Strong Tetrel Bonds: Theoretical Aspects and Experimental Evidence
Abstract
:1. Introduction
2. Systems and Methods
3. Results and Discussion
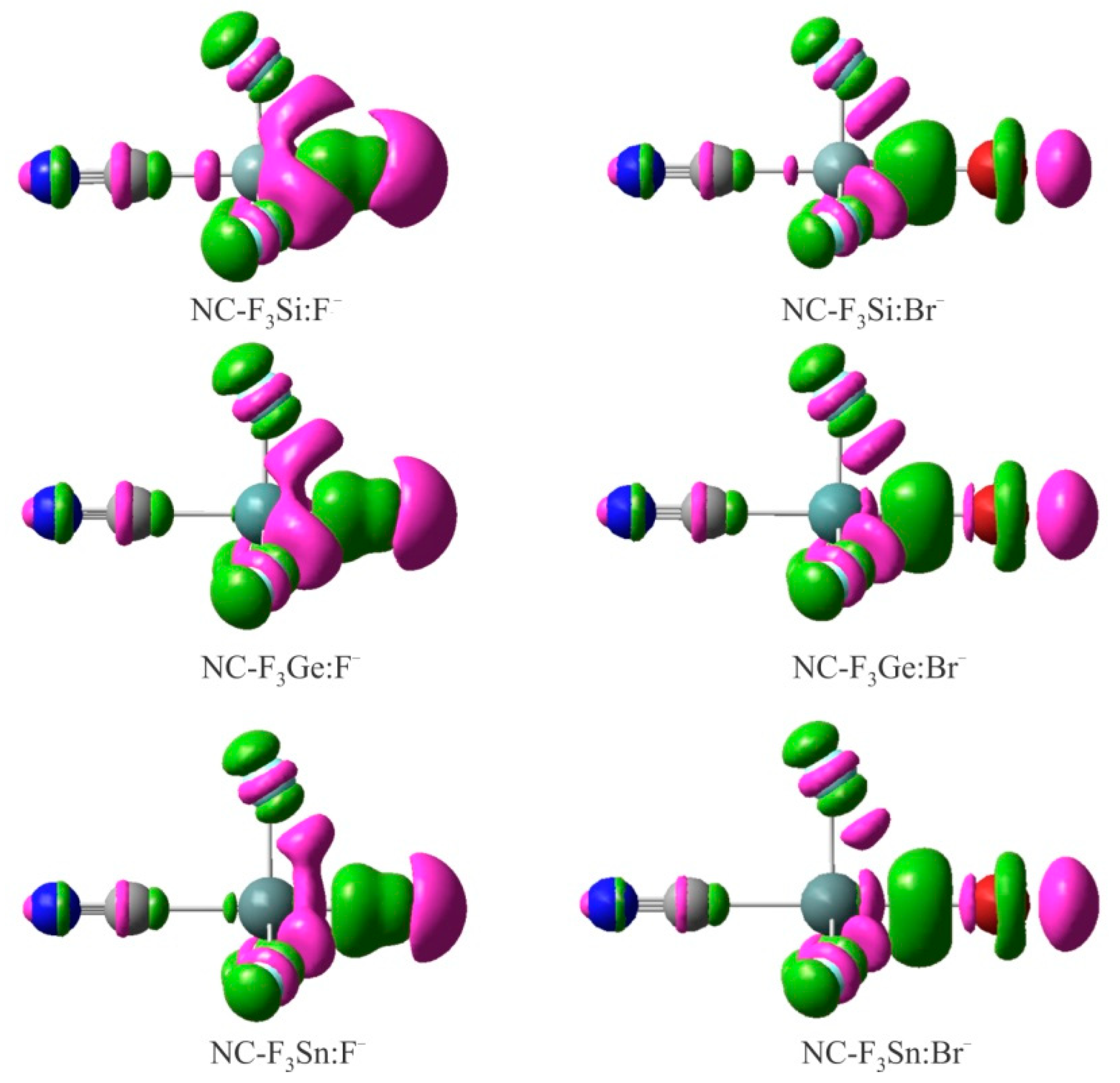
3.1. Anionic Tetrel-Bonds
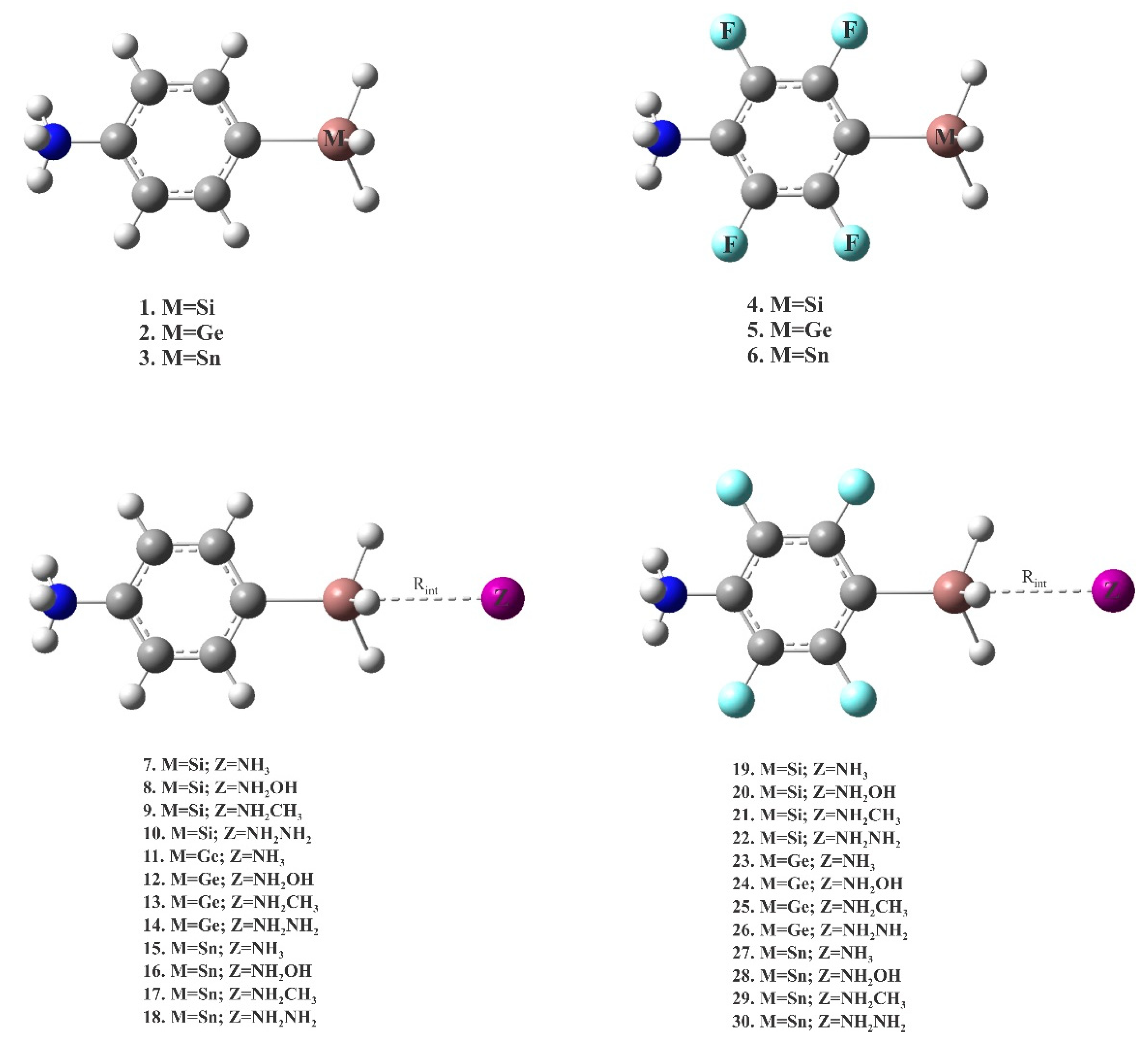
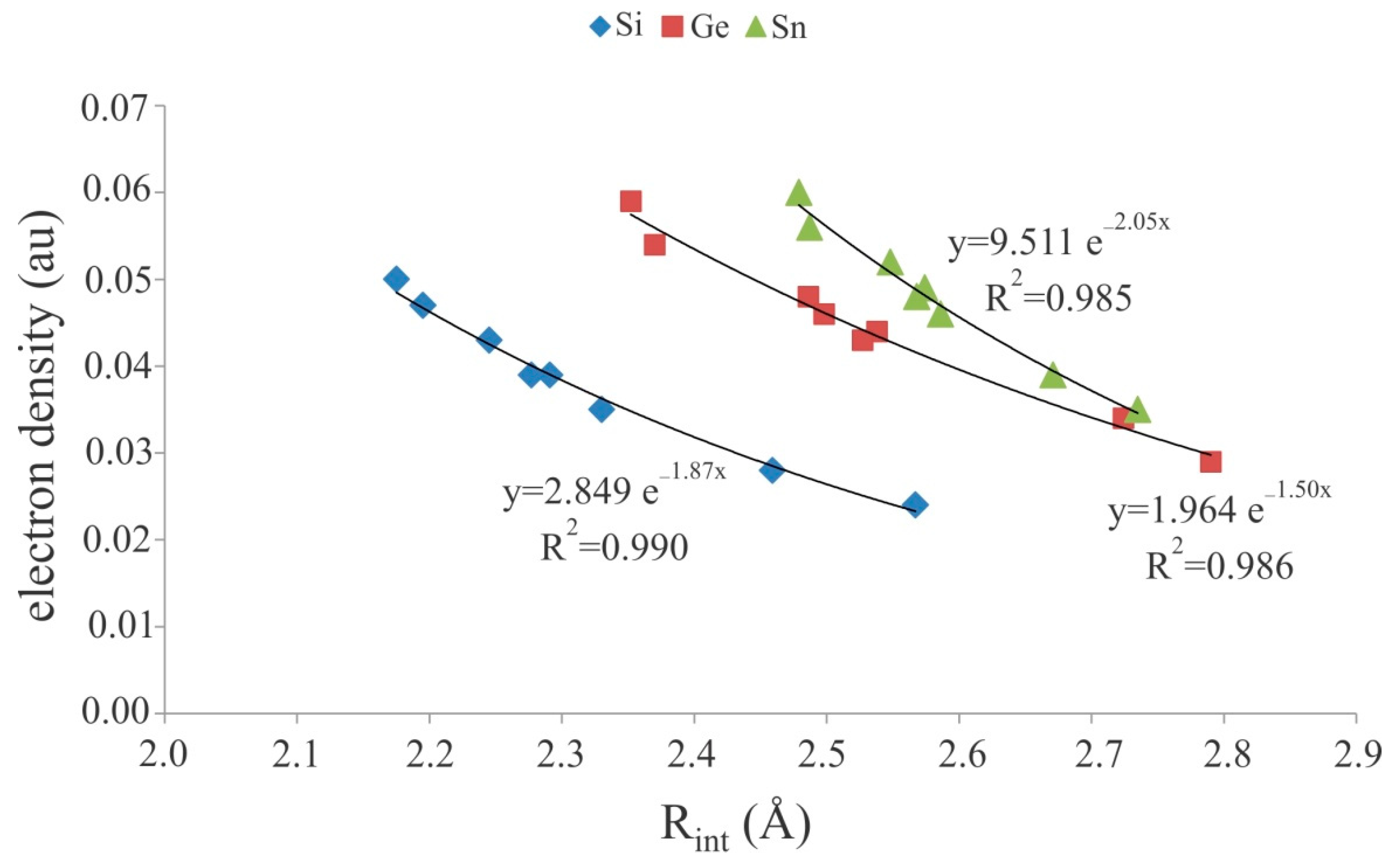
3.2. Cationic Tetrel-Bonds
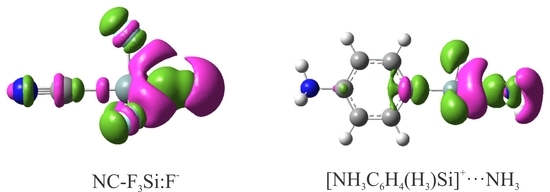
3.3. Experimental Evidencefor Charge-Assisted Tetrel-Bonds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Müller-Dethlefs, K.; Hobza, P. Noncovalent interactions: A challenge for experiment and theory. Chem. Rev. 2000, 100, 143–168. [Google Scholar] [CrossRef] [PubMed]
- Strekowski, L.; Wilson, B. Noncovalent interactions with DNA: An overview. Mutat. Res.-Fund. Mol. M. 2007, 623, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Riley, K.E.; Hobza, P. Noncovalent interactions in biochemistry. WIREs Comput. Mol. Sci. 2011, 1, 3–17. [Google Scholar] [CrossRef] [Green Version]
- Alkorta, I.; Elguero, J. Carbenes and silylenes as hydrogen bond acceptors. J. Phys. Chem. 1996, 100, 19367–19370. [Google Scholar] [CrossRef]
- Scheiner, S. Hydrogen Bonding. A Theoretical Perspective; Oxford University Press: New York, NY, USA, 1997. [Google Scholar]
- Rozas, I.; Alkorta, I.; Elguero, J. Behavior of ylides containing N, O, and C atoms as hydrogen bond acceptors. J. Am. Chem. Soc. 2000, 122, 11154–11161. [Google Scholar] [CrossRef]
- Dannenberg, J.J. The nature of the hydrogen bond: Outline of a comprehensive hydrogen bond theory. J. Am. Chem. Soc. 2010, 132, 3229–3230. [Google Scholar] [CrossRef]
- Jing, B.; Li, Q.; Gong, B.; Li, R.; Liu, Z.; Li, W.; Cheng, J.; Sun, J. Hydrogen bond and σ-hole interaction in M2C=S··· HCN (M=H, F, Cl, Br, HO, H3C, H2N) complex: Dual roles of C=S group and substitution effect. Int. J. Quantum Chem. 2012, 112, 1491–1498. [Google Scholar] [CrossRef]
- Metrangolo, P.; Resnati, G.; Pilati, T.; Biella, S. Halogen Bonding in Crystal Engineering; Springer: Berlin, Germany, 2008. [Google Scholar]
- Eskandari, K.; Zariny, H. Halogen bonding: A lump–hole interaction. Chem. Phys. Lett. 2010, 492, 9–13. [Google Scholar] [CrossRef]
- Ji, B.; Wang, W.; Deng, D.; Zhang, Y. Symmetrical bifurcated halogen bond: Design and synthesis. Cryst. Growth Des. 2011, 11, 3622–3628. [Google Scholar] [CrossRef]
- Metrangolo, P.; Murray, J.S.; Pilati, T.; Politzer, P.; Resnati, G.; Terraneo, G. The fluorine atom as a halogen bond donor, viz. a positive site. CrystEngComm 2011, 13, 6593–6596. [Google Scholar] [CrossRef] [Green Version]
- Politzer, P.; Riley, K.E.; Bulat, F.A.; Murray, J.S. Perspectives on halogen bonding and other σ-hole interactions: Lex parsimoniae (Occam’s Razor). Comput. Theor. Chem. 2012, 998, 2–8. [Google Scholar] [CrossRef]
- Stone, A.J. Are halogen bonded structures electrostatically driven? J. Am. Chem. Soc. 2013, 135, 7005–7009. [Google Scholar] [CrossRef] [PubMed]
- Ho, P.S. Biomolecular halogen bonds. In Halogen Bonding I; Springer: Weinheim, Germany, 2014; pp. 241–276. [Google Scholar]
- Lv, H.; Zhuo, H.-Y.; Li, Q.-Z.; Yang, X.; Li, W.-Z.; Cheng, J.-B. Halogen bonds with N-heterocyclic carbenes as halogen acceptors: A partially covalent character. Mol. Phys. 2014, 112, 3024–3032. [Google Scholar] [CrossRef]
- Novák, M.; Foroutan-Nejad, C.; Marek, R. Asymmetric bifurcated halogen bonds. Phys. Chem. Chem. Phys. 2015, 17, 6440–6450. [Google Scholar] [CrossRef] [PubMed]
- Clark, T.; Hennemann, M.; Murray, J.S.; Politzer, P. Halogen bonding: The σ-hole. J. Mol. Model. 2007, 13, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.S.; Lane, P.; Clark, T.; Politzer, P. σ-hole bonding: Molecules containing group VI atoms. J. Mol. Model. 2007, 13, 1033–1038. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.S.; Concha, M.C.; Lane, P.; Hobza, P.; Politzer, P. Blue shifts vs. red shifts in σ-hole bonding. J. Mol. Model. 2008, 14, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Politzer, P.; Murray, J.S.; Concha, M.C. σ-hole bonding between like atoms; a fallacy of atomic charges. J. Mol. Model. 2008, 14, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.S.; Lane, P.; Politzer, P. Expansion of the σ-hole concept. J. Mol. Model. 2009, 15, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.S.; Lane, P.; Clark, T.; Riley, K.E.; Politzer, P. σ-Holes, π-holes and electrostatically-driven interactions. J. Mol. Model. 2012, 18, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Bundhun, A.; Ramasami, P.; Murray, J.; Politzer, P. Trends in σ-hole strengths and interactions of F3MX molecules (M=C, Si, Ge and X=F, Cl, Br, I). J. Mol. Model. 2013, 19, 2739–2746. [Google Scholar] [CrossRef] [PubMed]
- Hassel, O.; Hvoslef, J. The structure of bromine 1, 4-dioxanate. Acta Chem. Scand. 1954, 8, 873. [Google Scholar] [CrossRef]
- Hassel, O. Structural aspects of interatomic charge-transfer bonding. Science 1970, 170, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Abate, A.; Saliba, M.; Hollman, D.J.; Stranks, S.D.; Wojciechowski, K.; Avolio, R.; Grancini, G.; Petrozza, A.; Snaith, H.J. Supramolecular halogen bond passivation of organic–inorganic halide perovskite solar cells. Nano Lett. 2014, 14, 3247–3254. [Google Scholar] [CrossRef] [PubMed]
- Saccone, M.; Dichiarante, V.; Forni, A.; Goulet-Hanssens, A.; Cavallo, G.; Vapaavuori, J.; Terraneo, G.; Barrett, C.J.; Resnati, G.; Metrangolo, P. Supramolecular hierarchy among halogen and hydrogen bond donors in light-induced surface patterning. J. Mater. Chem. C 2015, 3, 759–768. [Google Scholar] [CrossRef] [Green Version]
- Jungbauer, S.H.; Huber, S.M. Cationic multidentate halogen-bond donors in halide abstraction organocatalysis: Catalyst optimization by preorganization. J. Am. Chem. Soc. 2015, 137, 12110–12120. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, G.; Metrangolo, P.; Milani, R.; Pilati, T.; Priimagi, A.; Resnati, G.; Terraneo, G. The halogen bond. Chem. Rev. 2016, 116, 2478–2601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasylyeva, V.; Catalano, L.; Nervi, C.; Gobetto, R.; Metrangolo, P.; Resnati, G. Characteristic redshift and intensity enhancement as far-IR fingerprints of the halogen bond involving aromatic donors. CrystEngComm 2016, 18, 2247–2250. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, V.; Kraka, E.; Cremer, D. The intrinsic strength of the halogen bond: Electrostatic and covalent contributions described by coupled cluster theory. Phys. Chem. Chem. Phys. 2016, 18, 33031–33046. [Google Scholar] [CrossRef] [PubMed]
- Řezáč, J.; de la Lande, A. On the role of charge transfer in halogen bonding. Phys. Chem. Chem. Phys. 2017, 19, 791–803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bauzá, A.; Mooibroek, T.J.; Frontera, A. Tetrel-Bonding Interaction: Rediscovered Supramolecular Force? Angew. Chem. Int. Ed. 2013, 125, 12543–12547. [Google Scholar] [CrossRef]
- Bauzá, A.; Ramis, R.; Frontera, A. Computational study of anion recognition based on tetrel and hydrogen bonding interaction by calix [4] pyrrole derivatives. Comput. Theor. Chem. 2014, 1038, 67–70. [Google Scholar] [CrossRef]
- Servati Gargari, M.; Vladimir, S.; Bauzá, A.; Frontera, A.; McArdle, P.; Van Derveer, D.; Weng Ng, S.; Mahmoudi, G. Design of lead (II) metal–organic frameworks based on covalent and tetrel-bonding. Chem. Eur. J. 2015, 21, 17951–17958. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Guo, X.; Yang, X.; Li, W.; Cheng, J.; Li, H.-B. A σ-hole interaction with radical species as electron donors: Does single-electron tetrel-bonding exist? Phys. Chem. Chem. Phys. 2014, 16, 11617–11625. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, Z.; Solimannejad, M.; Esrafili, M.D. Interplay between hydrogen bond and single-electron tetrel-bond: H3C··· COX2··· HY and H3C··· CSX2··· HY (X=F, Cl; Y=CN, NC) complexes as a working model. Comput. Theor. Chem. 2015, 1074, 101–106. [Google Scholar] [CrossRef]
- Scheiner, S. Assembly of effective halide receptors from components. comparing hydrogen, halogen, and tetrel-bonds. J. Phys. Chem. A 2017, 121, 3606–3615. [Google Scholar] [CrossRef] [PubMed]
- Alkorta, I.; Sánchez-Sanz, G.; Elguero, J.; Del Bene, J.E. Influence of hydrogen bonds on the P··· P pnicogen bond. J. Chem. Theory Comput. 2012, 8, 2320–2327. [Google Scholar] [CrossRef] [PubMed]
- Del Bene, J.E.; Alkorta, I.; Sánchez-Sanz, G.; Elguero, J. Interplay of F–H…F hydrogen bonds and P…N pnicogen bonds. J. Phys. Chem. A 2012, 116, 9205–9213. [Google Scholar] [CrossRef] [PubMed]
- Alkorta, I.; Elguero, J.; Del Bene, J.E. Pnicogen bonded complexes of PO2X (X=F, Cl) with nitrogen bases. J. Phys. Chem. A 2013, 117, 10497–10503. [Google Scholar] [CrossRef] [PubMed]
- Alkorta, I.; Elguero, J.; Solimannejad, M. Single electron pnicogen bonded complexes. J. Phys. Chem. A 2014, 118, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Alkorta, I.; Elguero, J.; Grabowski, S.J. Pnicogen and hydrogen bonds: Complexes between PH3X(+) and PH2X systems. Phys. Chem. Chem. Phys. 2015, 17, 3261–3272. [Google Scholar] [CrossRef] [PubMed]
- Scheiner, S. Sensitivity of noncovalent bonds to intermolecular separation: Hydrogen, halogen, chalcogen, and pnicogen bonds. CrystEngComm 2013, 15, 3119–3124. [Google Scholar] [CrossRef]
- Scheiner, S. Detailed comparison of the pnicogen bond with chalcogen, halogen, and hydrogen bonds. Int. J. Quantum Chem. 2013, 113, 1609–1620. [Google Scholar] [CrossRef]
- Esrafili, M.D.; Mohammadian-Sabet, F. An ab initio study on chalcogen–chalcogen bond interactions in cyclic (SHX)3 complexes (X=F, Cl, CN, NC, CCH, OH, OCH3, NH2). Chem. Phys. Lett. 2015, 628, 71–75. [Google Scholar] [CrossRef]
- Esrafili, M.D.; Mohammadian-Sabet, F. Does single-electron chalcogen bond exist? Some theoretical insights. J. Mol. Model. 2015, 21, 65. [Google Scholar] [CrossRef] [PubMed]
- Esrafili, M.D.; Mohammadian-Sabet, F.; Baneshi, M.M. An ab initio investigation of chalcogen–hydride interactions involving HXeH as a chalcogen bond acceptor. Struct. Chem. 2016, 27, 785–792. [Google Scholar] [CrossRef]
- Bauzá, A.; Frontera, A. Aerogen bonding interaction: A new supramolecular force? Angew. Chem. Int. Ed. 2015, 54, 7340–7343. [Google Scholar] [CrossRef] [PubMed]
- Bauzá, A.; Frontera, A. π-Hole aerogen bonding interactions. Phys. Chem. Chem. Phys. 2015, 17, 24748–24753. [Google Scholar] [CrossRef] [PubMed]
- Esrafili, M.D.; Asadollahi, S.; Vakili, M. Investigation of substituent effects in aerogen-bonding interaction between ZO3 (Z=Kr, Xe) and nitrogen bases. Int. J. Quantum Chem. 2016, 116, 1254–1260. [Google Scholar] [CrossRef]
- Frontera, A.; Bauzá, A. Concurrent aerogen bonding and lone pair/anion–π interactions in the stability of organoxenon derivatives: A combined CSD and ab initio study. Phys. Chem. Chem. Phys. 2017, 19, 30063–30068. [Google Scholar] [CrossRef] [PubMed]
- Brezgunova, M.E.; Lieffrig, J.; Aubert, E.; Dahaoui, S.; Fertey, P.; Lebègue, S.b.; Ángyán, J.n.G.; Fourmigué, M.; Espinosa, E. Chalcogen bonding: Experimental and theoretical determinations from electron density analysis. Geometrical preferences driven by electrophilic–nucleophilic interactions. Cryst. Growth Des. 2013, 13, 3283–3289. [Google Scholar] [CrossRef]
- Xu, Z.; Yang, Z.; Liu, Y.; Lu, Y.; Chen, K.; Zhu, W. Halogen bond: Its role beyond drug–target binding affinity for drug discovery and development. J. Chem. Inf. Model. 2014, 54, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, U.; Scheiner, S. Effects of charge and substituent on the S···N chalcogen bond. J. Phys. Chem. A 2014, 118, 3183–3192. [Google Scholar] [CrossRef] [PubMed]
- Gilday, L.C.; Robinson, S.W.; Barendt, T.A.; Langton, M.J.; Mullaney, B.R.; Beer, P.D. Halogen bonding in supramolecular chemistry. Chem. Rev. 2015, 115, 7118–7195. [Google Scholar] [CrossRef] [PubMed]
- Bauzá, A.; Mooibroek, T.J.; Frontera, A. Tetrel-bonding interactions. Chem. Rec. 2016, 16, 473–487. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Li, Q. Interplay between tetrel-bonding and hydrogen bonding interactions in complexes involving F2XO (X=C and Si) and HCN. Comput. Theor. Chem. 2014, 1050, 51–57. [Google Scholar] [CrossRef]
- Mahmoudi, G.; Bauzá, A.; Frontera, A. Concurrent agostic and tetrel-bonding interactions in lead (II) complexes with an isonicotinohydrazide based ligand and several anions. Dalton Trans. 2016, 45, 4965–4969. [Google Scholar] [CrossRef] [PubMed]
- Nziko, P.V.; Scheiner, S. Comparison of π-hole tetrel-bonding with σ-hole halogen bonds in complexes of XCN (X=F, Cl, Br, I) and NH3. Phys. Chem. Chem. Phys. 2016, 18, 3581–3590. [Google Scholar] [CrossRef] [PubMed]
- Del Bene, J.E.; Alkorta, I.; Elguerob, J. Anionic complexes of F− and Cl− with substituted methanes: Hydrogen, halogen, and tetrel-bonds. Chem. Phys. Lett. 2016, 655–656, 115–119. [Google Scholar] [CrossRef]
- Esrafili, M.D.; Asadollahi, S.; Mousavian, P. Anionic tetrel-bonds: An ab initio study. Chem. Phys. Lett. 2018, 691, 394–400. [Google Scholar] [CrossRef]
- Solimannejad, M.; Orojloo, M.; Amani, S. Effect of cooperativity in lithium bonding on the strength of halogen bonding and tetrel-bonding:(LiCN)n···ClYF3 and (LiCN)n···YF3Cl (Y=C, Si and n = 1–5) complexes as a working model. J. Mol. Model. 2015, 21, 183. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Li, Q.; Li, W.; Cheng, J. Tetrel-bonds between PySiX3 and some nitrogenated bases: Hybridization, substitution, and cooperativity. J. Mol. Graph. Model. 2016, 65, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Li, Q.; Scheiner, S. Comparison of tetrel-bonds in neutral and protonated complexes of pyridine TF3 and furan TF3 (T=C, Si, and Ge) with NH3. Phys. Chem. Chem. Phys. 2017, 19, 5550–5559. [Google Scholar] [CrossRef] [PubMed]
- Grabowski, S.J. Tetrel-bonds with π-electrons acting as Lewis bases-theoretical results and experimental evidences. Molecules 2018, 23, 1183. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Zeng, Y.; Li, X.; Meng, L.; Zhang, X. Insight into the π-hole···π-electrons tetrel-bonds between F2ZO (Z=C, Si, Ge) and unsaturated hydrocarbons. Int. J. Quantum Chem. 2018, 118, e25521. [Google Scholar] [CrossRef]
- García-LLinás, X.; Bauzá, A.; Seth, S.K.; Frontera, A. Importance of R–CF3···O tetrel-bonding interactions in biological systems. J. Phys. Chem. A 2017, 121, 5371–5376. [Google Scholar] [CrossRef] [PubMed]
- Bauzá, A.; Frontera, A. Tetrel-bonding interactions in perchlorinated cyclopenta-and cyclohexatetrelanes: Acombined DFT and CSD study. Molecules 2018, 23, 1770. [Google Scholar] [CrossRef] [PubMed]
- Mitzel, N.W.; Losehand, U. β-donor bonds in compounds containing SiON fragments. Angew. Chem. Int. Ed. 1997, 36, 2807–2809. [Google Scholar] [CrossRef]
- Alkorta, I.; Rozas, I.; Elguero, J. Molecular complexes between silicon derivatives and electron-rich groups. J. Phys. Chem. A 2001, 105, 743–749. [Google Scholar] [CrossRef]
- Mani, D.; Arunan, E. The X–C⋯ Y (X=O/F, Y=O/S/F/Cl/Br/N/P)‘carbon bond’and hydrophobic interactions. Phys. Chem. Chem. Phys. 2013, 15, 14377–14383. [Google Scholar] [CrossRef] [PubMed]
- Mani, D.; Arunan, E. The X–C··· π (X=F, Cl, Br, Cn) Carbon Bond. J. Phys. Chem. A 2014, 118, 10081–10089. [Google Scholar] [CrossRef] [PubMed]
- Grabowski, S.J. Tetrel-bond–σ-hole bond as a preliminary stage of the SN2 reaction. Phys. Chem. Chem. Phys. 2014, 16, 1824–1834. [Google Scholar] [CrossRef] [PubMed]
- Scheiner, S. Comparison of halide receptors based on H, halogen, chalcogen, pnicogen, and tetrel-bonds. Faraday Discuss. 2017, 203, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Scheiner, S. Tetrel-bonding as a vehicle for strong and selective anion binding. Molecules 2018, 23, 1147. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Boys, S.F.; Bernardi, F. The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol. Phys. 1970, 19, 553–566. [Google Scholar] [CrossRef]
- Glendening, E.; Badenhoop, J.; Reed, A.; Carpenter, J.; Bohmann, J.; Morales, C.; Weinhold, F. NBO 5.0. Theoretical Chemistry Institute, University of Wisconsin: Madison, WI, USA, 2001. [Google Scholar]
- Bulat, F.; Toro-Labbé, A.; Brinck, T.; Murray, J.; Politzer, P. Quantitative analysis of molecular surfaces: Areas, volumes, electrostatic potentials and average local ionization energies. J. Mol. Model. 2010, 16, 1679–1691. [Google Scholar] [CrossRef] [PubMed]
- Biegler-König, F.; Schönbohm, J.; Derdau, R.; Bayles, D. AIM2000. J. Comput. Chem. 2001, 22, 545–559. [Google Scholar]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.-Z.; Zhuo, H.-Y.; Li, H.-B.; Liu, Z.-B.; Li, W.-Z.; Cheng, J.-B. Tetrel–hydride interaction between XH3F (X=C, Si, Ge, Sn) and HM (M=Li, Na, BeH, MgH). J. Phys. Chem. A 2014, 119, 2217–2224. [Google Scholar] [CrossRef] [PubMed]
- Del Bene, J.E.; Elguero, J.; Alkorta, I. Complexes of CO2 with the Azoles: Tetrel-bonds, hydrogen bonds and other secondary interactions. Molecules 2018, 23, 906. [Google Scholar] [CrossRef] [PubMed]
- Bondi, A. van der Waals volumes and radii. J. Phys. Chem. 1964, 68, 441–451. [Google Scholar] [CrossRef]
- Liu, M.; Li, Q.; Cheng, J.; Li, W.; Li, H.-B. Tetrel-bond of pseudohalide anions with XH3F (X=C, Si, Ge, and Sn) and its role in SN2 reaction. J. Chem. Phys. 2016, 145, 224310. [Google Scholar] [CrossRef] [PubMed]
- Scheiner, S. Highly selective halide receptors based on chalcogen, pnicogen, and tetrel-bonds. Chem. Eur. J. 2016, 22, 18850–18858. [Google Scholar] [CrossRef] [PubMed]
- Esrafili, M.D.; Mohammadian-Sabet, F. Exploring σ-hole bonding in XH3Si···HMY (X=H, F, CN; M=Be, Mg; Y=H, F, CH3) complexes: A “tetrel-hydride” interaction. J. Mol. Model. 2015, 21, 60. [Google Scholar] [CrossRef] [PubMed]
- Esrafili, M.D.; Mohammadian-Sabet, F. Cooperativity of tetrel-bonds tuned by substituent effects. Mol. Phys. 2016, 114, 1528–1538. [Google Scholar] [CrossRef]
- Esrafili, M.D.; Mohammadian-Sabet, F. σ-Hole bond tunability in YO2X2: NH3 and YO2X2: H2O complexes (X=F, Cl, Br; Y=S, Se): Trends and theoretical aspects. Struct. Chem. 2016, 27, 617–625. [Google Scholar] [CrossRef]
- Zierkiewicz, W.; Michalczyk, M. On the opposite trends of correlations between interaction energies and electrostatic potentials of chlorinated and methylated amine complexes stabilized by halogen bond. Theor. Chem. Acc. 2017, 136, 125. [Google Scholar] [CrossRef]
- Esrafili, M.D.; Mohammadian-Sabet, F. Tuning tetrel-bonds via cation–π interactions: Anab initiostudy on concerted interaction in M+–C6H5XH3–NCY complexes (M=Li, Na, K; X=Si, Ge; Y=H, F, OH). Mol. Phys. 2016, 114, 83–91. [Google Scholar] [CrossRef]
- Guo, X.; Liu, Y.-W.; Li, Q.-Z.; Li, W.-Z.; Cheng, J.-B. Competition and cooperativity between tetrel-bond and chalcogen bond in complexes involving F2CX (X=Se and Te). Chem. Phys. Lett. 2015, 620, 7–12. [Google Scholar] [CrossRef]
- Esrafili, M.D.; Mohammadirad, N.; Solimannejad, M. Tetrel-bond cooperativity in open-chain (CH3CN)n and (CH3NC)n clusters (n = 2–7): An ab initio study. Chem. Phys. Lett. 2015, 628, 16–20. [Google Scholar] [CrossRef]
- Bader, R.F. A bond path: A universal indicator of bonded interactions. J. Phys. Chem. A 1998, 102, 7314–7323. [Google Scholar] [CrossRef]
- Bader, R.F.W. Atoms in Molecules: A Quantum Theory; Oxford University Press: New York, NY, USA, 1990. [Google Scholar]
- Dong, W.; Li, Q.; Scheiner, S. Comparative strengths of tetrel, pnicogen, chalcogen, and halogen bonds and contributing factors. Molecules 2018, 23, 1681. [Google Scholar] [CrossRef] [PubMed]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge structural database. Acta Crystallogr. B 2016, 72, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.G.; Bassindale, A.R.; El Aziz, Y.; Pourny, M.; Stevenson, R.; Hursthouse, M.B.; Coles, S.J. Further studies of fluoride ion entrapment in octasilsesquioxane cages; X-ray crystal structure studies and factors that affect their formation. Dalton Trans. 2012, 41, 2048–2059. [Google Scholar] [CrossRef] [PubMed]
- Bassindale, A.R.; Parker, D.J.; Pourny, M.; Taylor, P.G.; Horton, P.N.; Hursthouse, M.B. Fluoride ion entrapment in octasilsesquioxane cages as models for ion entrapment in zeolites. Further examples, X-ray crystal structure studies, and investigations into how and why they may be formed. Organometallics 2004, 23, 4400–4405. [Google Scholar] [CrossRef]
- Muhammad, S.; Bassindale, A.R.; Taylor, P.G.; Male, L.; Coles, S.J.; Hursthouse, M.B. Study of binuclear silicon complexes of diketopiperazine at SN2 reaction profile. Organometallics 2011, 30, 564–571. [Google Scholar] [CrossRef]
- Blaschette, A.; Hippel, I.; Krahl, J.; Wieland, E.; Jones, P.G.; Sebald, A. Polysulfonylamine: XXXV. Synthese, Röntgenstrukturanalysen und hochaufgelöste Festkörper-NMR-Spektren der ionischen Organozinn (IV)-dimesylamide [Me3Sn(NH3)2][N(SO2Me)2] und [Me2Sn(DMSO)4][N(SO2Me)2]2. J. Organomet. Chem. 1992, 437, 279–297. [Google Scholar] [CrossRef]
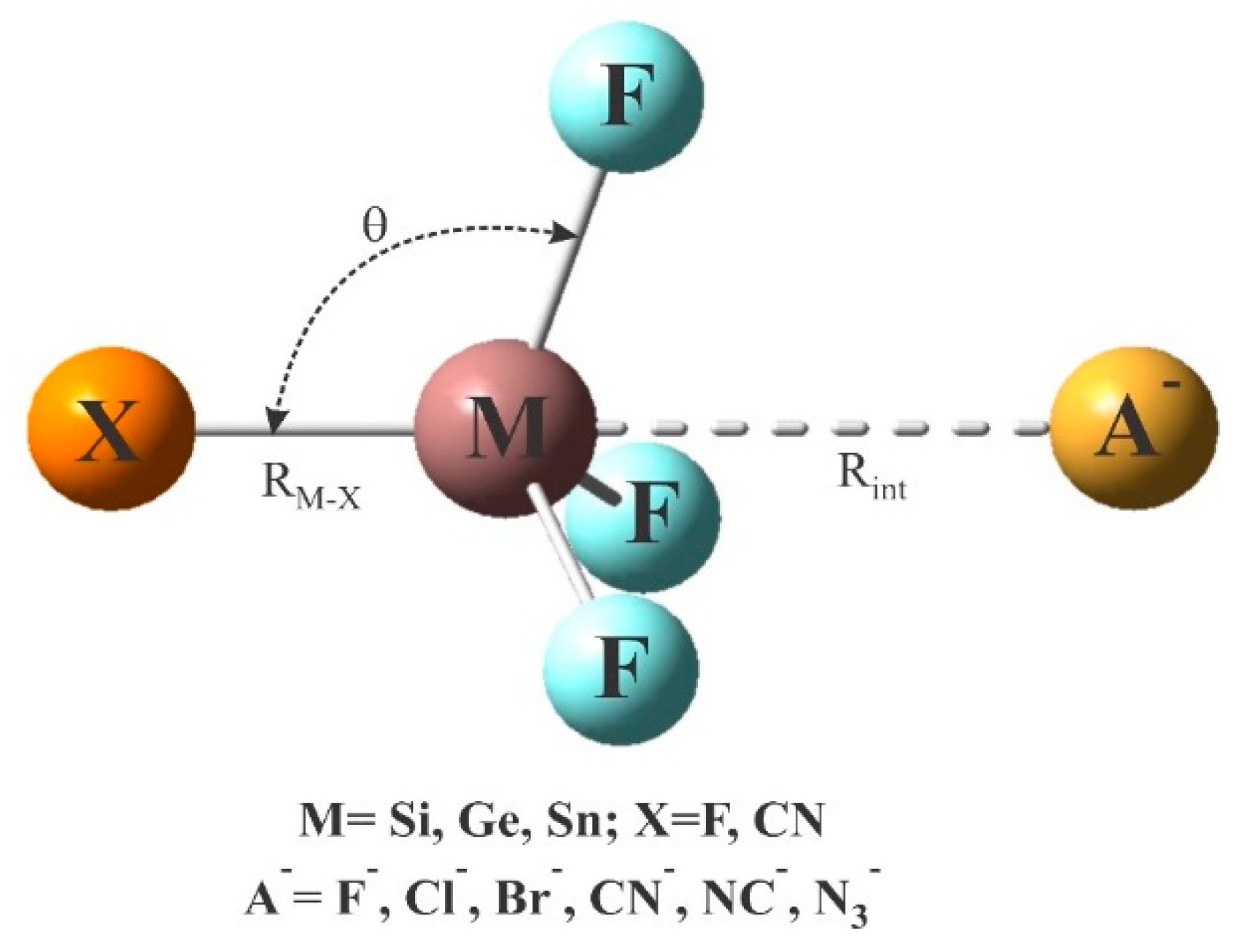
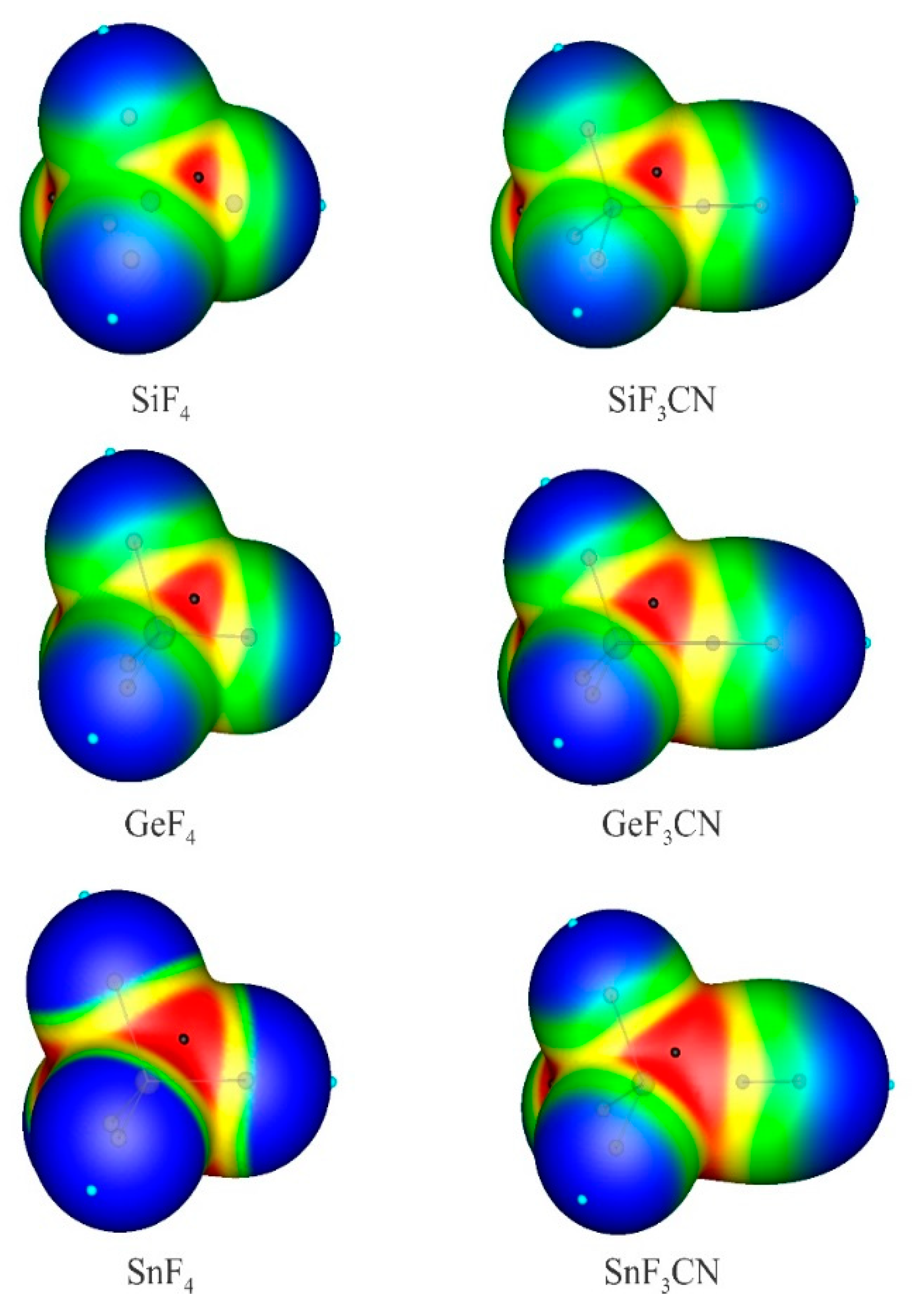
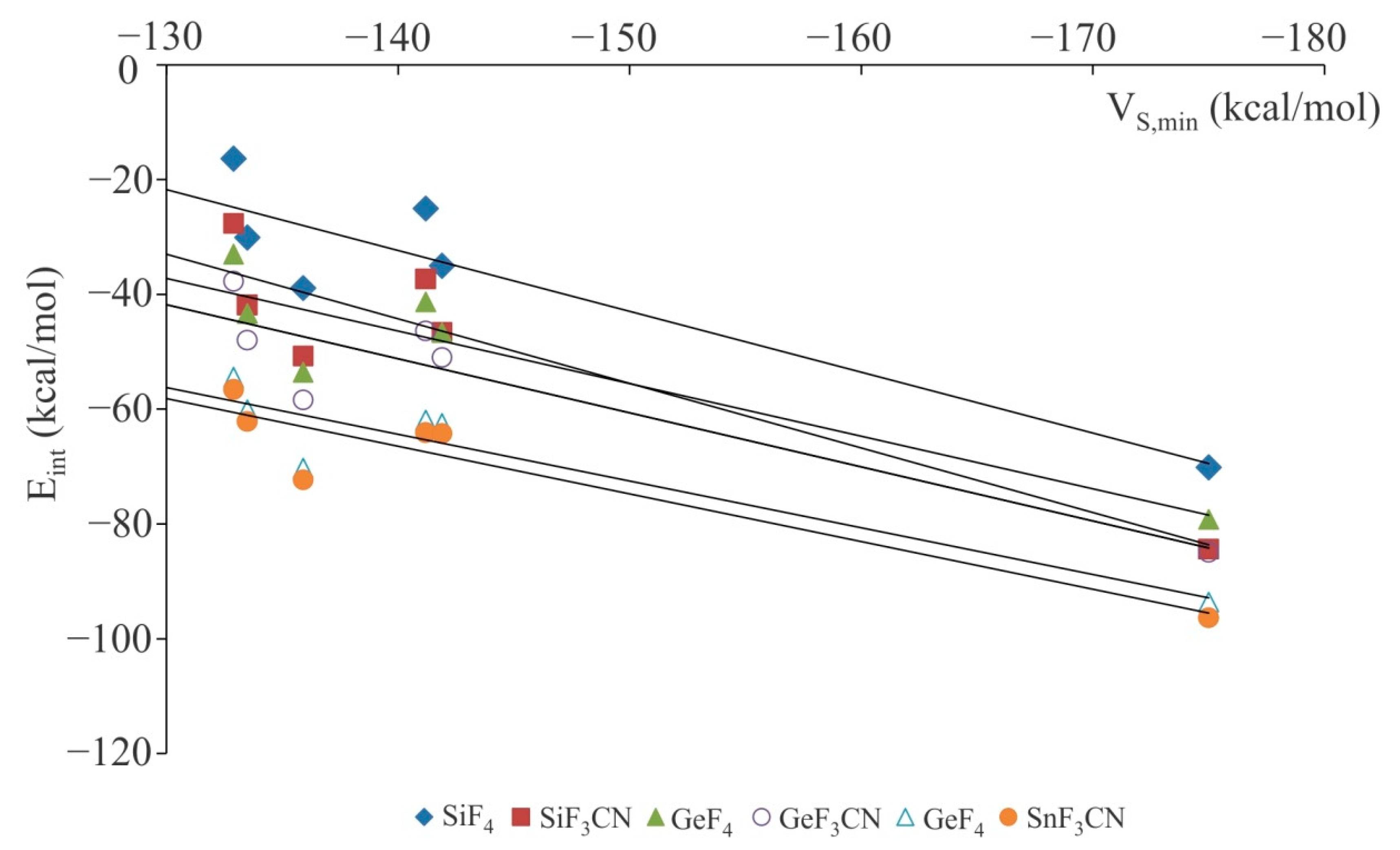
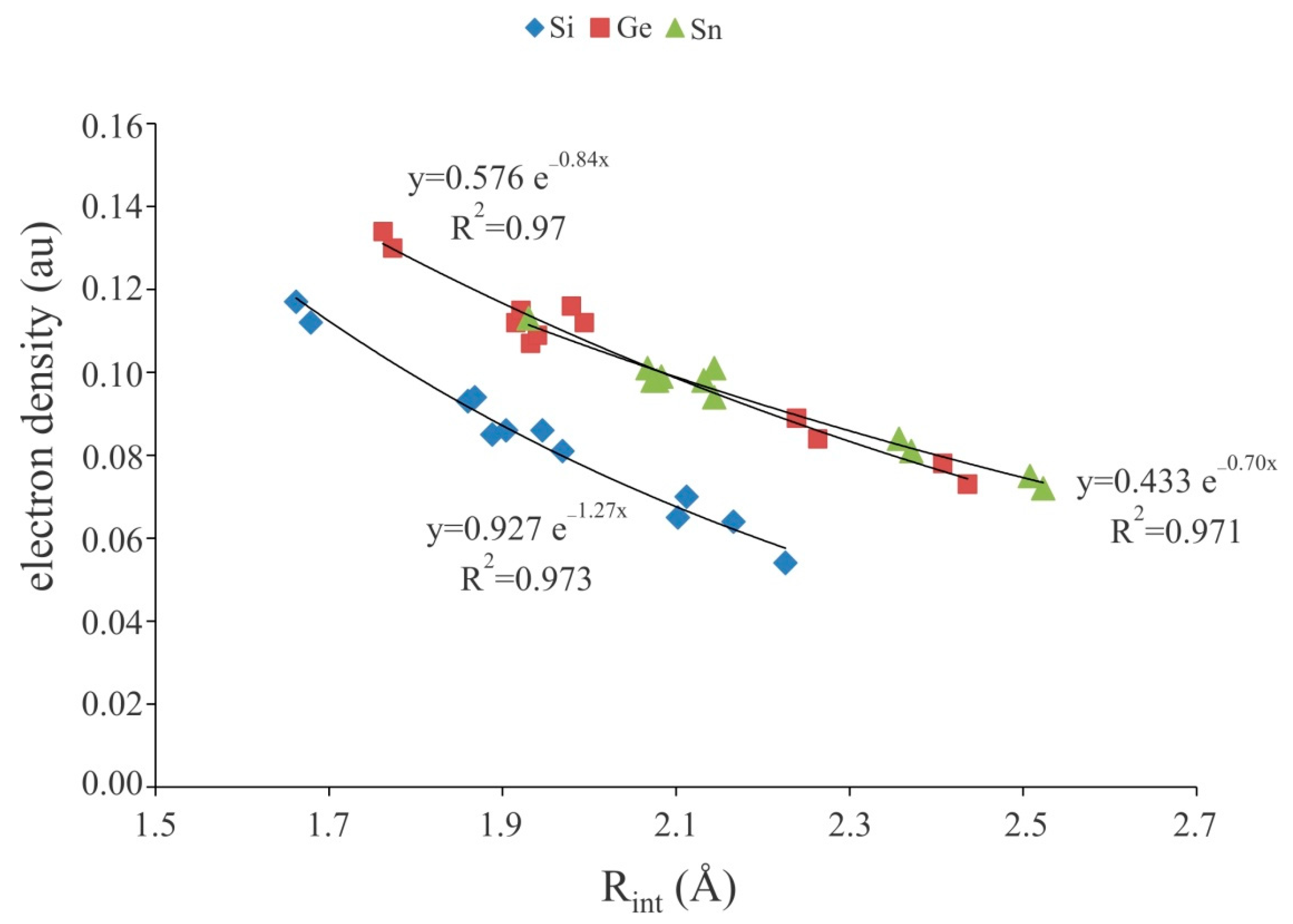

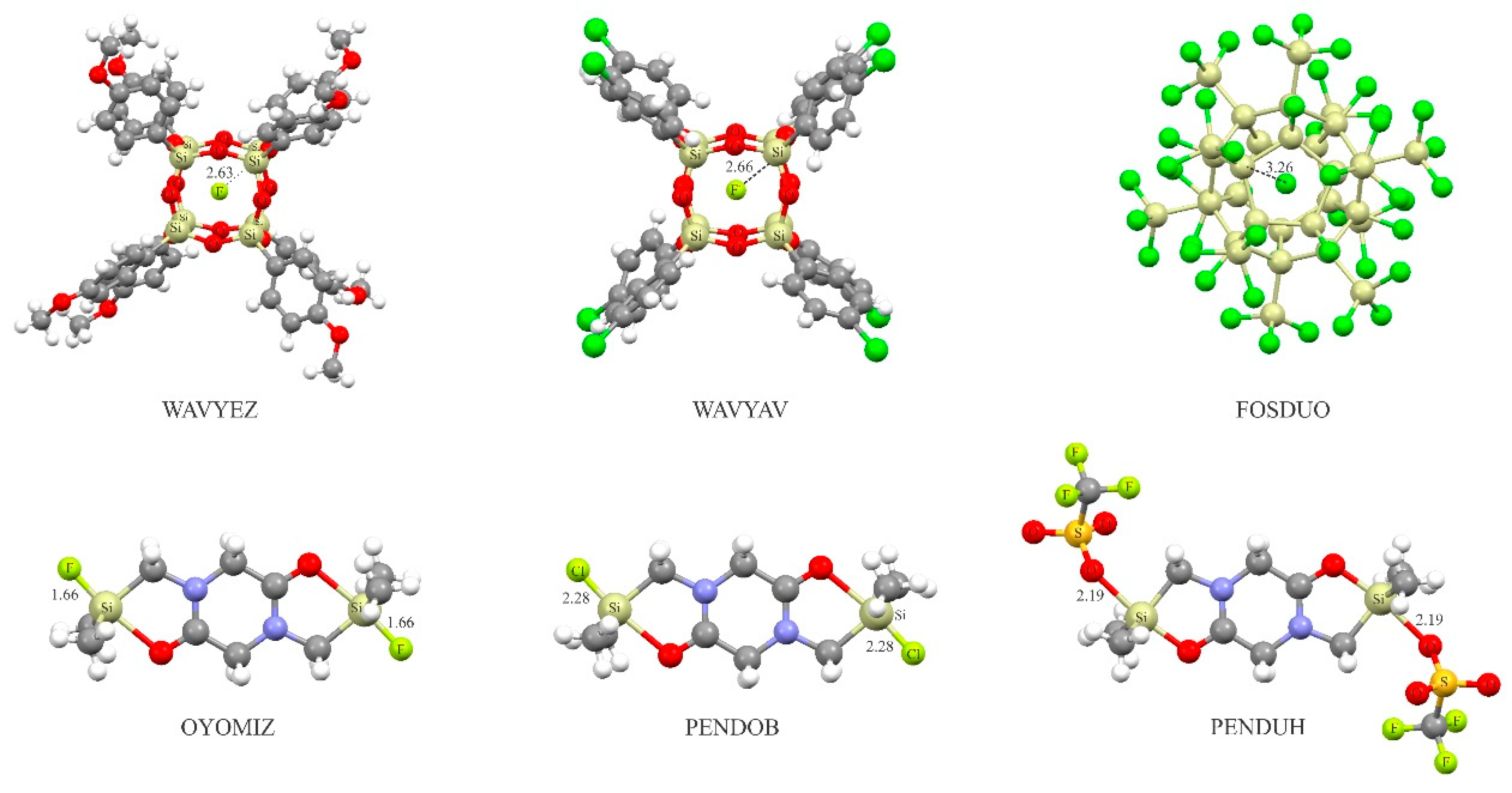
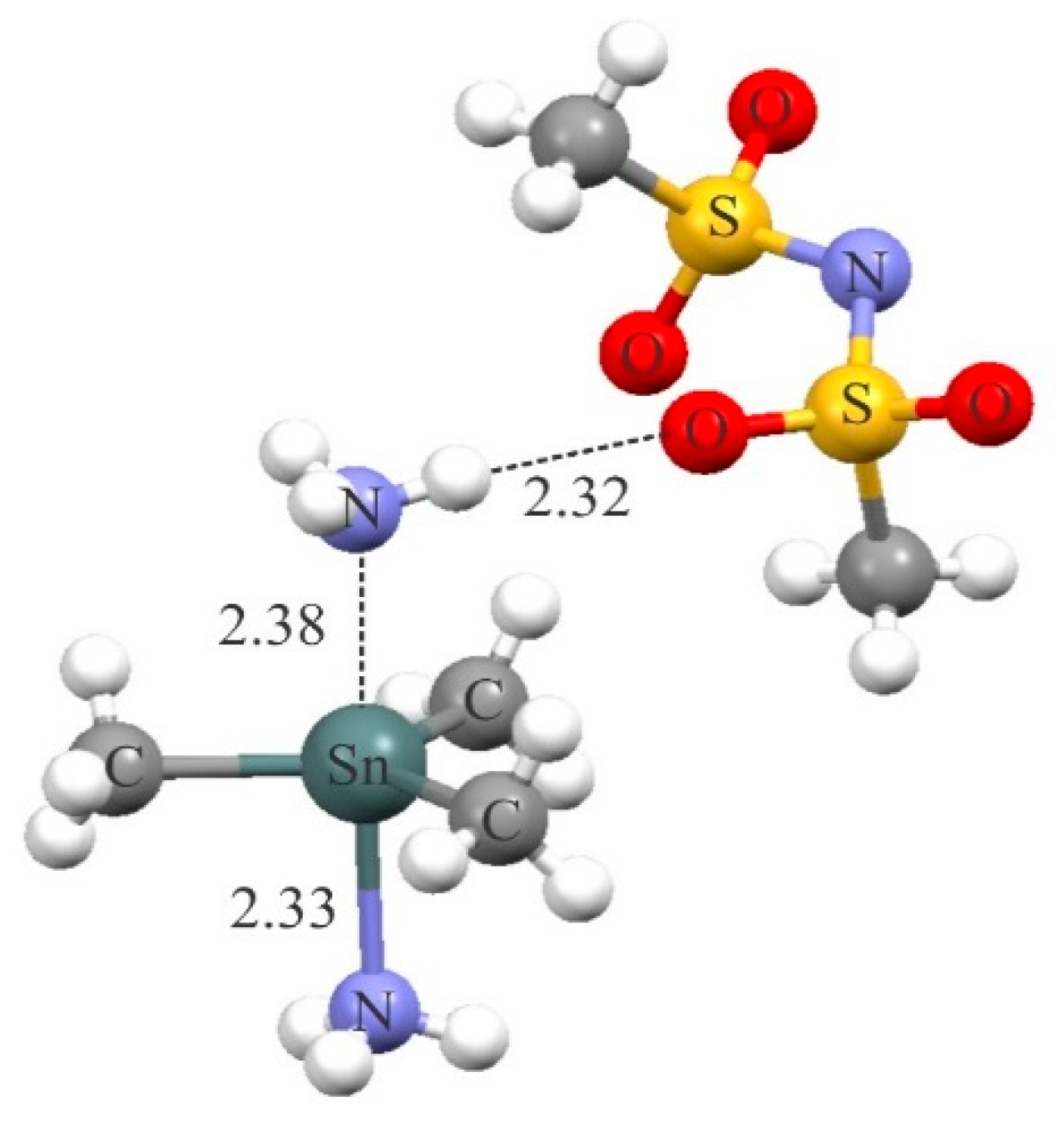
| Lewis Acid | Anion | Rint | θ | RM-X | ΔRM-X | Eint | VS,min |
|---|---|---|---|---|---|---|---|
| SiF4 | F− | 1.679 | 90.0 | 1.679 | 0.105 | −70.11 | −175.0 |
| Cl− | 2.102 | 91.7 | 1.665 | 0.091 | −25.00 | −141.2 | |
| Br− | 2.226 | 92.8 | 1.657 | 0.083 | −16.35 | −132.9 | |
| NC− | 1.888 | 92.0 | 1.661 | 0.087 | −35.03 | −141.9 | |
| CN− | 1.969 | 91.4 | 1.663 | 0.089 | −38.86 | −135.9 | |
| N3− | 1.904 | 91.7 | 1.661 | 0.087 | −30.08 | −133.5 | |
| SiF3CN | F− | 1.662 | 91.4 | 1.970 | 0.145 | −84.37 | −175.0 |
| Cl− | 2.112 | 89.9 | 1.951 | 0.126 | −37.29 | −141.2 | |
| Br− | 2.166 | 90.6 | 1.942 | 0.117 | −27.60 | −132.9 | |
| NC− | 1.860 | 90.5 | 1.943 | 0.118 | −46.59 | −141.9 | |
| CN− | 1.946 | 90.0 | 1.946 | 0.121 | −50.72 | −135.9 | |
| N3− | 1.868 | 89.9 | 1.945 | 0.12 | −41.87 | −133.5 | |
| GeF4 | F− | 1.773 | 90.0 | 1.773 | 0.085 | −79.17 | −175.0 |
| Cl− | 2.263 | 90.3 | 1.764 | 0.076 | −41.26 | −141.2 | |
| Br− | 2.436 | 90.7 | 1.760 | 0.072 | −32.99 | −132.9 | |
| NC− | 1.932 | 91.3 | 1.762 | 0.074 | −46.62 | −141.9 | |
| CN− | 1.994 | 90.5 | 1.762 | 0.074 | −53.57 | −135.9 | |
| N3− | 1.940 | 90.7 | 1.762 | 0.074 | −43.30 | −133.5 | |
| GeF3CN | F− | 1.762 | 90.5 | 1.994 | 0.118 | −84.92 | −175.0 |
| Cl− | 2.239 | 89.7 | 1.983 | 0.107 | −46.32 | −141.2 | |
| Br− | 2.407 | 90.0 | 1.977 | 0.101 | −37.73 | −132.9 | |
| NC− | 1.915 | 90.8 | 1.981 | 0.105 | −50.96 | −141.9 | |
| CN− | 1.979 | 90.0 | 1.979 | 0.103 | −58.35 | −135.9 | |
| N3− | 1.921 | 90.0 | 1.981 | 0.105 | −47.94 | −133.5 | |
| SnF4 | F− | 1.930 | 90.0 | 1.930 | 0.048 | −93.58 | −175.0 |
| Cl− | 2.371 | 89.5 | 1.936 | 0.054 | −61.84 | −141.2 | |
| Br− | 2.523 | 90.4 | 1.934 | 0.052 | −54.44 | −132.9 | |
| NC− | 2.078 | 90.1 | 1.933 | 0.051 | −62.50 | −141.9 | |
| CN− | 2.144 | 90.1 | 1.932 | 0.05 | −70.26 | −135.9 | |
| N3− | 2.083 | 90.3 | 1.932 | 0.05 | −60.07 | −133.5 | |
| SnF3CN | F− | 2.144 | 90.1 | 2.144 | 0.091 | −96.30 | −175.0 |
| Cl− | 2.357 | 90.8 | 2.141 | 0.088 | −64.11 | −141.2 | |
| Br− | 2.508 | 89.2 | 2.139 | 0.086 | −56.59 | −132.9 | |
| NC− | 2.067 | 90.9 | 2.134 | 0.081 | −64.21 | −141.9 | |
| CN− | 2.132 | 90.0 | 2.132 | 0.079 | −72.26 | −135.9 | |
| N3− | 2.073 | 90.1 | 2.133 | 0.080 | −62.09 | −133.5 |
| Lewis Acid | Anion | ρBCP | ∇2ρBCP | HBCP | E(2) | qM | qCT | WBI |
|---|---|---|---|---|---|---|---|---|
| SiF4 | F− | 0.112 | 0.879 | −0.025 | 53.63 | 2.64 | 0.26 | 0.48 |
| Cl− | 0.065 | 0.152 | −0.030 | 38.72 | 2.54 | 0.33 | 0.51 | |
| Br− | 0.054 | 0.060 | −0.028 | 32.06 | 2.55 | 0.31 | 0.49 | |
| NC− | 0.085 | 0.421 | −0.029 | 69.31 | 2.63 | 0.21 | 0.37 | |
| CN− | 0.081 | 0.280 | −0.045 | 47.92 | 2.50 | 0.34 | 0.56 | |
| N3− | 0.086 | 0.368 | −0.034 | 45.64 | 2.42 | 0.59 | 0.41 | |
| SiF3CN | F− | 0.117 | 0.936 | −0.028 | 65.86 | 2.50 | 0.27 | 0.56 |
| Cl− | 0.070 | 0.183 | −0.035 | 56.59 | 2.34 | 0.39 | 0.60 | |
| Br− | 0.064 | 0.080 | −0.034 | 49.40 | 2.32 | 0.40 | 0.60 | |
| NC− | 0.093 | 0.301 | −0.049 | 89.12 | 2.45 | 0.25 | 0.60 | |
| CN− | 0.086 | 0.299 | −0.049 | 61.17 | 2.30 | 0.39 | 0.60 | |
| N3− | 0.094 | 0.415 | −0.040 | 60.75 | 2.42 | 0.58 | 0.46 | |
| GeF4 | F− | 0.130 | 0.774 | −0.050 | 54.69 | 2.68 | 0.27 | 0.42 |
| Cl− | 0.084 | 0.130 | −0.038 | 51.64 | 2.52 | 0.38 | 0.57 | |
| Br− | 0.073 | 0.055 | −0.032 | 46.01 | 2.50 | 0.40 | 0.58 | |
| NC− | 0.107 | 0.364 | −0.048 | 78.64 | 2.66 | 0.22 | 0.38 | |
| CN− | 0.112 | 0.191 | −0.058 | 55.20 | 2.51 | 0.38 | 0.58 | |
| N3− | 0.109 | 0.308 | −0.053 | 53.60 | 2.60 | 0.58 | 0.44 | |
| GeF3CN | F− | 0.134 | 0.800 | −0.053 | 58.67 | 2.51 | 0.27 | 0.44 |
| Cl− | 0.089 | 0.135 | −0.042 | 60.69 | 2.31 | 0.42 | 0.63 | |
| Br− | 0.078 | 0.053 | −0.036 | 55.20 | 2.28 | 0.46 | 0.65 | |
| NC− | 0.112 | 0.381 | −0.052 | 91.00 | 2.45 | 0.25 | 0.42 | |
| CN− | 0.116 | 0.194 | −0.062 | 61.82 | 2.29 | 0.42 | 0.63 | |
| N3− | 0.115 | 0.323 | −0.058 | 59.72 | 2.40 | 0.58 | 0.48 | |
| SnF4 | F− | 0.113 | 0.687 | −0.029 | 34.37 | 2.93 | 0.21 | 0.36 |
| Cl− | 0.081 | 0.195 | −0.027 | 42.18 | 2.75 | 0.36 | 0.37 | |
| Br− | 0.072 | 0.117 | −0.024 | 40.60 | 2.71 | 0.40 | 0.60 | |
| NC− | 0.098 | 0.378 | −0.031 | 53.92 | 2.90 | 0.19 | 0.34 | |
| CN− | 0.094 | 0.229 | −0.038 | 37.04 | 2.77 | 0.33 | 0.53 | |
| N3− | 0.099 | 0.384 | −0.066 | 34.80 | 2.84 | 0.27 | 0.40 | |
| SnF3CN | F− | 0.101 | 0.702 | −0.031 | 33.81 | 2.77 | 0.22 | 0.38 |
| Cl− | 0.084 | 0.199 | −0.029 | 44.15 | 2.55 | 0.40 | 0.61 | |
| Br− | 0.075 | 0.118 | −0.026 | 42.58 | 2.50 | 0.45 | 0.66 | |
| NC− | 0.101 | 0.385 | −0.034 | 53.18 | 2.71 | 0.22 | 0.37 | |
| CN− | 0.098 | 0.230 | −0.040 | 34.38 | 2.55 | 0.37 | 0.58 | |
| N3− | 0.098 | 0.341 | −0.037 | 31.10 | 2.65 | 0.54 | 0.44 |
| Complex | Rint | θ | Eint | |
|---|---|---|---|---|
| 7 | 1+NH3 | 2.567 | 101.1 | −7.69 |
| 8 | 1+NH2OH | 2.459 | 101.4 | −9.25 |
| 9 | 1+NH2CH3 | 2.291 | 98.2 | −13.58 |
| 10 | 1+NH2NH2 | 2.245 | 97.5 | −15.05 |
| 11 | 2+NH3 | 2.790 | 102.6 | −9.82 |
| 12 | 2+NH2OH | 2.724 | 103.0 | −11.86 |
| 13 | 2+NH2CH3 | 2.538 | 100.2 | −17.44 |
| 14 | 2+NH2NH2 | 2.486 | 99.9 | −20.8 |
| 15 | 3+NH3 | 2.735 | 100.5 | −13.36 |
| 16 | 3+NH2OH | 2.671 | 100.2 | −16.65 |
| 17 | 3+NH2CH3 | 2.568 | 97.7 | −19.11 |
| 18 | 3+NH2NH2 | 2.548 | 98.1 | −22.46 |
| 19 | 4+NH3 | 2.330 | 99.4 | −10.89 |
| 20 | 4+NH2OH | 2.277 | 99.0 | −13.47 |
| 21 | 4+NH2CH3 | 2.195 | 96.7 | −17.42 |
| 22 | 4+NH2NH2 | 2.175 | 97.0 | −20.86 |
| 23 | 5+NH3 | 2.527 | 100.3 | −14.25 |
| 24 | 5+NH2OH | 2.498 | 101.1 | −18.48 |
| 25 | 5+NH2CH3 | 2.370 | 98.4 | −22.55 |
| 26 | 5+NH2NH2 | 2.352 | 98.6 | −25.5 |
| 27 | 6+NH3 | 2.586 | 99.0 | −17.02 |
| 28 | 6+NH2OH | 2.574 | 100.3 | −20.08 |
| 29 | 6+NH2CH3 | 2.487 | 98.1 | −23.66 |
| 30 | 6+NH2NH2 | 2.479 | 98.7 | −26.98 |
| Complex | ρBCP | ∇2ρBCP | HBCP | E(2) | qCT | WBI | |
|---|---|---|---|---|---|---|---|
| 7 | 1+NH3 | 0.024 | 0.049 | −0.003 | 10.42 | 0.072 | 0.114 |
| 8 | 1+NH2OH | 0.028 | 0.066 | −0.017 | 11.20 | 0.075 | 0.129 |
| 9 | 1+NH2CH3 | 0.039 | 0.084 | −0.021 | 13.45 | 0.078 | 0.135 |
| 10 | 1+NH2NH2 | 0.043 | 0.111 | −0.024 | 14.52 | 0.080 | 0.144 |
| 11 | 2+NH3 | 0.029 | 0.055 | −0.005 | 12.28 | 0.076 | 0.134 |
| 12 | 2+NH2OH | 0.034 | 0.062 | −0.019 | 14.55 | 0.079 | 0.155 |
| 13 | 2+NH2CH3 | 0.044 | 0.088 | −0.026 | 17.70 | 0.082 | 0.167 |
| 14 | 2+NH2NH2 | 0.048 | 0.099 | −0.030 | 19.14 | 0.084 | 0.176 |
| 15 | 3+NH3 | 0.035 | 0.090 | −0.007 | 15.25 | 0.079 | 0.155 |
| 16 | 3+NH2OH | 0.039 | 0.072 | −0.008 | 17.10 | 0.081 | 0.168 |
| 17 | 3+NH2CH3 | 0.048 | 0.083 | −0.022 | 18.75 | 0.084 | 0.182 |
| 18 | 3+NH2NH2 | 0.052 | 0.111 | −0.026 | 20.40 | 0.086 | 0.193 |
| 19 | 4+NH3 | 0.035 | 0.053 | −0.008 | 13.28 | 0.079 | 0.149 |
| 20 | 4+NH2OH | 0.039 | 0.090 | −0.023 | 14.48 | 0.084 | 0.189 |
| 21 | 4+NH2CH3 | 0.047 | 0.136 | −0.030 | 16.05 | 0.087 | 0.201 |
| 22 | 4+NH2NH2 | 0.050 | 0.141 | −0.033 | 18.60 | 0.090 | 0.228 |
| 23 | 5+NH3 | 0.043 | 0.113 | −0.030 | 15.08 | 0.082 | 0.165 |
| 24 | 5+NH2OH | 0.046 | 0.097 | −0.021 | 16.69 | 0.085 | 0.172 |
| 25 | 5+NH2CH3 | 0.054 | 0.121 | −0.028 | 18.82 | 0.089 | 0.191 |
| 26 | 5+NH2NH2 | 0.059 | 0.123 | −0.032 | 20.80 | 0.094 | 0.206 |
| 27 | 6+NH3 | 0.046 | 0.089 | −0.010 | 17.04 | 0.092 | 0.180 |
| 28 | 6+NH2OH | 0.049 | 0.093 | −0.026 | 18.92 | 0.095 | 0.196 |
| 29 | 6+NH2CH3 | 0.056 | 0.131 | −0.033 | 19.77 | 0.096 | 0.212 |
| 30 | 6+NH2NH2 | 0.060 | 0.141 | −0.036 | 22.50 | 0.098 | 0.225 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esrafili, M.D.; Mousavian, P. Strong Tetrel Bonds: Theoretical Aspects and Experimental Evidence. Molecules 2018, 23, 2642. https://doi.org/10.3390/molecules23102642
Esrafili MD, Mousavian P. Strong Tetrel Bonds: Theoretical Aspects and Experimental Evidence. Molecules. 2018; 23(10):2642. https://doi.org/10.3390/molecules23102642
Chicago/Turabian StyleEsrafili, Mehdi D., and Parisasadat Mousavian. 2018. "Strong Tetrel Bonds: Theoretical Aspects and Experimental Evidence" Molecules 23, no. 10: 2642. https://doi.org/10.3390/molecules23102642