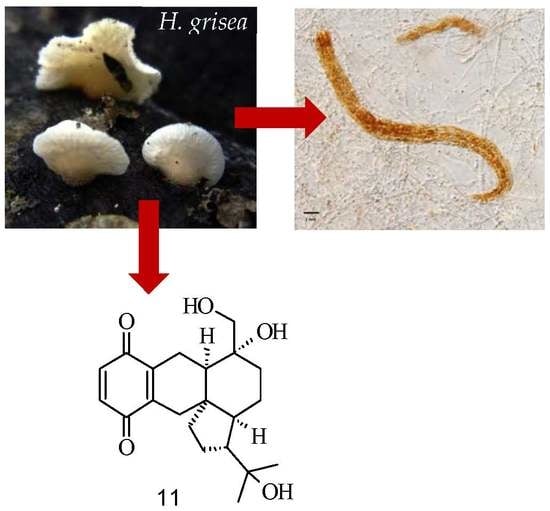
Antiviral 4-Hydroxypleurogrisein and Antimicrobial Pleurotin Derivatives from Cultures of the Nematophagous Basidiomycete Hohenbuehelia grisea
Abstract
:1. Introduction
2. Results and Discussion
Biological Activities
3. Materials and Methods
3.1. General
3.2. Fungal Material
3.3. Fermentation and Extraction
3.4. Isolation of Metabolites 5–11
3.5. Biological Activities
3.5.1. Antimicrobial Activities
3.5.2. Cytotoxicity Assay
3.5.3. Nematicidal Activity Assay
3.5.4. Inhibitory Effects on HCV Infectivity
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bills, G.F.; Gloer, J.B. Biologically Active Secondary Metabolites from the Fungi. Microbiol. Spectr. 2016, 4, 1087–1119. [Google Scholar] [CrossRef]
- Karwehl, S.; Stadler, M. Exploitation of Fungal Biodiversity for Discovery of Novel Antibiotics. Curr. Top Microbiol. Immunol. 2016, 398, 303–338. [Google Scholar] [CrossRef] [PubMed]
- Novak, R. Are pleuromutilin antibiotics finally fit for human use? Ann. N. Y. Acad. Sci. 2011, 1241, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Robbins, W.J.; Kavanagh, F.; Hervey, A. Antibiotic Substances From Basidiomycetes I. Pleurotus griseus*. Proc. Natl. Acad. Sci. USA 1947, 33, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Stadler, M.; Sheldrick, W.S.; Dasenbrock, J.; Steglich, W.; Anke, H. Antibiotics from the nematode-trapping basidiomycete Nematoctonus robustus. Nat. Prod. Lett. 1994, 4, 209–216. [Google Scholar] [CrossRef]
- Berdicevsky, I.; Kaufman, G.; Newman, D.J.; Horwitz, B.A. Preliminary study of activity of the thioredoxin inhibitor pleurotin against Trichophyton mentagrophytes: A novel anti-dermatophyte possibility. Mycoses 2009, 52, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Riondel, J.; Beriel, H.; Dardas, A.; Carraz, G.; Oddoux, L. Studies of antitumor activity of the culture filtrate of Hohenbuehelia geogenius (D.C. ex Fr.) Sing (basidiomycete). Arzneimittelforschung 1981, 31, 293–299. [Google Scholar] [PubMed]
- Hart, D.J.; Huang, H.-C.; Krishnamurthy, R.; Schwartz, T. free-radical Cyclizations: Application to the Total Synthesis of dl-Pleurotin and dl-dihydropleurotinic acid. J. Am. Chem. Soc. 1989, 111, 7507–7519. [Google Scholar] [CrossRef]
- Shipley, S.M.; Barr, A.L.; Graf, S.J.; Collins, R.P.; McCloud, T.G.; Newman, D.J. Development of a process for the production of the anticancer lead compound pleurotin by fermentation of Hohenbuehelia atrocaerulea. J. Ind. Microbiol. Biotechnol. 2006, 33, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Wipf, P.; Hopkins, T.D.; Jung, J.K.; Rodriguez, S.; Birmingham, A.; Southwick, E.C.; Lazo, J.S.; Powis, G. New inhibitors of the thioredoxin-thioredoxin reductase system based on a naphthoquinone spiroketal natural product lead. Bioorg. Med. Chem. Lett. 2001, 11, 2637–2641. [Google Scholar] [CrossRef]
- Holmgren, A.; Lu, J. Thioredoxin and thioredoxin reductase: Current research with special reference to human disease. Biochem. Biophys. Res. Commun. 2010, 396, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Sandargo, B.; Thongbai, B.; Stadler, M.; Surup, F. Cysteine-Derived Pleurotin Congeners from the Nematode-Trapping Basidiomycete Hohenbuehelia grisea. J. Nat. Prod. 2018, 81, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Capaul, B. Untersuchungen zur Biosynthese von Pleurotin. Ph.D. Thesis, ETH Zurich, Zürich, Switzerland, 1992. [Google Scholar] [CrossRef]
- Ciesek, S.; von Hahn, T.; Colpitts, C.C.; Schang, L.M.; Friesland, M.; Steinmann, J.; Manns, M.P.; Ott, M.; Wedemeyer, H.; Meuleman, P.; et al. The green tea polyphenol, epigallocatechin-3-gallate, inhibits hepatitis C virus entry. Hepatology 2011, 54, 1947–1955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuhnert, E.; Surup, F.; Herrmann, J.; Huch, V.; Müller, R.; Stadler, M. Rickenyls A-E, antioxidative terphenyls from the fungus Hypoxylon rickii (Xylariaceae, Ascomycota). Phytochemistry 2015, 118, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Richter, C.; Helaly, S.E.; Thongbai, B.; Hyde, K.D.; Stadler, M. Pyristriatins A and B: Pyridino-Cyathane Antibiotics from the Basidiomycete Cyathus cf. striatus. J. Nat. Prod. 2016, 79, 1684–1688. [Google Scholar] [CrossRef] [PubMed]
- Kuephadungphan, W.; Helaly, S.E.; Daengrot, C.; Phongpaichit, S.; Luangsa-Ard, J.J.; Rukachaisirikul, V.; Stadler, M. Akanthopyrones A–D, α-pyrones bearing a 4-O-methyl-β-D-glucopyranose moiety from the spider-associated ascomycete Akanthomyces novoguineensis. Molecules 2017, 22, 1202. [Google Scholar] [CrossRef] [PubMed]
- Mulwa, L.S.; Jansen, R.; Praditya, D.F.; Mohr, K.I.; Okanya, P.W.; Wink, J.; Steinmann, E.; Stadler, M. Lanyamycin, a macrolide antibiotic from Sorangium cellulosum, strain Soce 481 (Myxobacteria). Beilstein J. Org. Chem. 2018, 481, 1554–1562. [Google Scholar] [CrossRef] [PubMed]
- Mulwa, L.S.; Jansen, R.; Praditya, D.F.; Mohr, K.I.; Wink, J.; Steinmann, E.; Stadler, M. Six heterocyclic metabolites from the myxobacterium Labilithrix luteola. Molecules 2018, 23, 542. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |




| Pos | 5 | 6 | 7 | 8 | ||||
|---|---|---|---|---|---|---|---|---|
| δC, Type | δH (J in Hz) | δC, Type | δH (J in Hz) | δC, Type | δH (J in Hz) | δC, Type | δH (J in Hz) | |
| 1 | 31.9, CH2 | 1.50, m | 36.9, CH2 | 2.27, ddd (12.2, 9, 2.6) | 34.4, CH2 | 1.35, m | 33.6, CH2 | 1.29, m |
| 1.93, m | 1.90, m | 1.94, m | ||||||
| 2 | 34.8, CH2 | 1.53, m | 25.9, CH2 | 1.69, m | 26.4, CH2 | 1.77, m | 25.0, CH2 | 1.78, m |
| 2.30, m | 1.75, m | 1.90, m | 1.93, m | |||||
| 3 | 82.8, C | 46.0, CH | 2.08, m | 46.3, CH | 2.31, m | 43.9, CH | 2.34, m | |
| 4 | 39.1, CH | 2.14, m | 32.4, CH | 2.20, m | 34.8, CH | 2.14, m | 33.7, CH | 2.11, m |
| 5 | 59.3, CH | 1.94, m | 52.6, CH | 1.65, m | 53.3, CH | 1.86, m | 50.6, CH | 1.81, m |
| 6 | 19.3, CH2 | 1.78, m | 22.6, CH2 | 1.78, m 1.86, | 23.2, CH2 | 1.85, m | 21.7, CH2 | 1.87, m |
| 1.94, m | 1.86, td (12.9, 3.9) | 2.06, m | 1.98, m | |||||
| 7 | 30.2, CH2 | 1.63, m | 31.2, CH2 | 1.58, m | 32.3, CH2 | 1.67, qd (12.8, 4.7) | 29.5, CH2 | 1.87, m |
| 2.20, m | 2.10, m | 2.15, m | 2.23, m | |||||
| 8 | 42.9, CH | 2.13, m | 43.3, CH | 1.94, td (12.2, 4.0) | 44.9, CH | 2.35, m | 42.4, CH | 2.62, m |
| 9 | 43.5, CH | 2.05, dd (12, 3.2) | 51.4, CH | 2.06, m | 46.2, CH | 1.99, dd (12, 5.8) | 58.9, CH | 2.63, m |
| 10 | 44.5, C | 46.1, C | 48.0, C | 49.8, C | ||||
| 11 | 75.9, CH2 | 3.69, dd (13.2, 3.6) | 75.3, CH2 | 3.34, dd (12.3, 6.9) | 77.6, CH2 | 3.91, dd (12.8, 8.0) | 77.6, CH2 | 4.02, br d (12.8) |
| 4.02, dd (13.2, 8.3) | 3.96, dd (12.3, 8.6) | 4.18, dd (12.8, 2.0) | 4.12, dd (12.8, 8.0) | |||||
| 12 | 16.6, CH3 | 1.09, d (6.9) | 21.3, CH3 | 0.93, d (7.0) | 21.8, CH3 | 1.07, d (7.3) | 20.7, CH3 | 1.10, d (7.3) |
| 13 | 179.6, C | 176.8, C | 177.5, C | 176.8, C | ||||
| 14 | 24.2, CH2 | 2.51, m | 63.9, CH | 4.55, br s | 26.5, CH2 | 2.66, dd (17.5, 6.2) | 202.7, C | |
| 2.74, br d (17.5) | ||||||||
| 15 | 74.2, CH | 4.43, s | 73.2, CH | 4.46, s | 82.9, CH | 5.0, s | 82.2, CH | 5.21, s |
| 16 | 140.7, C | 141.8, C | 123.8, C | 113.6, C | ||||
| 17 | 139.8, C | 139.9, C | 121.6, C | 121.6, C | ||||
| 18 | 186.3, C | 187.6, C | 152.8, C | 149.7, C | ||||
| 19 | 137.7, CH | 6.70 *, d (10.2) | 138.6, CH | 6.79, d (10.2) | 114.9, CH | 6.43, d (8.5) | 128.0, CH | 7.09, d (9.0) |
| 20 | 135.6, CH | 6.70 *, d (10.2) | 136.8, CH | 6.78, d (10.2) | 115.8, CH | 6.62, d (8.5) | 119.0, CH | 6.88, d (9.0) |
| 21 | 186.8, C | 187.8, C | 148.8, C | 157.4, C | ||||
| 14-OH | 4.16, br s | |||||||
| 18-OH | 8.17, s | 8.71 | ||||||
| Pos | 9 | 10 | 11 | |||
|---|---|---|---|---|---|---|
| δC, Type | δH (J in Hz) | δC, Type | δH (J in Hz) | δC, Type | δH (J in Hz) | |
| 1 | 32.0, CH2 | 2.10, m | 36.9, CH2 | 1.89, m | 39.4, CH2 | 1.11, m |
| 1.33, m | 1.59, m | 1.54, dd (11.6, 7.1) | ||||
| 2 | 30.0, CH2 | 1.9, m | 28.5, CH2 | 1.41, m | 27.22, CH2 | 1.73, m |
| 2.12, m | 1.95, m | 1.83, m | ||||
| 3 | 47.3, CH | 3.04, m | 41.6, CH | 2.11, m | 50.9, CH | 2.31, m |
| 4 | 147.7, C | 37.0, CH | 2.38, m | 72.8, C | ||
| 5 | 52.5, CH | 1.69, m | 50.3, CH | 1.90, m | 54.55, CH | 1.94, m |
| 6 | 22.9, CH2 | 1.9, m | 23.3, CH2 | 2.11, m | 23.2, CH2 | 1.72, m |
| 1.56, m | 1.90, m | 2.05, m | ||||
| 7 | 24.0, CH2 | 1.47, dd (12.3, 3.9) 2.27, m | 30.3, CH2 | 2.14, m | 39.4, CH2 | 1.23, m |
| 1.74, m | 2.29, m | |||||
| 8 | 39.3, CH | 2.27, m | 45.9, CH | 2.54, td (12.1, 4.0) | 74.8, C | |
| 9 | 50.4, CH | 2.44, d (7.1) | 60.5, CH | 2.81, d (12.1) | 51.3, CH | 1.8, m |
| 10 | 48.9, C | 59.4, C | 43.8, C | |||
| 11 | 73.7, CH2 | 4.87, d (16.0) | 67.4, CH2 | 3.64, dd (10.6, 3.3) | 29.9, CH3 | 1.18, s |
| 4.50, d (16.0) | 3.46, dd (10.6, 6.1) | |||||
| 12 | 110.0, CH2 | 4.79, br s 4.88, br s | 18.4, CH3 | 1.14, d (6.6) | 31.5, CH3 | 1.26, s |
| 13 | 174.3, C | 174.9, C | 63.9, CH2 | 3.12, dd (5, 1.7) | ||
| 3.40, dd (10.6, 5.0) | ||||||
| 14 | 74.9, CH | 5.64, d (7.3) | 202.3, C | 22.3, CH2 | 2.29, m | |
| 3.03, m | ||||||
| 15 | 80.0, CH | 4.97, s | 205.3, C | 27.16 CH2 | 2.15, m | |
| 2.61, m | ||||||
| 16 | 118.4, C | 112.2, C | 142.6, C | |||
| 17 | 118.4, C | 112.9, C | 142.9, C | |||
| 18 | 152.2, C | 155.8, C | 188.1, C | |||
| 19 | 118.8, CH | 6.87, d (8.8) | 129.0, CH | 7.24 *, d (0.6) | 137.4, CH | 6.73 *, s |
| 20 | 119.5, CH | 6.82, d (8.2) | 128.5, CH | 7.24 *, d (0.6) | 137.2, CH | 6.73 *, s |
| 21 | 149.4, C | 155.3, C | 187.8, C | |||
| 4-OH | 3.14, br s | |||||
| 8-OH | 3.48, br s | |||||
| 13-OH | 2.84, br s | |||||
| 18-OH | 8.61, s | 11.88, s | ||||
| 21-OH | 11.64, s | |||||
| Organism | MIC (µg/mL) | Reference (MIC) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 * | 2 * | 3 * | 5 | 6 | 7 | 8 | 9 | 10 | 11 | ||
| Mucor plumbeus MUCL49355 | 100 | - | 100 | - | - | - | - | - | - | - | Nystatin (12.5) |
| Candida tenuis MUCL29892 | 25 | 100 | 100 | 100 | - | - | - | 25 | - | - | Nystatin (12.5) |
| Bacillus subtilis DSM10 | 50 | 25 | 100 | 100 | - | - | 50 | - | 100 | 50 | Penicillin (6.3) |
| Pichia anomala DSM6766 | 66.7 | 66.7 | 66.7 | - | - | - | - | - | - | - | Nystatin (16.7) |
| Candida albicans DSM1665 | 33.3 | - | - | - | - | - | - | - | - | - | Nystatin (16.7) |
| Rhodotorula glutinis DSM10134 | 16.7 | 33.3 | 33.3 | 33.3 | - | - | - | - | - | - | Nystatin (16.7) |
| Mucor hiemalis DSM2656 | 8.3 | 33.3 | 16.7 | - | - | - | - | - | - | - | Nystatin (16.7) |
| Micrococcus luteus DSM1790 | 66.7 | 66.7 | - | - | - | - | - | - | - | 66.7 | Oxytetracycline (0.4) |
| Staphylococcus aureus DSM346 | 33.3 | 33.3 | 66.7 | - | - | - | - | - | - | 33.3 | Oxytetracycline (6.7) |
| Cell line | IC50 (µg/mL) | Reference (IC50) | |||||||||
| L929 (IC50) | 7.5 | 2.2 | 18 | 22 | 23 | 17 | 22 | 21 | 22 | 6.9 | Epothilone B (0.00062) |
| KB3.1 (IC50) | 8.5 | 2.8 | 18 | 22 | 22 | 20 | 22 | 18 | 22 | 7.5 | Epothilone B (0.00003) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sandargo, B.; Thongbai, B.; Praditya, D.; Steinmann, E.; Stadler, M.; Surup, F. Antiviral 4-Hydroxypleurogrisein and Antimicrobial Pleurotin Derivatives from Cultures of the Nematophagous Basidiomycete Hohenbuehelia grisea. Molecules 2018, 23, 2697. https://doi.org/10.3390/molecules23102697
Sandargo B, Thongbai B, Praditya D, Steinmann E, Stadler M, Surup F. Antiviral 4-Hydroxypleurogrisein and Antimicrobial Pleurotin Derivatives from Cultures of the Nematophagous Basidiomycete Hohenbuehelia grisea. Molecules. 2018; 23(10):2697. https://doi.org/10.3390/molecules23102697
Chicago/Turabian StyleSandargo, Birthe, Benjarong Thongbai, Dimas Praditya, Eike Steinmann, Marc Stadler, and Frank Surup. 2018. "Antiviral 4-Hydroxypleurogrisein and Antimicrobial Pleurotin Derivatives from Cultures of the Nematophagous Basidiomycete Hohenbuehelia grisea" Molecules 23, no. 10: 2697. https://doi.org/10.3390/molecules23102697