Enantioseparation, Stereochemical Assignment and Chiral Recognition Mechanism of Sulfoxide-Containing Drugs
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chiral Resolution of 1–4
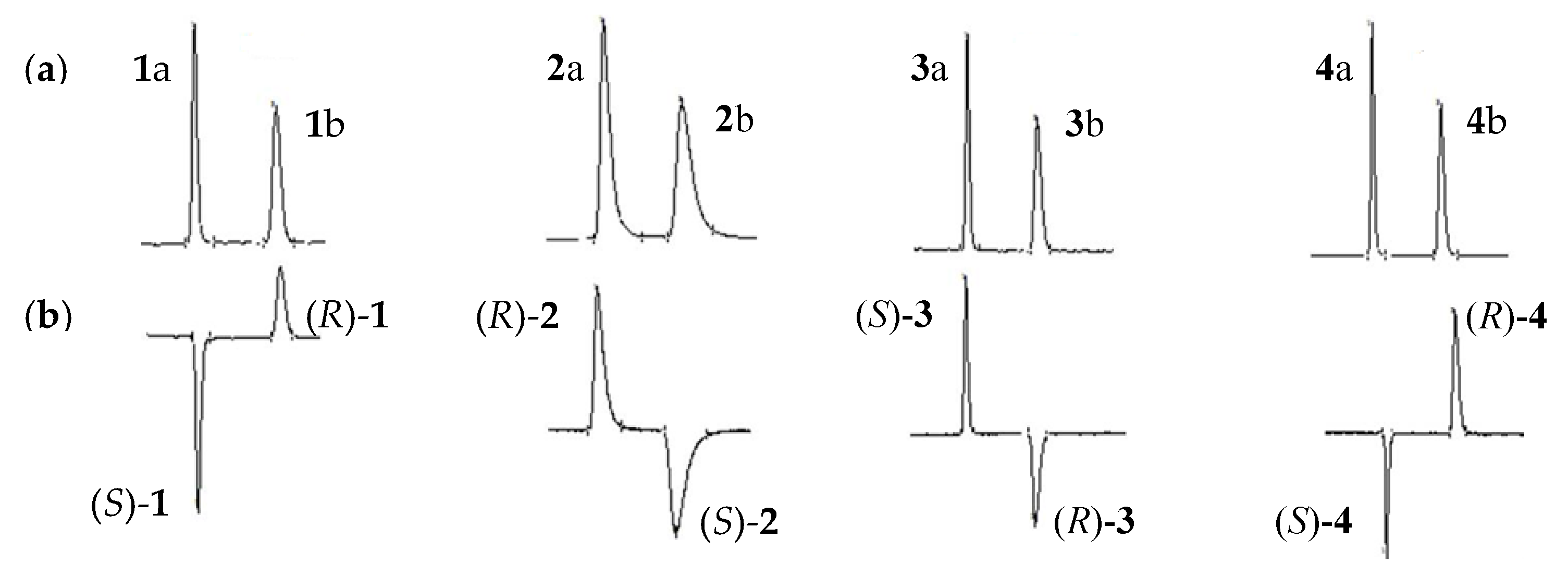
2.1.1. Factors Affecting Optical Resolution
2.1.2. The Optimal Resolution and Enantiomeric Elution Order
2.2. AC Assignments of 1–4
2.2.1. Experimental UV and ECD Spectra
2.2.2. TDDFT Calculation of UV/ECD Spectra
2.2.3. Electron Transitions
2.3. Chiral Separation Mechanism and Molecular Docking
2.3.1. Common Structural Features
2.3.2. Molecular Interactions between Analytes and CSPs
2.3.3. Mean Binding Energy
3. Materials and Methods
3.1. Materials and Reagents
3.2. Chromatographic Conditions
3.3. ECD Experiments
3.4. Computational Methods
3.4.1. TDDFT Computations
3.4.2. Molecular Docking
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bentley, R. Role of sulfur chirality in the chemical processes of biology. Chem. Soc. Rev. 2005, 34, 609–624. [Google Scholar] [CrossRef] [PubMed]
- Matsui, T.; Dekishima, Y.; Ueda, M. Biotechnological production of chiral organic sulfoxides: Current state and perspectives. Appl. Microbiol. Biotechnol. 2014, 98, 7699–7706. [Google Scholar] [CrossRef] [PubMed]
- Goundry, W.R.F.; Adams, B.; Benson, H.; Demeritt, J.; McKown, S.; Mulholland, K.; Robertson, A.; Siedlecki, P.; Tomlin, P.; Vare, K. Development and Scale-up of a Biocatalytic Process to Form a Chiral Sulfoxide. Org. Process Res. Dev. 2017, 21, 107–113. [Google Scholar] [CrossRef]
- Otocka, S.; Kwiatkowska, M.; Madalińska, L.; Kiełbasiński, P. Chiral organosulfur ligands/catalysts with a stereogenic sulfur atom: Applications in asymmetric synthesis. Chem. Rev. 2017, 117, 4147–4181. [Google Scholar] [CrossRef] [PubMed]
- Trost, B.M.; Rao, M. Development of chiral sulfoxide ligands for asymmetric catalysis. Angew. Chem. Int. Ed. 2015, 54, 5026–5043. [Google Scholar] [CrossRef] [PubMed]
- Andersson, T.; Weidolf, L. Stereoselective disposition of proton pump inhibitors. Clin. Drug Investig. 2008, 28, 263–279. [Google Scholar] [CrossRef] [PubMed]
- Katsuki, H.; Yagi, H.; Arimori, K.; Nakamura, C.; Nakano, M.; Katafuchi, S.; Fujioka, Y.; Fujiyama, S. Determination of R (+)-and S (–)-lansoprazole using chiral stationary-phase liquid chromatography and their enantioselective pharmacokinetics in humans. Pharm. Res. 1996, 13, 611–615. [Google Scholar] [CrossRef] [PubMed]
- Donovan, J.L.; Malcolm, R.J.; Markowitz, J.S.; DeVane, C.L. Chiral analysis of d-and l-modafinil in human serum: Application to human pharmacokinetic studies. Ther. Drug Monit. 2003, 25, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Bravi, L.; Rudini, N.; Cuttano, R.; Giampietro, C.; Maddaluno, L.; Ferrarini, L.; Adams, R.H.; Corada, M.; Boulday, G.; Tournier-Lasserve, E.; et al. Sulindac metabolites decrease cerebrovascular malformations in CCM3-knockout mice. Proc. Natl. Acad. Sci. USA 2015, 112, 8421–8426. [Google Scholar] [CrossRef] [PubMed]
- Hwu, J.R.; Tsay, S.C.; Chuang, K.S.; Kapoor, M.; Lin, J.Y.; Yeh, C.S.; Su, W.C.; Wu, P.C.; Tsai, T.L.; Wang, P.W.; et al. Syntheses of Platinum-Sulindac Complexes and Their Nanoparticles as Targeted Anticancer Drugs. Chemistry (Easton) 2016, 22, 1926–1930. [Google Scholar] [CrossRef] [PubMed]
- Scriba, G.K. Cyclodextrins in capillary electrophoresis enantioseparations--recent developments and applications. J. Sep. Sci. 2008, 31, 1991–2011. [Google Scholar] [CrossRef] [PubMed]
- Jac, P.; Scriba, G.K. Recent advances in electrodriven enantioseparations. J. Sep. Sci. 2013, 36, 52–74. [Google Scholar] [CrossRef] [PubMed]
- Hein, J.E.; Cao, B.H.; van der Meijden, M.W.; Leeman, M.; Kellogg, R.M. Resolution of Omeprazole Using Coupled Preferential Crystallization: Efficient Separation of a Nonracemizable Conglomerate Salt under Near-Equilibrium Conditions. Org. Process Res. Dev. 2013, 17, 946–950. [Google Scholar] [CrossRef] [Green Version]
- Bortolini, O.; Fantin, G.; Fogagnolo, M.; Medici, A.; Pedrini, P. Optical resolution of sulfoxides by inclusion in host dehydrocholic acid. Chem. Commun. 2000, 365–366. [Google Scholar] [CrossRef]
- Ferretti, R.; Carradori, S.; Guglielmi, P.; Pierini, M.; Casulli, A.; Cirilli, R. Enantiomers of triclabendazole sulfoxide: Analytical and semipreparative HPLC separation, absolute configuration assignment, and transformation into sodium salt. J. Pharm. Biomed. Anal. 2017, 140, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Gegenava, M.; Chankvetadze, L.; Farkas, T.; Chankvetadze, B. Enantioseparation of selected chiral sulfoxides in high-performance liquid chromatography with polysaccharide-based chiral selectors in polar organic mobile phases with emphasis on enantiomer elution order. J. Sep. Sci. 2014, 37, 1083–1088. [Google Scholar] [CrossRef] [PubMed]
- Sardella, R.; Ianni, F.; Di Michele, A.; Di Capua, A.; Carotti, A.; Anzini, M.; Natalini, B. Enantioresolution and stereochemical characterization of two chiral sulfoxides endowed with COX-2 inhibitory activity. Chirality 2017, 29, 536–540. [Google Scholar] [CrossRef] [PubMed]
- Cavazzini, A.; Pasti, L.; Massi, A.; Marchetti, N.; Dondi, F. Recent applications in chiral high performance liquid chromatography: A review. Anal. Chim. Acta 2011, 706, 205–222. [Google Scholar] [CrossRef] [PubMed]
- del Nozal, M.J.; Toribio, L.; Bernal, J.L.; Alonso, C.; Jiménez, J.J. Chiral separation of omeprazole and several related benzimidazoles using supercritical fluid chromatography. J. Sep. Sci. 2004, 27, 1023–1029. [Google Scholar] [CrossRef] [PubMed]
- West, C.; Konjaria, M.L.; Shashviashvili, N.; Lemasson, E.; Bonnet, P.; Kakava, R.; Volonterio, A.; Chankvetadze, B. Enantioseparation of novel chiral sulfoxides on chlorinated polysaccharide stationary phases in supercritical fluid chromatography. J. Chromatogr. A 2017, 1499, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Okamoto, Y. Efficient separation of enantiomers using stereoregular chiral polymers. Chem. Rev. 2016, 116, 1094–1138. [Google Scholar] [CrossRef] [PubMed]
- Dixit, S.; Dubey, R.; Bhushan, R. Normal and polar-organic-phase high-performance liquid chromatographic enantioresolution of omeprazole, rabeprazole, lansoprazole and pantoprazole using monochloro-methylated cellulose-based chiral stationary phase and determination of dexrabeprazole. Biomed. Chromatogr. 2014, 28, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Balmér, K.; Persson, B.-A.; Lagerström, P.-O. Stereoselective effects in the separation of enantiomers of omeprazole and other substituted benzimidazoles on different chiral stationary phases. J. Chromatogr. A 1994, 660, 269–273. [Google Scholar] [CrossRef]
- Cirilli, R.; Ferretti, R.; Gallinella, B.; De Santis, E.; Zanitti, L.; La Torre, F. High-performance liquid chromatography enantioseparation of proton pump inhibitors using the immobilized amylose-based Chiralpak IA chiral stationary phase in normal-phase, polar organic and reversed-phase conditions. J. Chromatogr. A 2008, 1177, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, R.; Zanitti, L.; Casulli, A.; Cirilli, R. Green high-performance liquid chromatography enantioseparation of lansoprazole using a cellulose-based chiral stationary phase under ethanol/water mode. J. Sep. Sci. 2016, 39, 1418–1424. [Google Scholar] [CrossRef] [PubMed]
- Cass, Q.B.; Degani, A.L.G.; Cassiano, N.M. Effects on enantioselectivity by the use of polysaccharide-based columns by multimodal elution. J. Liq. Chromatogr. Relat. Technol. 2003, 26, 2083–2101. [Google Scholar] [CrossRef]
- Cass, Q.B.; Kohn, C.K.; Calafatti, S.A.; Aboul-Enein, H.Y. An enantioselective assay for (±)-modafinil. J. Pharm. Biomed. Anal. 2001, 26, 123–130. [Google Scholar] [CrossRef]
- Harvanová, M.; Gondová, T. New enantioselective LC method development and validation for the assay of modafinil. J. Pharm. Biomed. Anal. 2017, 138, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Nageswara Rao, R.; Shinde, D.D.; Kumar Talluri, M.V. Enantioselective HPLC resolution of synthetic intermediates of armodafinil and related substances. J. Sep. Sci. 2008, 31, 981–989. [Google Scholar] [CrossRef] [PubMed]
- Hauck, W.; Adam, P.; Bobier, C.; Landmesser, N. Use of large−scale chromatography in the preparation of armodafinil. Chirality 2008, 20, 896–899. [Google Scholar] [CrossRef] [PubMed]
- Naso, F.; Cardellicchio, C.; Affortunato, F.; Capozzi, M.A.M. Asymmetric synthesis of Sulindac esters by enantioselective sulfoxidation in the presence of chiral titanium complexes. Tetrahedron-Asymmetr. 2006, 17, 3226–3229. [Google Scholar] [CrossRef]
- Liao, S.; Čorić, I.; Wang, Q.; List, B. Activation of H2O2 by chiral confined Brønsted acids: A highly enantioselective catalytic sulfoxidation. J. Am. Chem. Soc. 2012, 134, 10765–10768. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Q.; Han, X.; He, L.; Beesley, T.E.; Trahanovsky, W.S.; Armstrong, D.W. Chromatographic evaluation of poly (trans-1,2-cyclohexanediyl-bis acrylamide) as a chiral stationary phase for HPLC. J. Chromatogr. A 2005, 1066, 55–70. [Google Scholar] [CrossRef] [PubMed]
- Slovakova, A.; Von Maltzan, X.F.; Patel, B.; Drake, A.; Hutt, A. Chromatographic resolution, chiroptical characterization and urinary excretion of the enantiomers of sulindac. Chromatographia 1998, 48, 369–376. [Google Scholar] [CrossRef]
- Barnhart, W.W.; Gahm, K.H.; Hua, Z.; Goetzinger, W. Supercritical fluid chromatography comparison of the poly (trans-1,2-cyclohexanediyl-bis acrylamide) (P-CAP) column with several derivatized polysaccharide-based stationary phases. J. Chromatogr. B 2008, 875, 217–229. [Google Scholar] [CrossRef] [PubMed]
- West, C.; Guenegou, G.; Zhang, Y.; Morin-Allory, L. Insights into chiral recognition mechanisms in supercritical fluid chromatography. II. Factors contributing to enantiomer separation on tris-(3,5-dimethylphenylcarbamate) of amylose and cellulose stationary phases. J. Chromatogr. A 2011, 1218, 2033–2057. [Google Scholar] [CrossRef] [PubMed]
- Von Unge, S.; Langer, V.; Sjölin, L. Stereochemical assignment of the enantiomers of omeprazole from X-ray analysis of a fenchyloxymethyl derivative of (+)-(R)-omeprazole. Tetrahedron-Asymmetr. 1997, 8, 1967–1970. [Google Scholar] [CrossRef]
- Prisinzano, T.; Podobinski, J.; Tidgewell, K.; Luo, M.; Swenson, D. Synthesis and determination of the absolute configuration of the enantiomers of modafinil. Tetrahedron-Asymmetr. 2004, 15, 1053–1058. [Google Scholar] [CrossRef]
- Andersson, S.; Nelander, H.; Öhlén, K. Preparative chiral chromatography and chiroptical characterization of enantiomers of omeprazole and related benzimidazoles. Chirality 2007, 19, 706–715. [Google Scholar] [CrossRef] [PubMed]
- Berova, N.; Di Bari, L.; Pescitelli, G. Application of electronic circular dichroism in configurational and conformational analysis of organic compounds. Chem. Soc. Rev. 2007, 36, 914–931. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, L.; Si, Y.K. Electronic circular dichroism behavior of chiral Phthiobuzone. Acta Pharm. Sin. B 2014, 4, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Si, Y.K. Study on the absolute configuration of levetiracetam via density functional theory calculations of electronic circular dichroism and optical rotatory dispersion. J. Pharm. Biomed. Anal. 2011, 56, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Si, Y.K. Study on the absolute configurations of 3-alkylphthalides using TDDFT calculations of chiroptical properties. Chirality 2012, 24, 987–993. [Google Scholar] [CrossRef] [PubMed]
- Rossi, D.; Nasti, R.; Collina, S.; Mazzeo, G.; Ghidinelli, S.; Longhi, G.; Memo, M.; Abbate, S. The role of chirality in a set of key intermediates of pharmaceutical interest, 3-aryl-substituted-γ-butyrolactones, evidenced by chiral HPLC separation and by chiroptical spectroscopies. J. Pharm. Biomed. Anal. 2017, 144, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Lai, W.; Sun, J.; Hu, G.; Liu, W. Probing the chiral separation mechanism and the absolute configuration of malathion, malaoxon and isomalathion enantiomers by chiral high performance liquid chromatography coupled with chiral detector-binding energy computations. J. Chromatogr. A 2013, 1281, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Persson, B.-A.; Andersson, S. Unusual effects of separation conditions on chiral separations. J. Chromatogr. A 2001, 906, 195–203. [Google Scholar] [CrossRef]
- Wang, T.; Chen, Y.W.; Vailaya, A. Enantiomeric separation of some pharmaceutical intermediates and reversal of elution orders by high-performance liquid chromatography using cellulose and amylose tris (3,5-dimethylphenyl- carbamate) derivatives as stationary phases. J. Chromatogr. A 2000, 902, 345–355. [Google Scholar] [CrossRef]
- Donnoli, M.I.; Giorgio, E.; Superchi, S.; Rosini, C. Circular dichroism spectra and absolute configuration of some aryl methyl sulfoxides. Org. Biomol. Chem. 2003, 1, 3444–3449. [Google Scholar] [CrossRef] [PubMed]
- Pescitelli, G.; Bruhn, T. Good computational practice in the assignment of absolute configurations by TDDFT calculations of ECD spectra. Chirality 2016, 28, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Pescitelli, G.; Di Pietro, S.; Cardellicchio, C.; Capozzi, M.A.M.; Di Bari, L. Systematic investigation of CD spectra of aryl benzyl sulfoxides interpreted by means of TDDFT calculations. J. Org. Chem. 2010, 75, 1143–1154. [Google Scholar] [CrossRef] [PubMed]
- Lammerhofer, M. Chiral recognition by enantioselective liquid chromatography: Mechanisms and modern chiral stationary phases. J. Chromatogr. A 2010, 1217, 814–856. [Google Scholar] [CrossRef] [PubMed]
- Chankvetadze, B. Recent developments on polysaccharide-based chiral stationary phases for liquid-phase separation of enantiomers. J. Chromatogr. A 2012, 1269, 26–51. [Google Scholar] [CrossRef] [PubMed]
- Pirkle, W.H.; Pochapsky, T.C. Considerations of chiral recognition relevant to the liquid chromatographic separation of enantiomers. Chem. Rev. 1989, 89, 347–362. [Google Scholar] [CrossRef]
- Barreiro, J.C.; de Campos Lourenço, T.; Silva, L.M.A.; Venâncio, T.; Cass, Q.B. High resolution magic angle spinning NMR as a tool for unveiling the molecular enantiorecognition of omeprazole by amylose-based chiral phase. Analyst 2014, 139, 1350–1354. [Google Scholar] [CrossRef] [PubMed]
- MOE2009.10, Chemical Computing Group Inc. Available online: www.chemcomp.com (accessed on 15 October 2018).
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2010. [Google Scholar]
- Ye, Y.K.; Bai, S.; Vyas, S.; Wirth, M.J. NMR and computational studies of chiral discrimination by amylose tris (3,5-dimethylphenylcarbamate). J. Phys. Chem. B 2007, 111, 1189–1198. [Google Scholar] [CrossRef] [PubMed]
- AutoDock. Available online: http://autodock.scripps.edu (accessed on 15 October 2018).
Sample Availability: Samples of the enantiopure isomers of compounds 1–4 are available from the authors. |



| CSP | Compound | Mobile Phase (v/v) | tR1 (min) | tR2 (min) | k1 | k2 | α | Rs | Sign e |
|---|---|---|---|---|---|---|---|---|---|
| AD-H | 1 | n-Hex:EtOH (60:40) a,c | 12.97 | 20.26 | 3.32 | 5.80 | 1.75 | 6.63 | (S)-(−) |
| 2 | n-Hex:IPA (80:20) a,d | 18.89 | 21.04 | 5.30 | 6.01 | 1.13 | 2.02 | (S)-(−) | |
| 3 | n-Hex:EtOH (80:20) a,c | 19.88 | 26.00 | 2.98 | 4.20 | 1.45 | 5.85 | (R)-(−) | |
| 4 | n-Hex:FA:EtOH (80:0.1:20) a,c | 14.96 | 22.87 | 1.99 | 3.57 | 1.79 | 8.39 | (S)-(−) | |
| AS-H | 1 | n-Hex:EtOH (60:40) b,c | 8.11 | 12.45 | 1.70 | 3.15 | 1.85 | 5.54 | (R)-(+) |
| 3 | n-Hex:EtOH (60:40) b,d | 13.64 | 21.83 | 1.73 | 3.37 | 1.95 | 7.46 | (S)-(+) | |
| OD-H | 1 | n-Hex:EtOH (90:10) a,d | 18.16 | 22.40 | 5.05 | 6.47 | 1.28 | 2.54 | (S)-(−) |
| 2 | n-Hex:IPA (80:20) a,c | 18.48 | 27.43 | 5.16 | 8.14 | 1.58 | 3.48 | (R)-(+) | |
| OJ-H | 2 | n-Hex:EtOH (95:5) a,d | 33.95 | 36.68 | 10.31 | 11.22 | 1.09 | 1.67 | (R)-(+) |
| 3 | n-Hex:EtOH (55:45) b,d | 16.06 | 21.01 | 2.21 | 3.20 | 1.46 | 5.43 | (S)-(+) |
| Entry | Mean Binding Energy (kcal/mol) | The Length of Hydrogen Bond (Å) | Elution Time (min) | Rs |
|---|---|---|---|---|
| (R)-1 | −5.87 | 1.932 | 20.26 | 6.63 |
| (S)-1 | −5.43 | 2.003 | 12.97 | |
| (R)-2 | −6.71 | 2.054 | 21.04 | 2.02 |
| (S)-2 | −6.01 | 2.232 | 18.89 | |
| (R)-3 | −7.19 | 2.195 | 19.88 | 5.85 |
| (S)-3 | −7.82 | 2.090 | 26.00 | |
| (R)-4 | −7.92 | 1.762 | 22.87 | 8.39 |
| (S)-4 | −6.52 | 2.016 | 14.96 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiong, F.; Yang, B.-B.; Zhang, J.; Li, L. Enantioseparation, Stereochemical Assignment and Chiral Recognition Mechanism of Sulfoxide-Containing Drugs. Molecules 2018, 23, 2680. https://doi.org/10.3390/molecules23102680
Xiong F, Yang B-B, Zhang J, Li L. Enantioseparation, Stereochemical Assignment and Chiral Recognition Mechanism of Sulfoxide-Containing Drugs. Molecules. 2018; 23(10):2680. https://doi.org/10.3390/molecules23102680
Chicago/Turabian StyleXiong, Fei, Bei-Bei Yang, Jie Zhang, and Li Li. 2018. "Enantioseparation, Stereochemical Assignment and Chiral Recognition Mechanism of Sulfoxide-Containing Drugs" Molecules 23, no. 10: 2680. https://doi.org/10.3390/molecules23102680






