Ultrasound-Assisted Metal-Mediated Method for the Formation of Tetrahydro-3,3′-Disubstituted Biscoumarins
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of 3,3′,4,4′-Tetrahydro-3,3′-disubstituted-4,4′-biscoumarins
2.2. Spectroscopic Data Interpretation of Homodimers 3
3. Conclusions
4. Experimental Section
4.1. Materials
4.2. General Methods
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pratap, R.; Ram, V.J. Natural and synthetic chromenes, fused chromenes, and versatility of dihydrobenzo[h]chromenes in organic synthesis. Chem. Rev. 2014, 114, 10476–10526. [Google Scholar] [CrossRef] [PubMed]
- Borges, F.; Roleira, F.; Milhazes, N.; Santana, L.; Uriarte, E. Simple coumarins and analogues in medicinal chemistry: Occurrence, synthesis and biological activity. Curr. Med. Chem. 2005, 12, 887–916. [Google Scholar] [CrossRef] [PubMed]
- Kancheva, V.D.; Boranova, P.V.; Nechev, J.T.; Manolov, I.I. Structure-activity relationships of new 4-hydroxy bis-coumarins as radical scavengers and chain-breaking antioxidants. Biochimie 2010, 92, 1138–1146. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Singh, B.K.; Sharma, N.K.; Gyanda, K.; Jain, S.K.; Tyagi, Y.K.; Baghel, A.S.; Pandey, M.; Sharma, S.K.; Prasad, A.K.; et al. Specificities of acetoxy derivatives of coumarins, biscoumarins, chromones, flavones, isoflavones and xanthones for acetoxy drug: Protein transacetylase. Eur. J. Med. Chem. 2007, 42, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Dutra, P.K.; Majumder, P.C.; Dutia, N.L. Synthetic approaches towards bicoumarins: Synthesis of euphorbetin and isoeuphorbetin. Tetrahedron 1975, 31, 1167–1170. [Google Scholar] [CrossRef]
- Spencer, R.R.; Witt, S.C.; Lundin, R.E.; Bickoff, E.M. Bicoumol, a new bicoumarinyl, from ladino clover. J. Agric. Food Chem. 1967, 3, 536–538. [Google Scholar] [CrossRef]
- Hussain, H.; Hussain, J.; Al-Harrasi, A.; Krohn, K. The chemistry and biology of bicoumarins. Tetrahedron 2012, 68, 2553–2578. [Google Scholar] [CrossRef]
- Koleva, A.I.; Petkova, N.I.; Nikolova, R.D. Ultrasound-assisted conjugate addition of organometallic reagents to 3-diethylphosphonocoumarin. Synlett 2016, 27, 2676–2680. [Google Scholar] [CrossRef]
- Luche, J.-L. Synthetic Organic Sonochemistry; Springer: New York, NY, USA, 1998; ISBN 978-1-4899-1910-6. [Google Scholar]
- Mason, T.J.; Lorimer, P.J. Applied Sonochemistry: The Uses of Power Ultrasound in Chemistry and Processing, 1st ed.; Wiley-VCH: Weinheim, Germany, 2002. [Google Scholar]
- Mason, T.J.; Peters, D. Practical Sonochemistry: Uses and Applications of Ultrasound, 2nd ed.; Woodhead Publishing: Cambridge, UK, 2002. [Google Scholar]
- Gallego-Juáres, A.J.; Graff, F.K. Power Ultrasonics: Applications of High-Intensity Ultrasound, 1st ed.; Woodhead Publishing: Cambridge, UK, 2014. [Google Scholar]
- Nielsen, M.F.; Batanero, B.; Löhl, T.; Schafer, H.J.; Wurthwein, E.; Frohlich, R. Enantioselective cathodic reduction of 4-methylcoumarin: Dependence of selectivity on reaction conditions and investigation of the mechanism. Chem. Eur. J. 1997, 3, 2011–2024. [Google Scholar] [CrossRef]
- Pasciak, E.M.; Rittichier, J.T.; Chen, C.; Mubarak, M.S.; VanNieuwenhze, M.S.; Peters, D.G.J. Electroreductive dimerization of coumarin and coumarin analogues at carbon cathodes. Org. Chem. 2015, 80, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Tuğral, S.; Berkemb, M.L. Electrochemical behavior of some ethylenedioxycoumarins: Cathodic dimerization. J. Mol. Liq. 2014, 196, 363–369. [Google Scholar] [CrossRef]
- Kise, N. Density functional theory study of electroreductive hydrocoupling of α,β-unsaturated carbonyl compounds. J. Org. Chem. 2006, 71, 9203–9207. [Google Scholar] [CrossRef] [PubMed]
- Ohno, T.; Sakai, M.; Ishino, Y.; Shibata, T.; Maekawa, H.; Nishiguchi, I. Mg-promoted regio- and stereoselective C-acylation of aromatic α,β-unsaturated carbonyl compounds. Org. Lett. 2001, 3, 3439–3442. [Google Scholar] [CrossRef] [PubMed]
- Gourley, R.N.; Grimshaw, J.; Millar, P.G. Electrochemical reactions. Part VIII. Asymmetric induction during the reduction of coumarins modified by the presence of tertiary amines. J. Chem. Soc. (C) 1970, 17, 2318–2323. [Google Scholar] [CrossRef]
- Kise, N.; Iitaka, S.; Iwasaki, K.; Ueda, N. Stereoselective hydrocoupling of cinnamic acid esters by electroreduction: Application to asymmetric synthesis of hydrodimers. J. Org. Chem. 2002, 67, 8305–8315. [Google Scholar] [CrossRef] [PubMed]
- Schoo, N.; Schäfer, H.-J. Electroorganic synthesis, 54. Enantioselective cathodic reduction of 4-substituted coumarins with alkaloids as catalysts, 1. Liebigs Ann. Chem. 1993, 1993, 601–607. [Google Scholar] [CrossRef]
- Pfoertner, K.-H. Photoreaktionen von 3-substituierten cumarinen. Helv. Chim. Acta 1976, 59, 834–840. [Google Scholar] [CrossRef]
- Petkov, I.; Bojilova, A.; Markov, P. Photochemical dehydrogenation of 3-acetyl-3,4-dihydrocoumarin. Monatshefte fur Chemie 1990, 121, 85–87. [Google Scholar] [CrossRef]
- Kawata, H.; Ichikawa, S.; Kumagai, T.; Niizuma, S. A new type of photodimerization reaction for coumarin derivatives. Tetrahedron Lett. 2002, 43, 5161–5163. [Google Scholar] [CrossRef]
- Konde Deshmukh, R.S.; Paradkar, M.V. A facile synthesis of new [4,4′-BI-2H-1-Benzopyran]-2,2′-Diones. Synth. Commun. 1988, 18, 589–596. [Google Scholar] [CrossRef]
- Panichayupakaranant, P.; Noguchi, H.; Ke-Eknamkul, W. A new biscoumarin from Impatiens balsamina root cultures. Planta Med. 1998, 64, 774–775. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.G.; Lin, G.Q. The first total synthesis of 4,4′-biisofraxidin. Chin. J. Chem. 2002, 20, 1263–1267. [Google Scholar] [CrossRef]
- Lei, J.G.; Xu, M.H.; Lin, G.Q. Nickel-catalyzed cross-coupling reactions of 4-mesylcoumarins with aryl halides: Facile synthesis of 4-substituted coumarins. Synlett 2004, 13, 2364–2368. [Google Scholar] [CrossRef]
- Ivanov, C.; Bojilova, A. On the reaction of 3-phenylcoumarin with organomagnesium compounds. Synthesis 1974, 1974, 708–709. [Google Scholar] [CrossRef]
- Bojilova, A.; Ivanov, C. Two new routes to esters of 2-oxochroman-4-acetic acid. Synthesis 1976, 1976, 267–268. [Google Scholar] [CrossRef]
- Silverman, G.S.; Rakita, P.E. Handbook of Grignard Reagents; Marcel Dekker, Inc.: New York, NY, USA, 1996; ISBN 0-8247-9545-8. [Google Scholar]
- Gustafsson, B. Case of radical formation in the reactions between ethyl 3-coumarincarboxylate and Grignard reagents. Finn. Chem. Lett. 1975, 2, 49–50. [Google Scholar]
- Eistert, B.; Klein, L. Das verschiedene verhalten einiger o-chinone und des benzils gegen dimethyl- und diäthylzink; Analogie zum verhalten gegen diazomethan und -äthan. Chem. Ber. 1968, 101, 900–907. [Google Scholar] [CrossRef]
- Rappoport, Z.; Marek, I. The Chemistry of Organozinc Compounds: R-Zn 2 Part Set (Patai’s Chemistry of Functional Groups), 1st ed.; John Wiley & Sons Ltd.: New York, NY, USA, 2006; Print ISBN 9780470093375, Online ISBN 9780470093399. [Google Scholar] [CrossRef]
- Corey, E.J.; Pyne, S.G. Conversion of ketones having δ, ϵ-π-functions to cyclopentanols by zinc-trimethylchlorosilane. Tetrahedron Lett. 1983, 24, 2821–2824. [Google Scholar] [CrossRef]
- Blomberg, C.; Mosher, H. A radical process in a reaction of a Grignard compound. J. Organomet. Chem. 1968, 13, 519–522. [Google Scholar] [CrossRef]
- Blomberg, C.; Grootveld, H.H.; Gerner, T.H.; Bickelhaupt, F. Radical formation during reactions of Grignard reagents with quinones. J. Organomet. Chem. 1970, 24, 549–553. [Google Scholar] [CrossRef]
- Streuff, J.; Gansauer, A. Metal-catalyzed β-functionalization of Michael acceptors through reductive radical addition reactions. Angew. Chem. Int. Ed. Engl. 2015, 54, 14232–14242. [Google Scholar] [CrossRef] [PubMed]
- Nikolova, R.D.; Bojilova, A.G.; Rodios, N.A. A new and efficient method for conjugate addition of trialkylphosphites to 3-acylsubstituted coumarins. Tetrahedron 2004, 60, 10335–10342. [Google Scholar] [CrossRef]
- Simeonov, M.F.; Spassov, S.L.; Bojilova, A.; Ivanov, C. Conformation and tautomeric equilibria of 3-acyl-3,4-dihydrocoumarins: A 1H and 13C NMR study. J. Mol. Struct. 1985, 127, 127–133. [Google Scholar] [CrossRef]
- Ilieva, E.D.; Petkova, N.I.; Nikolova, R.D. A new and efficient method for the synthesis of 3,4-disubstituted pyrrolidine-2,5-diones. Molecules 2012, 17, 4936–4949. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Burla, M.C.; Caliandro, R.; Camalli, M.; Carrozzini, B.; Cascarano, G.L.; De Caro, L.; Giacovazzo, C.; Polidori, G.; Siliqi, D.; Spagna, R. IL MILIONE: A suite of computer programs for crystal structure solution of proteins. J. Appl. Cryst. 2007, 40, 609–613. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Bojilova, A.; Nikolova, R.; Ivanov, C.; Rodios, N.A.; Terzis, A.; Raptopoulou, C.P. A comparative study of the interaction of salicylaldehydes with phosphonoacetates under Knoevenagel reaction conditions. Synthesis of 1,2-benzoxaphosphorines and their dimers. Tetrahedron 1996, 52, 12597–12612. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds will be available on request from the authors. |
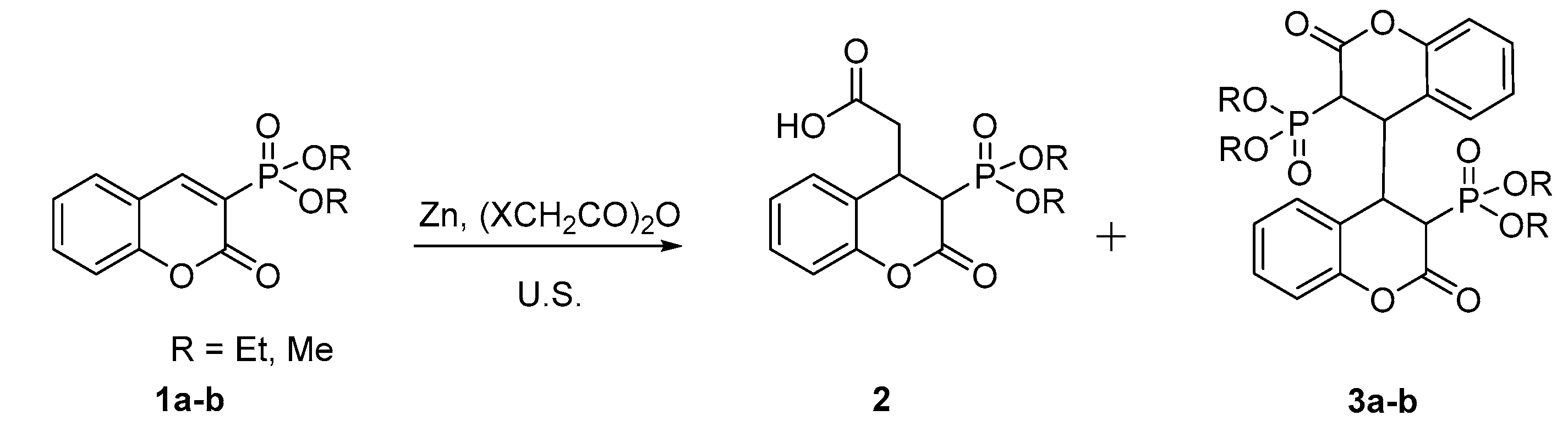

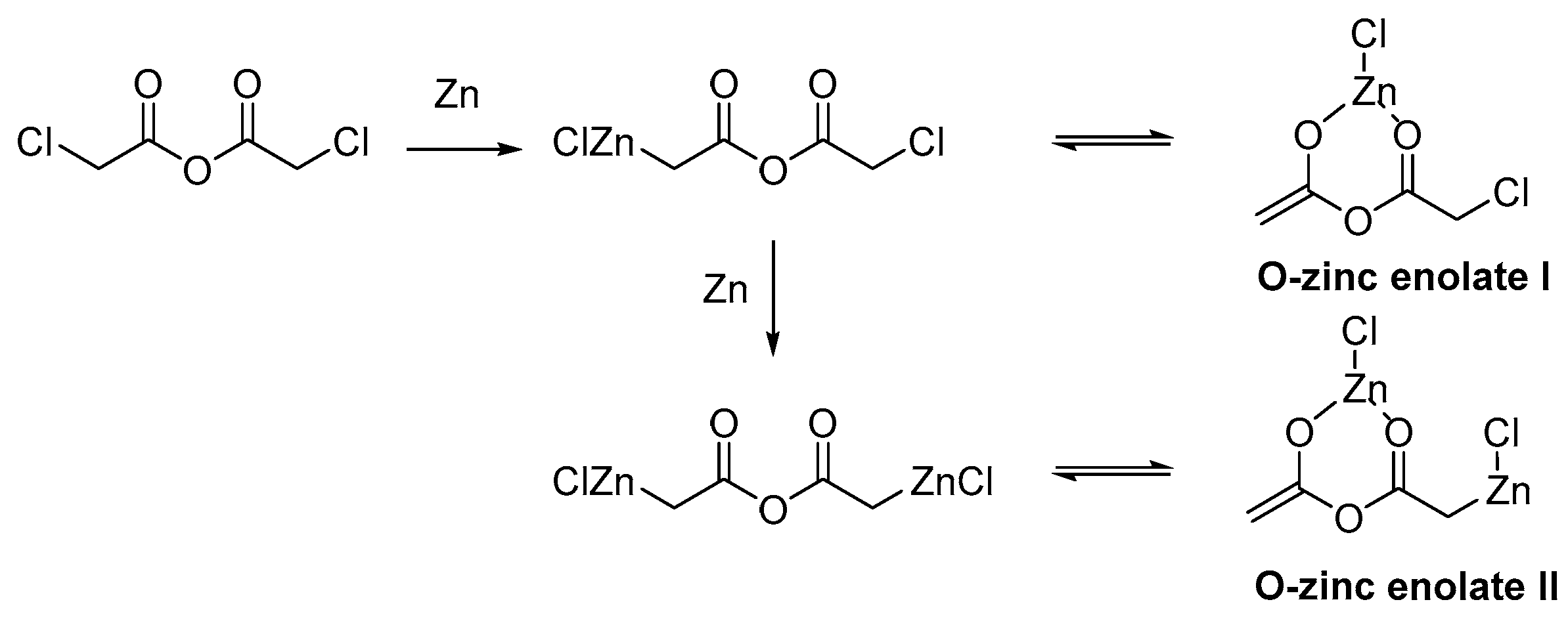
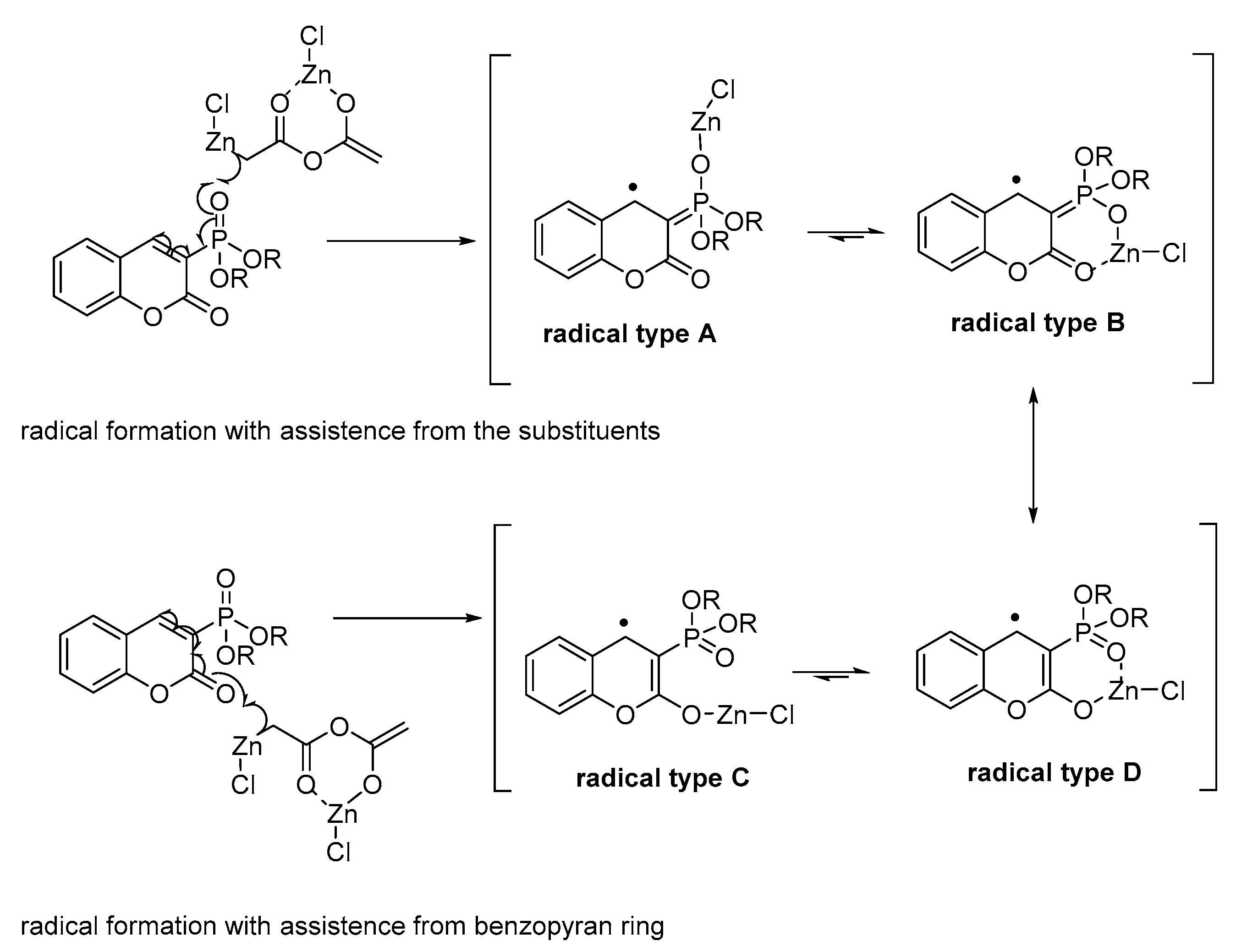
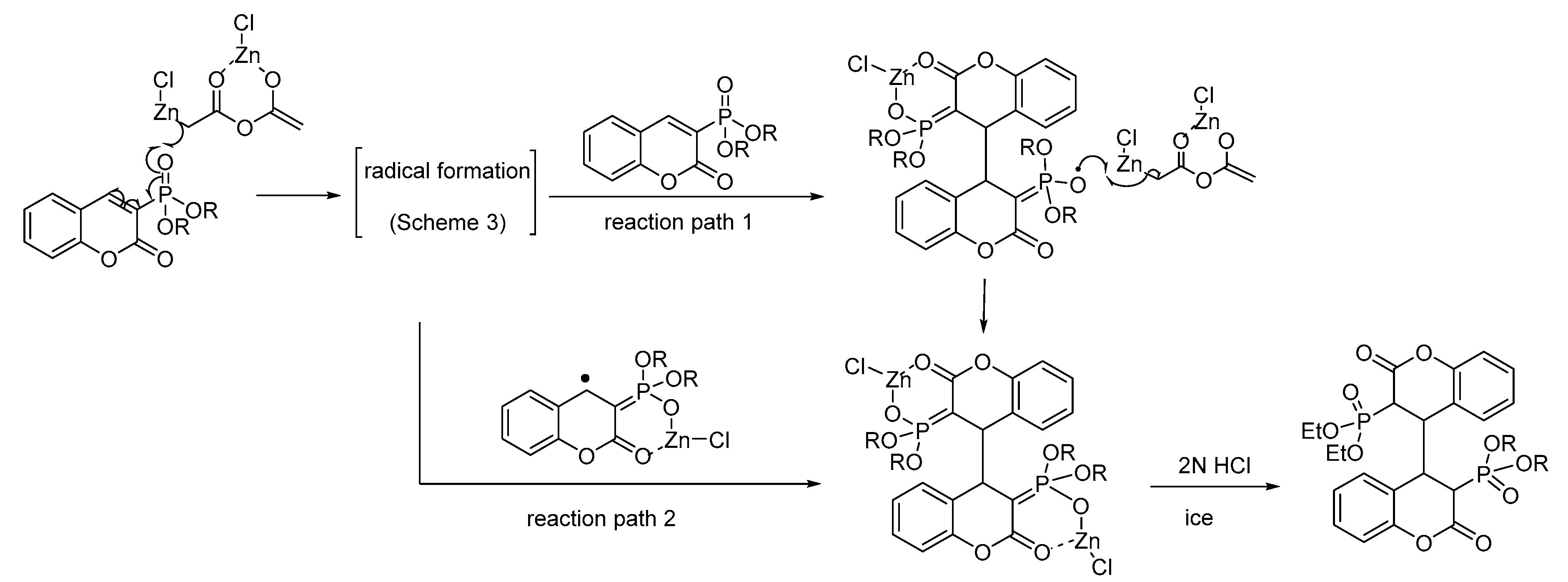
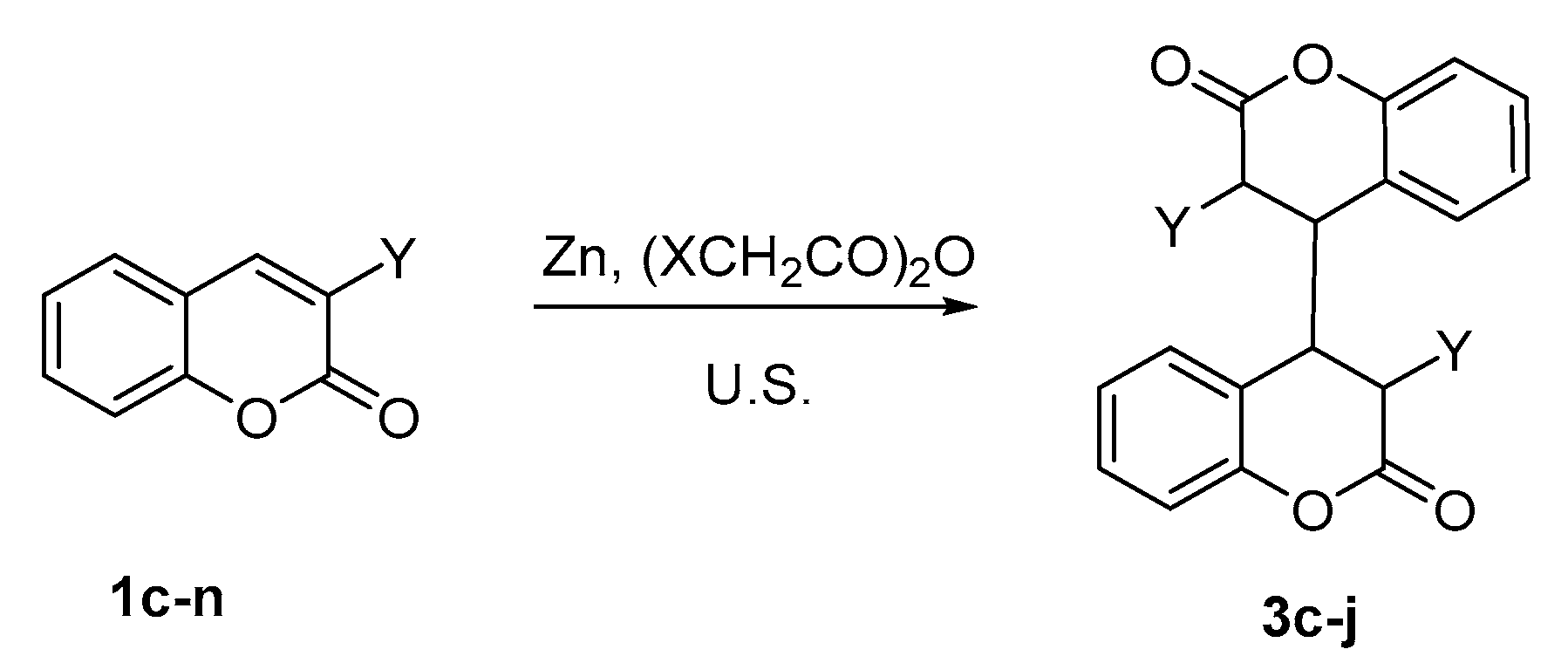

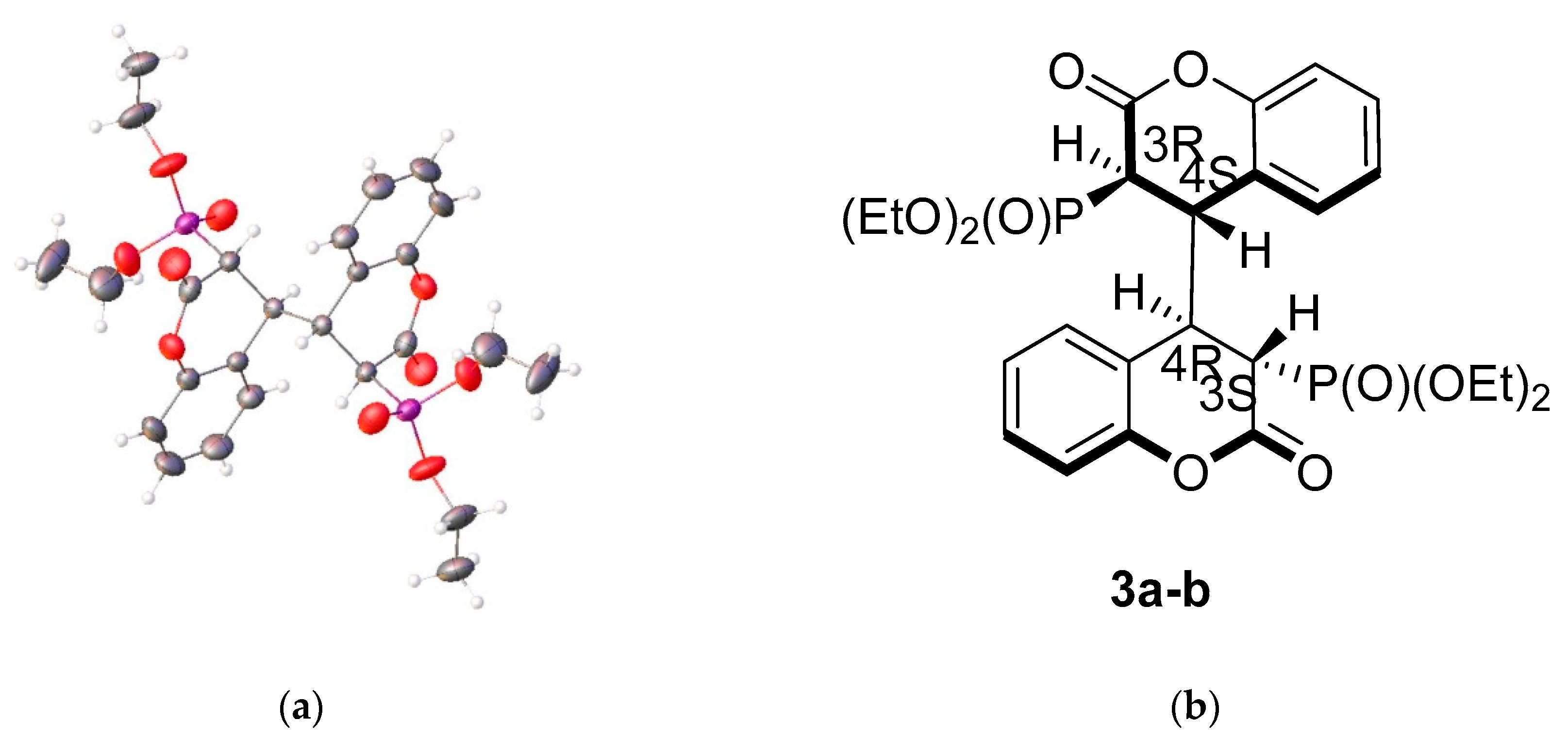
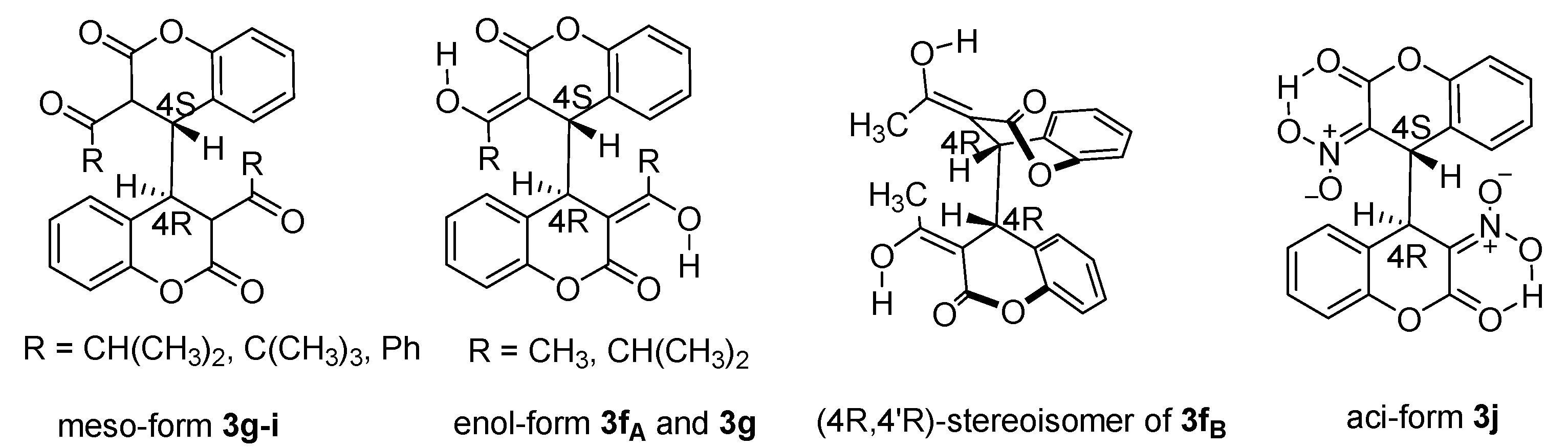
| Product | Substituent | Metal | Reaction Conditions–1: Metal: (ClCH2CO)2O | |||||
|---|---|---|---|---|---|---|---|---|
| Method A 1:2.8:2.4 | Method B 1:5.6:2.4 | Method C 1:3.4:1.5 | ||||||
| Time [min] | Yield [%] | Time [min] | Yield [%] | Time [min] | Yield [%] | |||
| 3a | P(O)(OEt)2 | Mg | 420 | 14 | - | - | - | - |
| 3a | P(O)(OEt)2 | Zn | 420 | 70 | 180 | 89 | 2040 | 30 a |
| 3b | P(O)(OMe)2 | Zn | 420 | 25 a | 200 | 61 | - | - |
| Solvent | Reaction Time [min] | Yield |
|---|---|---|
| THF | 120 | Complex mixture |
| Et2O | 420 | N/A |
| THF:Et2O (3.5:5) | 420 | 70% |
| Coumarin | Product | Y | Method A | Method B | ||
|---|---|---|---|---|---|---|
| Reaction time [min] | Yield [%] | Reaction time [min] | Yield [%] | |||
| 1c | 3c | COOEt | 300 | 60 | 30 | 73 |
| 1d | 3d | COOMe | 240 | 51 | 30 | 73 |
| 1e | 3e | COOPh | 120 | 55 | 40 | 67 |
| 1f | 3f | COMe | 180 | Complex mixture | 10 | 92 a |
| 1g | 3g | COiPr | 1020 | Partial conversion | 15 | 86 a |
| 1h | 3h | COtBu | 390 | 71 | 180 | 92 a |
| 1i | 3i | COPh | 270 | 52 | 10 | 84 |
| 1j | 3j | NO2 | 840 | Complex mixture | 90 | 45 |
| 1k | 3k | CN | 840 | Complex mixture | 270 | Complex mixture |
| 1l | 3l | H | 1020 | N/A | 120 | N/A |
| 1m | 3m | Ph | 1020 | N/A | 120 | N/A |
| 1n | 3n | NHCOMe | 720 | N/A | - | - |
| Entry | Mixed Coumarin Reaction (Method B, Equimolar Ratio for the Coumarins) | Reaction Time [min] | Products |
|---|---|---|---|
| 1 | 1a:1m | 180 | 3a:1m |
| 2 | 1a:1c | 40 | 1a:3c |
| 3 | 1f:1i | 10 | 3f:3i |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koleva, A.I.; Petkova-Yankova, N.I.; Nikolova, R.D. Ultrasound-Assisted Metal-Mediated Method for the Formation of Tetrahydro-3,3′-Disubstituted Biscoumarins. Molecules 2018, 23, 2810. https://doi.org/10.3390/molecules23112810
Koleva AI, Petkova-Yankova NI, Nikolova RD. Ultrasound-Assisted Metal-Mediated Method for the Formation of Tetrahydro-3,3′-Disubstituted Biscoumarins. Molecules. 2018; 23(11):2810. https://doi.org/10.3390/molecules23112810
Chicago/Turabian StyleKoleva, Ana I., Nevena I. Petkova-Yankova, and Rositca D. Nikolova. 2018. "Ultrasound-Assisted Metal-Mediated Method for the Formation of Tetrahydro-3,3′-Disubstituted Biscoumarins" Molecules 23, no. 11: 2810. https://doi.org/10.3390/molecules23112810






