391. [Google Scholar] [CrossRef]
- Douglas, T.; Strable, E.; Willits, D.; Aitochen, A.; Libera, M.; Young, M. Protein engineering of a viral cage for constrained nanomaterials synthesis. Adv. Mater. 2002, 14, 415–418. [Google Scholar] [CrossRef]
- Radloff, C.; Vaia, R.A.; Brunton, J.; Bouwer, G.T.; Ward, V.K. Metal nanoshell assembly on a virus bioscaffold. Nano Lett. 2005, 5, 1187–1191. [Google Scholar] [CrossRef] [PubMed]
- Prodan, E.; Radloff, C.; Halas, N.J.; Nordlander, P. A hybridization model for the plasmon response of complex nanostructures. Science 2003, 302, 419–422. [Google Scholar] [CrossRef] [PubMed]
- Bardhan, R.; Mukherjee, S.; Mirin, N.A.; Levit, S.D.; Nordlander, P.; Halas, N.J. Nanosphere-in-a-nanoshell: A simple nanomatryushka. J. Phys. Chem. C 2010, 114, 7378–7383. [Google Scholar] [CrossRef]
- Zhang, T.; Lu, G.; Li, W.; Liu, J.; Hou, L.; Perriat, P.; Martini, M.; Tillement, O.; Gong, Q. Optimally designed nanoshell and matryoshka-nanoshell as a plasmonic-enhanced fluorescence Probe. J. Phys. Chem. C 2012, 116, 8804–8812. [Google Scholar] [CrossRef]
- Kircher, M.F.; de la Zerda, A.; Jokerst, J.V.; Zavaleta, C.L.; Kempen, P.J.; Mittra, E.; Pitter, K.; Huang, R.; Campos, C.; Habte, F.; et al. A brain tumor molecular imaging strategy using a new triple-modality MRI-photoacoustic-Raman nanoparticle. Nat. Med. 2012, 18, 829. [Google Scholar] [CrossRef] [PubMed]
- Keren, S.; Zavaleta, C.; Cheng, Z.; de la Zerda, A.; Gheysens, O.; Gambhir, S.S. Noninvasive molecular imaging of small living subjects using Raman spectroscopy. Proc. Natl. Acad. Sci. USA 2008, 105, 5844–5849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayala-Orozco, C.; Liu, J.G.; Knight, M.W.; Wang, Y.; Day, J.K.; Nordlander, P.; Halas, N.J. Fluorescence enhancement of molecules inside a gold nanomatryoshka. Nano Lett. 2014, 14, 2926–2933. [Google Scholar] [CrossRef] [PubMed]
- Liz-Marzán, L.M.; Giersig, M.; Mulvaney, P. Synthesis of nanosized gold–silica core–shell particles. Langmuir 1996, 12, 4329–4335. [Google Scholar] [CrossRef]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Xiaohu, X.; Yang, L.; Vadim, B.; Guillermo, A.A. Engineering sub-100 nm multi-layer nanoshells. Nanotechnology 2006, 17, 5435. [Google Scholar]
- Yongping, G.; Yongsheng, L.; Yao, W.; Yi, C.; Jinlou, G.; Wenru, Z.; Jian, D.; Jianlin, S. Controlled synthesis of multilayered gold nanoshells for enhanced photothermal therapy and SERS detection. Small 2015, 11, 77–83. [Google Scholar]
- Chen, H.M.; Liu, R.-S.; Asakura, K.; Lee, J.-F.; Jang, L.-Y.; Hu, S.-F. Fabrication of nanorattles with passive Shell. J. Phys. Chem. B 2006, 110, 19162–19167. [Google Scholar] [CrossRef] [PubMed]
- Mazzucco, S.; Stéphan, O.; Colliex, C.; Pastoriza-Santos, I.; Liz-Marzan, L.M.; de Abajo, J.G.; Kociak, M. Spatially resolved measurements of plasmonic eigenstates in complex-shaped, asymmetric nanoparticles: gold nanostars. Eur. Phys. J. Appl. Phys. 2011, 54, 33512. [Google Scholar] [CrossRef] [Green Version]
- Pandian Senthil, K.; Isabel, P.-S.; Benito, R.-G.; de Abajo, J.G.; Luis, M.L.-M. High-yield synthesis and optical response of gold nanostars. Nanotechnology 2008, 19, 015606. [Google Scholar] [CrossRef] [PubMed]
- Yuanyuan, J.; Xue-Jun, W.; Qi, L.; Jingjian, L.; Dongsheng, X. Facile synthesis of gold nanoflowers with high surface-enhanced Raman scattering activity. Nanotechnology 2011, 22, 385601. [Google Scholar]
- Gersten, J.; Nitzan, A. Electromagnetic theory of enhanced Raman scattering by molecules adsorbed on rough surfaces. J. Phys. Chem. 1980, 73, 3023–3037. [Google Scholar] [CrossRef]
- Jana, D.; Gorunmez, Z.; He, J.; Bruzas, I.; Beck, T.; Sagle, L. Surface enhanced Raman spectroscopy of a Au@Au core–shell structure containing a spiky Shell. J. Phys. Chem. C 2016, 120, 20814–20821. [Google Scholar] [CrossRef]
- Bedford, E.E.; Boujday, S.; Pradier, C.-M.; Gu, F.X. Spiky gold shells on magnetic particles for DNA biosensors. Talanta 2018, 182, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Lorenzo, L.; de la Rica, R.; Álvarez-Puebla, R.A.; Liz-Marzán, L.M.; Stevens, M.M. Plasmonic nanosensors with inverse sensitivity by means of enzyme-guided crystal growth. Nat. Mater. 2012, 11, 604. [Google Scholar] [CrossRef] [PubMed]
- Ray, P.C. Size and shape dependent second order nonlinear optical properties of nanomaterials and their application in biological and chemical sensing. Chem. Rev. 2010, 110, 5332–5365. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Fales, A.M.; Vo-Dinh, T. TAT Peptide-functionalized gold nanostars: Enhanced intracellular delivery and efficient NIR photothermal therapy using ultralow irradiance. J. Am. Chem. Soc. 2012, 134, 11358–11361. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Gaytan, B.L.; Park, S.-J. Spiky gold nanoshells. Langmuir 2010, 26, 19170–19174. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Gaytan, B.L.; Swanglap, P.; Lamkin, T.J.; Hickey, R.J.; Fakhraai, Z.; Link, S.; Park, S.-J. Spiky gold nanoshells: Synthesis and enhanced scattering properties. J. Phys. Chem. C 2012, 116, 10318–10324. [Google Scholar] [CrossRef]
- Sanchez-Gaytan, B.L.; Qian, Z.; Hastings, S.P.; Reca, M.L.; Fakhraai, Z.; Park, S.-J. Controlling the topography and surface plasmon resonance of gold nanoshells by a templated surfactant-assisted seed growth method. J. Phys. Chem. C 2013, 117, 8916–8923. [Google Scholar] [CrossRef]
- Kwizera, E.A.; Chaffin, E.; Shen, X.; Chen, J.; Zou, Q.; Wu, Z.; Gai, Z.; Bhana, S.; O’Connor, R.; Wang, L.; et al. Size- and shape-controlled synthesis and properties of magnetic-plasmonic core-shell nanoparticles. J. Phys. Chem. C 2016, 120, 10530–10546. [Google Scholar] [CrossRef] [PubMed]
- Hastings, S.P.; Swanglap, P.; Qian, Z.; Fang, Y.; Park, S.-J.; Link, S.; Engheta, N.; Fakhraai, Z. Quadrupole-enhanced Raman scattering. ACS Nano 2014, 8, 9025–9034. [Google Scholar] [CrossRef] [PubMed]
- Pedireddy, S.; Li, A.; Bosman, M.; Phang, I.Y.; Li, S.; Ling, X.Y. Synthesis of spiky Ag–Au octahedral nanoparticles and their tunable optical properties. J. Phys. Chem. C 2013, 117, 16640–16649. [Google Scholar] [CrossRef]
- Topete, A.; Alatorre-Meda, M.; Iglesias, P.; Villar-Alvarez, E.M.; Barbosa, S.; Costoya, J.A.; Taboada, P.; Mosquera, V. Fluorescent drug-Loaded, polymeric-based, branched gold nanoshells for localized multimodal therapy and imaging of tumoral cells. ACS Nano 2014, 8, 2725–2738. [Google Scholar] [CrossRef] [PubMed]
- Shanavas, A.; Rengan, A.K.; Chauhan, D.; George, L.; Vats, M.; Kaur, N.; Yadav, P.; Mathur, P.; Chakraborty, S.; Tejaswini, A.; et al. Glycol chitosan assisted in situ reduction of gold on polymeric template for anti-cancer theranostics. Int. J. Biol. Macromol. 2018, 110, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Kim, J.-P.; Bahng, J.H.; Kotov, N.A.; Lee, J. Self-Assembly mechanism of spiky magnetoplasmonic supraparticles. Adv. Funct. Mater. 2014, 24, 1439–1448. [Google Scholar] [CrossRef]
- Topete, A.; Alatorre-Meda, M.; Villar-Álvarez, E.M.; Cambón, A.; Barbosa, S.; Taboada, P.; Mosquera, V. Simple control of surface topography of gold nanoshells by a surfactant-less seeded-growth method. ACS Appl. Mater. Interfaces 2014, 6, 11142–11157. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Kim, K.H.; Choi, N.; Chon, H.; Lee, S.; Choo, J. Preparation of silica-encapsulated hollow gold nanosphere tags using layer-by-layer method for multiplex surface-enhanced Raman scattering detection. Langmuir 2011, 27, 10228–10233. [Google Scholar] [CrossRef] [PubMed]
- Hermes, J.P.; Sander, F.; Fluch, U.; Peterle, T.; Thompson, D.; Urbani, R.; Pfohl, T.; Mayor, M. Monofunctionalized gold nanoparticles stabilized by a single dendrimer form dumbbell structures upon homocoupling. J. Am. Chem. Soc. 2012, 134, 14674–14677. [Google Scholar] [CrossRef] [PubMed]
- Kah, J.C.Y.; Wong, K.Y.; Neoh, K.G.; Song, J.H.; Fu, J.W.P.; Mhaisalkar, S.; Olivo, M.; Sheppard, C.J.R. Critical parameters in the pegylation of gold nanoshells for biomedical applications: An in vitro macrophage study. J. Drug Targeting 2009, 17, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Conroy, S.; Raymond, S.; Miqin, Z. Folic acid-PEG conjugated superparamagnetic nanoparticles for targeted cellular uptake and detection by MRI. J. Biomed. Mater. Res. 2006, 78, 550–557. [Google Scholar]
- Zhongshi, L.; Yun, L.; Xiangyang, L.; Qinge, W.; Jiahui, Y.; Shufang, L.; Lihui, L.; Shunying, L. Surface-modified gold nanoshells for enhanced cellular uptake. J. Biomed. Mater. Res. 2011, 98, 479–487. [Google Scholar]
- Wang, Z.L. Transmission electron microscopy and spectroscopy of nanoparticles. In Characterization of Nanophase Materials; Wiley-VCH Verlag: Weinheim, Germany, 2001; pp. 37–83. [Google Scholar]
- Patri, A.K.; Stephan, M.A.D.; Stern, T.; McNeil, S.E. Preclinical characterization of engineered nanoparticles intended for cancer therapeutics. In Nanotechnology for Cancer Therapy; CRC Press: Boca Raton, FL, USA, 2006; pp. 105–138. [Google Scholar]
- Ratner, B.D.; Hoffman, A.S.; Schoen, F.J.; Lemons, J.E. Biomaterials Science: An Introduction to Materials in Medicine; Academic Press: Cambridge, MA, USA, 2004. [Google Scholar]
- Hall, J.B.; Dobrovolskaia, M.A.; Patri, A.K.; McNeil, S.E. Characterization of nanoparticles for therapeutics. Nanomedicine 2007, 2, 789–803. [Google Scholar] [CrossRef] [PubMed]
- Johal, M.S. Understanding Nanomaterials; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Mayle, K.M.; Dern, K.R.; Wong, V.K.; Sung, S.; Ding, K.; Rodriguez, A.R.; Taylor, Z.; Zhou, Z.H.; Grundfest, W.S.; Deming, T.J.; et al. Polypeptide-based gold nanoshells for photothermal therapy. SLAS Technol. 2017, 22, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Sapsford, K.E.; Tyner, K.M.; Dair, B.J.; Deschamps, J.R.; Medintz, I.L. Analyzing nanomaterial bioconjugates: A review of current and emerging purification and characterization techniques. Anal. Chem. 2011, 83, 4453–4488. [Google Scholar] [CrossRef] [PubMed]
- Pons, T.; Uyeda, H.T.; Medintz, I.L.; Mattoussi, H. Hydrodynamic dimensions, electrophoretic mobility, and stability of hydrophilic quantum dots. J. Phys. Chem. B 2006, 110, 20308–20316. [Google Scholar] [CrossRef] [PubMed]
- Brar, S.K.; Verma, M. Measurement of nanoparticles by light-scattering techniques. TrAC Trends Anal. Chem. 2011, 30, 4–17. [Google Scholar] [CrossRef]
- Jans, H.; Liu, X.; Austin, L.; Maes, G.; Huo, Q. Dynamic light scattering as a powerful tool for gold nanoparticle bioconjugation and biomolecular binding studies. Anal. Chem. 2009, 81, 9425–9432. [Google Scholar] [CrossRef] [PubMed]
- Park, H.H.; Srisombat, L.-O.; Jamison, A.C.; Liu, T.; Marquez, M.D.; Park, H.; Lee, S.; Lee, T.-C.; Lee, T.R. Temperature-responsive hydrogel-coated gold nanoshells. Gels 2018, 4, 28. [Google Scholar] [CrossRef]
- Bootz, A.; Vogel, V.; Schubert, D.; Kreuter, J. Comparison of scanning electron microscopy, dynamic light scattering and analytical ultracentrifugation for the sizing of poly(butyl cyanoacrylate) nanoparticles. Eur. J. Pharm. Biopharm. 2004, 57, 369–375. [Google Scholar] [CrossRef]
- Clogston, J.D.; Patri, A.K. Zeta potential measurement. In Characterization of Nanoparticles Intended for Drug Delivery; Humana Press: Totowa, NJ, USA, 2011; pp. 63–70. [Google Scholar]
- Kumar, A.; Dixit, C.K. Methods for characterization of nanoparticles. In Advances in Nanomedicine for the Delivery of Therapeutic Nucleic Acids; Nimesh, S., Chandra, R., Gupta, N., Eds.; Woodhead Publishing: Sawston, CA, USA, 2017; pp. 43–58. [Google Scholar]
- Wang, Y.; Shen, Y.; Xie, A.; Li, S.; Wang, X.; Cai, Y. A Simple method to construct bifunctional Fe3O4/Au hybrid nanostructures and tune their optical properties in the near-infrared region. J. Phys. Chem. C 2010, 114, 4297–4301. [Google Scholar] [CrossRef]
- Sastry, T.P.; Sundaraseelan, J.; Swarnalatha, K.; Sobhana, S.S.L.; Makheswari, M.U.; Sekar, S.; Mandal, A.B. Growth of hydroxyapatite on physiologically clotted fibrin capped gold nanoparticles. Nanotechnology 2008, 19, 245604. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Yu, C.; Chu, M. A gold nanoshell with a silica inner shell synthesized using liposome templates for doxorubicin loading and near-infrared photothermal therapy. Int. J. Nanomed. 2011, 6, 807–813. [Google Scholar]
- Bédard, M.; Avti, P.K.; Lam, T.; Rouleau, L.; Tardif, J.-C.; Rhéaume, É.; Lesage, F.; Kakkar, A. Conjugation of multivalent ligands to gold nanoshells and designing a dual modality imaging probe. J. Mater. Chem. B 2015, 3, 1788–1800. [Google Scholar] [CrossRef]
- Amirthalingam, T.; Kalirajan, J.; Chockalingam, A. Use of silica-gold core shell structured nanoparticles for targeted drug delivery system. J. Nanomed. Nanotechnol. 2011, 2, 119–123. [Google Scholar] [CrossRef]
- Khosroshahi, M.E.; Nourbakhsh, M.S.; Ghazanfari, L. Synthesis and biomedical application of SiO2/Au nanofluid based on laser-induced surface plasmon resonance thermal effect. J. Mod. Phys. 2011, 2, 944–953. [Google Scholar] [CrossRef]
- Castner, D.G.; Hinds, K.; Grainger, D.W. X-ray photoelectron spectroscopy sulfur 2p study of organic thiol and disulfide binding interactions with gold surfaces. Langmuir 1996, 12, 5083–5086. [Google Scholar] [CrossRef]
- Ng, V.W.K.; Avti, P.K.; Bédard, M.; Lam, T.; Rouleau, L.; Tardif, J.-C.; Rhéaume, É.; Lesage, F.; Kakkar, A. Miktoarm star conjugated multifunctional gold nanoshells: synthesis and an evaluation of biocompatibility and cellular uptake. J. Mater. Chem. B 2014, 2, 6334–6344. [Google Scholar] [CrossRef]
- Behzadi, S.; Ghasemi, F.; Ghalkhani, M.; Ashkarran, A.A.; Akbari, S.M.; Pakpour, S.; Hormozi-Nezhad, M.R.; Jamshidi, Z.; Mirsadeghi, S.; Dinarvand, R.; et al. Determination of nanoparticles using UV-Vis spectra. Nanoscale 2015, 7, 5134–5139. [Google Scholar] [CrossRef] [PubMed]
- Erickson, T.A.; Tunnell, J.W. Gold nanoshells in biomedical applications. In Nanotechnologies for the Life Sciences; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2010. [Google Scholar]
- Hassannejad, Z.; Khosroshahi, M.E.; Firouzi, M. Fabrication and characterization of magnetoplasmonic liposome carriers. Nanosci. Technol. 2014, 1, 1–9. [Google Scholar]
























| Zeta Potential Value (mV) | Stability Behavior |
|---|---|
| 0 to ±5 | Flocculation or coagulation |
| ±10 to ±30 | Incipient instability |
| ±30 to ±40 | Moderate stability |
| ±40 to ±60 | Good stability |
| Greater than ±60 | Excellent stability |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.-C.; Rhéaume, É.; Lesage, F.; Kakkar, A. Synthetic Methodologies to Gold Nanoshells: An Overview. Molecules 2018, 23, 2851. https://doi.org/10.3390/molecules23112851
Wang Y-C, Rhéaume É, Lesage F, Kakkar A. Synthetic Methodologies to Gold Nanoshells: An Overview. Molecules. 2018; 23(11):2851. https://doi.org/10.3390/molecules23112851
Chicago/Turabian StyleWang, Yu-Chen, Éric Rhéaume, Frédéric Lesage, and Ashok Kakkar. 2018. "Synthetic Methodologies to Gold Nanoshells: An Overview" Molecules 23, no. 11: 2851. https://doi.org/10.3390/molecules23112851