Phytochemical Profiling of Fruit Powders of Twenty Sorbus L. Cultivars
Abstract
:1. Introduction
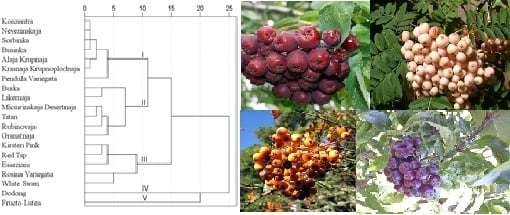
2. Results
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. Sample Preparation
4.2.1. Extraction of Phenolic Compounds
4.2.2. Extraction of Sugars and Organic Acids
4.2.3. Extraction of Carotenoids
4.3. Qualitative and Quantitative Analysis
4.3.1. HPLC Methods
4.3.2. Spectrophotometric Methods
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aldasoro, J.J.; Aedo, C.; Navarro, C.; Garmendia, F.M. The Genus Sorbus (Maloideae, Rosaceae) in Europe and in North Africa: Morphological Analysis and Systematics. Syst. Bot. 1998, 23, 189–212. [Google Scholar] [CrossRef]
- Baltacioglu, C.; Velioglu, S.; Karacabey, E. Changes in total phenolic and flavonoid contents of rowanberry fruit during posthatvest storage. J. Food Qual. 2011, 34, 278–283. [Google Scholar] [CrossRef]
- Berna, E.; Kampuse, S.; Dukalska, L.; Murniece, I. The chemical and physical properties of sweet rowanberries in powder sugar. In Proceedings of the 6th Baltic Conference on Food Science and Technology FOODBALT-2011, Jelgava, Latvia, 5–6 May 2011. [Google Scholar]
- Hukkanen, A.T.; Pölönen, S.S.; Kärenlampi, S.O.; Kokko, H.I. Antioxidant Capacity and Phenolic Content of Sweet Rowanberries. J. Agric. Food Chem. 2006, 54, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Poyrazoglu, E.S. Changes in ascorbic acid and sugar content of rowanberries during ripening. J. Food Qual. 2004, 27, 366–370. [Google Scholar] [CrossRef]
- Gil-Izquierdo, A.; Mellenthin, A. Identification and quantitation of flavonols in rowanberry (Sorbus aucuparia L.) juice. Eur. Food Res. Technol. 2001, 213, 12–17. [Google Scholar]
- Jurikova, T.; Sochor, J.; Mlcek, J.; Balla, S.; Klejdus, B.; Baron, M.; Ercisli, S.; Yilmaz, S.O. Polyphenolic profile of interspecific crosses of rowan (Sorbus aucuparia L.). Ital. J. Food Sci. 2014, 26, 317–324. [Google Scholar]
- Kylli, P.; Nohynek, L.; Puupponen-Pimiä, R.; Westerlund-Wikström, B.; McDougall, G.; Stewart, D.; Heinonen, M. Rowanberry Phenolics: Compositional Analysis and Bioactivities. J. Agric. Food Chem. 2010, 58, 11985–11992. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Lee, Y.J.; Jang, H.-J.; Kim, A.R.; Hong, S.; Kim, T.W.; Kim, M.-Y.; Lee, J.; Lee, Y.G.; Cho, J.Y. Anti-inflammatory activity of Sorbus commixta water extract and its molecular inhibitory mechanism. J. Ethnopharmacol. 2011, 134, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Olszewska, M.A.; Michel, P. Antioxidant activity of inflorescences, leaves and fruits of three Sorbus species in relation to their polyphenolic composition. Nat. Prod. Res. 2009, 23, 1507–1521. [Google Scholar] [CrossRef] [PubMed]
- Grussu, D.; Stewart, D.; McDougall, G.J. Berry Polyphenols Inhibit α-Amylase in vitro: Identifying Active Components in Rowanberry and Raspberry. J. Agric. Food Chem. 2011, 59, 2324–2331. [Google Scholar] [CrossRef] [PubMed]
- Mikulic-Petkovsek, M.; Krska, B.; Kiprovski, B.; Veberic, R. Bioactive Components and Antioxidant Capacity of Fruits from Nine Sorbus Genotypes. J. Food Sci. 2017, 82, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Rocchetti, G.; Chiodelli, G.; Giuberti, G.; Ghisoni, S.; Baccolo, G.; Blasi, F.; Montesano, D.; Trevisan, M.; Lucini, L. UHPLC-ESI-QTOF-MS profile of polyphenols in Goji berries (Lycium barbarum L.) and its dynamics during in vitro gastrointestinal digestion and fermentation. J. Funct. Foods 2018, 40, 564–572. [Google Scholar] [CrossRef]
- Raudone, L.; Raudonis, R.; Liaudanskas, M.; Viskelis, J.; Pukalskas, A.; Janulis, V. Phenolic Profiles and Contribution of Individual Compounds to Antioxidant Activity of Apple Powders. J. Food Sci. 2016, 81, 1055–1061. [Google Scholar] [CrossRef] [PubMed]
- Fiedor, J.; Burda, K. Potential role of carotenoids as antioxidants in human health and disease. Nutrients 2014, 6, 466–488. [Google Scholar] [CrossRef] [PubMed]
- Fattore, M.; Montesano, D.; Pagano, E.; Teta, R.; Borrelli, F.; Mangoni, A.; Seccia, S.; Albrizio, S. Carotenoid and flavonoid profile and antioxidant activity in “Pomodorino Vesuviano” tomatoes. J. Food Compos. Anal. 2016, 53, 61–68. [Google Scholar] [CrossRef]
- Raudonis, R.; Raudonė, L.; Gaivelytė, K.; Viškelis, P.; Janulis, V. Phenolic and antioxidant profiles of rowan (Sorbus L.) fruits. Nat. Prod. Res. 2014, 28, 1231–1240. [Google Scholar] [CrossRef] [PubMed]
- Liang, N.; Kitts, D. Role of Chlorogenic Acids in Controlling Oxidative and Inflammatory Stress Conditions. Nutrients 2015, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, R.; Mohan Rao, L.J. An Outlook on Chlorogenic Acids—Occurrence, Chemistry, Technology, and Biological Activities. Crit. Rev. Food Sci. Nutr. 2013, 53, 968–984. [Google Scholar] [CrossRef] [PubMed]
- Mikulic-Petkovsek, M.; Schmitzer, V.; Slatnar, A.; Stampar, F.; Veberic, R. Composition of Sugars, Organic Acids, and Total Phenolics in 25 Wild or Cultivated Berry Species. J. Food Sci. 2012, 77, C1064–C1070. [Google Scholar] [CrossRef] [PubMed]
- Kampuss, K.; Kampuse, S.; Berna, E.; Kruma, Z.; Krasnova, I.; Drudze, I. Biochemical composition and antiradical activity of rowanberry (Sorbus L.) cultivars and hybrids with different Rosaceae L. cultivars. Latvian J. Agron. 2009, 12, 59–65. (In Polish) [Google Scholar]
- Tomás-Barberán, F.A.; Espín, J.C. Phenolic compounds and related enzymes as determinants of quality in fruits and vegetables. J. Sci. Food Agric. 2001, 81, 853–876. [Google Scholar] [CrossRef]
- Ratti, C. Freese drying for food powder production. In Handbook of Food Powders, 1st ed.; Bhandari, B., Bansal, N., Zhang, M., Schuck, P., Eds.; Woodhead Publishing Limited: Oxford, UK, 2013; pp. 57–84. ISBN 978-0-85709-513-8. [Google Scholar]
- De Boer, A.; Urlings, M.J.E.; Bast, A. Active ingredients leading in health claims on functional foods. J. Funct. Foods 2016, 20, 587–593. [Google Scholar] [CrossRef]
- Razina, T.G.; Zueva, E.P.; Ulrich, A.V.; Rybalkina, O.Y.; Chaikovskii, A.V.; Isaikina, N.V.; Kalinkina, G.I.; Zhdanov, V.V.; Zyuz’kov, G.N. Antitumor Effects of Sorbus aucuparia L. Extract Highly Saturated with Anthocyans and Their Mechanisms. Bull. Exp. Biol. Med. 2016, 162, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Clifford, M.N. Chlorogenic acids and other cinnamates—Nature, occurrence and dietary burden. J. Sci. Food Agric. 1999, 79, 362–372. [Google Scholar] [CrossRef]
- Mattila, P.; Hellström, J.; Törrönen, R. Phenolic Acids in Berries, Fruits, and Beverages. J. Agric. Food Chem. 2006, 54, 7193–7199. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, T.; Ueno, Y.; Kojo, H.; Yoshikawa, T.; Osawa, T. Gene expression profile of isolated rat adipocytes treated with anthocyanins. Biochim. Biophys. Acta 2005, 1733, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhang, Y.; Liu, Y.; Sun, R.; Xia, M. Purified Anthocyanin Supplementation Reduces Dyslipidemia, Enhances Antioxidant Capacity, and Prevents Insulin Resistance in Diabetic Patients. J. Nutr. 2015, 145, 742–748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakaishi, H.; Matsumoto, H.; Tominaga, S.; Hirayama, M. Effects of black current anthocyanoside intake on dark adaptation and VDT work-induced transient refractive alteration in healthy humans. Altern. Med. Rev. 2000, 5, 553–562. [Google Scholar] [PubMed]
- Seeram, N.; Momin, R.A.; Nair, M.G.; Bourquin, L.D. Cyclooxygenase inhibitory and antioxidant cyanidin glycosides in cherries and berries. Phytomedicine 2001, 8, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Azimova, S.S.; Glushenkova, A.I. Sorbus aucuparia L. (S. boissieri Schneid.). In Lipids, Lipophilic Components and Essential Oils from Plant Sources; Azimova, S.S., Glushenkova, A.I., Eds.; Springer: London, UK, 2012; pp. 778–779. [Google Scholar]
- Montesano, D.; Rocchetti, G.; Putnik, P.; Lucini, L. Bioactive profile of pumpkin: An overniew on terpenoids and their health-promoting properties. Curr. Opin. Food Sci. 2018, 22, 81–87. [Google Scholar] [CrossRef]
- Lin, T.M.; Durance, T.D.; Scaman, C.H. Characterization of vacuum microwave, air and freeze dried carrot slices. Food Res. Int. 1998, 31, 111–117. [Google Scholar] [CrossRef]
- Viljakainen, S.; Visti, A.; Laakso, S. Concentrations of Organic Acids and Soluble Sugars in Juices from Nordic Berries. Acta Agric. Scand. Sect. B Soil Plant Sci. 2002, 52, 101–109. [Google Scholar] [CrossRef]
- Chukwuma, C.I.; Islam, M.S. Sorbitol increases muscle glucose uptake ex vivo and inhibits intestinal glucose absorption ex vivo and in normal and type 2 diabetic rats. Appl. Physiol. Nutr. Metab. 2017, 42, 377–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skrovankova, S.; Sumczynski, D.; Mlcek, J.; Jurikova, T.; Sochor, J. Bioactive Compounds and Antioxidant Activity in Different Types of Berries. Int. J. Mol. Sci. 2015, 16, 24673–24706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shofian, N.M.; Hamid, A.A.; Osman, A.; Saari, N.; Anwar, F.; Dek, M.S.P.; Hairuddin, M.R. Effect of freeze-drying on the antioxidant compounds and antioxidant activity of selected tropical fruits. Int. J. Mol. Sci. 2011, 12, 4678–4692. [Google Scholar] [CrossRef] [PubMed]
- Hallmann, E.; Orpel, E.; Rembiałkowska, E. The Content of Biologically Active Compounds in Some Fruits from Natural State. Veg. Crop. Res. Bull. 2011, 75, 81–90. [Google Scholar] [CrossRef]
- Gaivelyte, K.; Jakstas, V.; Razukas, A.; Janulis, V. Variation in the contents of neochlorogenic acid, chlorogenic acid and three quercetin glycosides in leaves and fruits of Rowan (Sorbus) species and varieties from collections in Lithuania. Nat. Prod. Commun. 2013, 8, 1105–1110. [Google Scholar] [PubMed]
- Gaivelyte, K. Research of Phenolic Compounds in Plants of Genus Sorbus L. Ph.D. Thesis, Lithuanian University of Health Sciences, Kaunas, Lithuania, 2014. [Google Scholar]
- Gaivelyte, K.; Jakstas, V.; Razukas, A.; Janulis, V. Variation of quantitative composition of phenolic compounds in rowan (Sorbus aucuparia L.) leaves during the growth season. Nat. Prod. Res. 2014, 28, 1018–1020. [Google Scholar] [CrossRef] [PubMed]
- Council of Europe. European Pharmacopoeia, 9th ed.; Council of Europe: Strasbourg, France, 2016. [Google Scholar]
- Raudone, L.; Raudonis, R.; Gaivelyte, K.; Pukalskas, A.; Viškelis, P.; Venskutonis, P.R.; Janulis, V. Phytochemical and antioxidant profiles of leaves from different Sorbus L. species. Nat. Prod. Res. 2015, 29, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |




| Cultivar | Compound | |||||
|---|---|---|---|---|---|---|
| Neochlorogenic Acid | Chlorogenic Acid | Cryptochlorogenic Acid | Caffeoylshikimic Acid | Coumaroylquinnic Acid Derivative | Dicaffeoylquinnic Acid | |
| ‘Alaja Krupnaja’ | 1588 ± 74 efg 1 | 1191 ± 61 k | 131 ± 6 ijk | nd 2 | nd | 69 ± 3 hi |
| ‘Burka’ | 2736 ± 55 bc | 2868 ± 70 fgh | 159 ± 1 ghi | nd | nd | 57 ± 0 i |
| ‘Businka’ | 1722 ± 51 ef | 3130 ± 90 def | 138 ± 7 hij | 37 ± 2 c | 39 ± 1 e | 302 ± 6 c |
| ‘Dodong’ | 13,351 ± 178 a | 3813 ± 77 b | 1025 ± 26 a | 520 ± 26 a | nd | 208 ± 9 ef |
| ‘Essenziani’ | 1526 ± 65 fg | 2848 ± 25 fghi | 286 ± 0 d | nd | 47 ± 2 d | 353 ± 3 b |
| ‘Fructo Lutea’ | 13,879 ± 689 a | 10,314 ± 143 a | 957 ± 11 b | 186 ± 11 b | 73 ± 1 a | 1327 ± 37 a |
| ‘Granatnaja’ | 2555 ± 73 c | 2425 ± 6 i | 142 ± 1 ghij | nd | nd | 85 ± 1 hi |
| ‘Kirsten Pink’ | 2574 ± 64 c | 3590 ± 97 bc | 268 ± 5 de | nd | 55 ± 2 c | 177 ± 1 f |
| ‘Konzentra’ | 820 ± 47 h | 1804 ± 12 j | 93 ± 2 l | nd | nd | 91 ± 1 ghi |
| ‘Krasnaja Krupnoplodnaja’ | 1660 ± 108 efg | 2798 ± 20 fghi | 119 ± 13 jkl | nd | nd | 196 ± 8 f |
| ‘Likernaja’ | 2710 ± 48 c | 2797 ± 58 fghi | 176 ± 3 fg | nd | nd | 59 ± 1 i |
| ‘Miciurinskaja Desertnaja’ | 1770 ± 12 edf | 2707 ± 57 fghi | 119 ± 1 jkl | nd | nd | 71 ± 2 hi |
| ‘Nevezinskaja’ | 892 ± 161 h | 3543 ± 451 bcd | 102 ± 15 kl | nd | nd | 212 ± 10 ef |
| ‘Pendula Variegata’ | 1922 ± 82 edf | 2690 ± 53 ghi | 268 ± 6 de | nd | nd | 241 ± 3 de |
| ‘Red Tip’ | 2149 ± 48 cde | 2918 ± 48 efgh | 276 ± 3 de | 40 ± 1 c | 39 ± 0 e | 298 ± 15 c |
| ‘Rosina Variegata’ | 1742 ± 321 edf | 3308 ± 245 cde | 170 ± 21 fgh | nd | nd | 214 ± 33 def |
| ‘Rubinovaja’ | 1575 ± 95 efg | 2761 ± 136 fghi | 200 ± 13 f | 40 ± 2 c | 39 ± 2 e | 128 ± 18 g |
| ‘Sorbinka’ | 1052 ± 149 gh | 2484 ± 144 hi | 245 ± 21 e | nd | nd | 103 ± 2 gh |
| ‘Titan’ | 2347 ± 30 cd | 2530 ± 116 hi | 132 ± 0 ijk | nd | nd | 91 ± 5 ghi |
| ‘White Swan’ | 3335 ± 236 b | 3087 ± 145 efg | 437 ± 14 c | 29 ± 0 c | 69 ± 2 b | 254 ± 2 d |
| Cultivar | Compound | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Quercetin Dihexoside1 | Quercetin Dihexoside2 | Quercetin Dihexoside3 | Rutin | Hyperoside | Isoquercitrin | Quercetin Malonyl Glucoside | Isorhamnetin Rutinoside | Astragalin | |
| ‘Alaja Krupnaja’ | 121 ± 10 ij 1 | 146 ± 10 e | nd 2 | 97 ± 7 d | 88 ± 6 e | 210 ± 15 bc | 69 ± 6 def | nd | 9 ± 1 d |
| ‘Burka’ | 113 ± 1 ij | 80 ± 0 hi | 52 ± 0 e | 62 ± 0 gh | 136 ± 1 c | 180 ± 5 d | 41 ± 1 f | 7 ± 0 h | nd |
| ‘Businka’ | 250±7 fg | 122±6 f | nd | 23 ± 1 ij | 35 ± 2 j | 61 ± 20 h | 84 ± 54 cde | nd | nd |
| ‘Dodong’ | nd | nd | 57 ± 0 d | 8 ± 0 k | 67 ± 2 fg | 41 ± 0 hi | 69 ± 2 def | 220 ± 7 b | nd |
| ‘Essenziani’ | 276 ± 9 ef | 68 ± 2 i | nd | 118 ± 2 c | 19 ± 0 k | 13 ± 1 j | nd | nd | nd |
| ‘Fructo Lutea’ | 212 ± 2 h | 39 ± 0 j | nd | 33 ± 7 i | 43 ± 0 ij | 50 ± 11 hi | 33 ± 1 fg | 25 ± 0 g | nd |
| ‘Granatnaja’ | 260 ± 7 f | 181 ± 7 d | 52 ± 0 e | 51 ± 1 h | 124 ± 0 d | 156 ± 2 e | 49 ± 0 ef | 3 ± 0 h | nd |
| ‘Kirsten Pink’ | 515 ± 12 b | 188 ± 5 d | nd | 138 ± 3 b | 173 ± 1 a | 115 ± 0 f | nd | nd | 13 ± 0 c |
| ‘Konzentra’ | 149 ± 4 i | 68 ± 2 i | nd | 24 ± 0 ij | 14 ± 0 k | 13 ± 0 j | nd | nd | nd |
| ‘Krasnaja Krupnoplodnaja’ | 261 ± 17 f | 124 ± 5 f | nd | 27 ± 5 ij | 42 ± 7 ij | 46 ± 5 hi | 37 ± 0 fg | 3 ± 1 h | nd |
| ‘Likernaja’ | 131 ± 3 i | 99 ± 2 g | 62 ± 1 c | 65 ± 1 g | 138 ± 1 c | 192 ± 2 cd | 34 ± 1 fg | 6 ± 0 h | nd |
| ‘Miciurinskaja Desertnaja’ | 218 ± 6 gh | 94 ± 2 gh | 99 ± 4 a | 33 ± 1 i | 172 ± 5 a | 235 ± 7 a | 322 ± 9 a | 176 ± 7 c | 27 ± 0 a |
| ‘Nevezinskaja’ | 147 ± 18 i | 43 ± 6 j | nd | 8 ± 0 k | 59 ± 4 gh | 36 ± 4 i | nd | nd | nd |
| ‘Pendula Variegata’ | 93 ± 1 j | 29 ± 0 j | nd | 31 ± 1 i | 121 ± 2 d | 139 ± 2 e | 233 ± 3 b | 144 ± 6 d | 24 ± 1 b |
| ‘Red Tip’ | 280 ± 7 ef | 45 ± 0 j | nd | 211 ± 6 a | 98 ± 5 e | 82 ± 3 g | 101 ± 3 cd | 46 ± 4 f | nd |
| ‘Rosina Variegata’ | 627 ± 14 a | 304 ± 8 a | nd | 84 ± 2 ef | 90 ± 0 e | 114 ± 0 f | 108 ± 3 c | 9 ± 1 h | 10 ± 0 d |
| ‘Rubinovaja’ | 320 ± 13 cd | 260 ± 1 b | 7 ± 0 f | 88 ± 9 de | 51 ± 6 hi | 105 ± 3 f | 60 ± 8 ef | 6 ± 2 h | nd |
| ‘Sorbinka’ | 308 ± 5 de | 30 ± 2 j | 3 ± 0 g | 17 ± 0 ik | 74 ± 2 f | 33 ± 1 ij | 44 ± 1 f | 64 ± 0 e | nd |
| ‘Titan’ | 352 ± 5 c | 221 ± 5 c | 76 ± 1 b | 74 ± 2 fg | 177 ± 0 a | 219 ± 2 ab | 66 ± 0 def | 7 ± 0 h | nd |
| ‘White Swan’ | 617 ± 35 a | 226 ± 15 c | 3 ± 0 g | 72 ± 1 fg | 152 ± 5 b | 148 ± 4 e | 332 ± 6 a | 264 ± 16 a | 10 ± 0 d |
| Cultivar | Compound | |||||||
|---|---|---|---|---|---|---|---|---|
| Cyanidin 3-Galactoside | Cyanidin 3-Glucoside | Cyanidin 3-Arabinoside | Peonidin 3-Arabinoside | Malvidin 3-Arabinoside | β-Carotene | Total Carotenoids | Antioxidant Activity | |
| ‘Alaja Krupnaja’ | 553 ± 14 f 1 | 5 ± 0 gh | 0.33 ± 0 f | nd 2 | 3 ± 0 g | 445 ± 23 ef | 1455 ± 73 c | 101 ± 3 k |
| ‘Burka’ | 5196 ± 130 a | 320 ± 6 a | 1827 ± 46 a | 100 ± 2 a | 28 ± 1 a | 150 ± 8 h | 498 ± 5 f | 464 ± 12 a |
| ‘Businka’ | 248 ± 6 gh | 13 ± 0 fg | 5 ± 0 f | nd | 2 ± 0 h | 435 ± 22 ef | 1119 ± 23 d | 196 ± 5 e |
| ‘Dodong’ | 91 ± 2 ijk | nd | 3 ± 0 f | nd | nd | 1262 ± 7 a | 2659 ± 133 a | 107 ± 3 jk |
| ‘Essenziani’ | 78 ± 2 ijk | nd | 4 ± 0 f | nd | nd | 467 ± 24 de | 743 ± 38 e | 66 ± 2 m |
| ‘Fructo Lutea’ | nd | nd | nd | nd | nd | 520 ± 13 c | 1323 ± 34 c | 323 ± 8 b |
| ‘Granatnaja’ | 3792 ± 95 b | 251 ± 5 c | 883 ± 22 b | 45 ± 1 c | 19 ± 0 c | 361 ± 18 g | 1097 ± 55 d | 154 ± 4 g |
| ‘Kirsten Pink’ | 41 ± 1 jk | nd | 3 ± 0 f | nd | nd | 23 ± 1 j | 39 ± 2 h | 138 ± 3 gh |
| ‘Konzentra’ | 90 ± 2 ijk | 1 ± 0 h | 4 ± 0 f | nd | nd | 525 ± 7 c | 1436 ± 18 c | 26 ± 1 o |
| ‘Krasnaja Krupnoplodnaja’ | 309 ± 8 g | 20 ± 0 f | 6 ± 0 f | nd | 2 ± 0 gh | 323 ± 4 g | 1049 ± 27 d | 107 ± 3 jk |
| ‘Likernaja’ | 5165 ± 129 a | 309 ± 6 b | 1849 ± 46 a | 92 ± 2 b | 24 ± 1 b | 102 ± 5 i | 237 ± 6 g | 476 ± 12 a |
| ‘Miciurinskaja Desertnaja’ | 1575 ± 39 d | 132 ± 3 d | 330 ± 8 d | 35 ± 1 d | 9 ± 0 e | 65 ± 1 ij | 143 ± 4 gh | 221 ± 6 d |
| ‘Nevezinskaja’ | 175 ± 4 ghij | nd | 6 ± 0 f | nd | 1 ± 0 i | 537 ± 27 c | 1440 ± 36 c | 77 ± 2 lm |
| ‘Pendula Variegata’ | 92 ± 2 hijk | 8 ± 0 gh | 5 ± 0 f | nd | nd | 815 ± 41 b | 2348 ± 118 b | 80 ± 2 lm |
| ‘Red Tip’ | 15 ± 0 k | nd | nd | nd | nd | 62 ± 3 ij | 124 ± 7 gh | 49 ± 1 n |
| ‘Rosina Variegata’ | 314 ± 8 g | 34 ± 1 e | 10 ± 0 ef | nd | 4 ± 0 f | 418 ± 11 f | 983 ± 15 d | 121 ± 3 ij |
| ‘Rubinovaja’ | 1101 ± 28 e | 129 ± 3 d | 56 ± 1 e | 5 ± 0 f | 3 ± 0 fg | 93 ± 2 i | 161 ± 8 gh | 245 ± 6 c |
| ‘Sorbinka’ | 202 ± 5 ghi | nd | 10 ± 0 ef | nd | nd | 505 ± 5 cd | 1423 ± 18 c | 85 ± 2 l |
| ‘Titan’ | 3175 ± 79 c | 245 ± 5 c | 632 ± 16 c | 32 ± 1 e | 10 ± 0 d | 100 ± 2 i | 217 ± 3 g | 132 ± 3 hi |
| ‘White Swan’ | 26 ± 1 jk | nd | nd | nd | nd | 87 ± 5 i | 108 ± 6 gh | 170 ± 4 f |
| Cultivar | Compound | Sugars/Acids Ratio | ||||||
|---|---|---|---|---|---|---|---|---|
| Xylose | Fructose | Glucose | Sucrose | Sorbitol | Ascorbic Acid | Malic Acid | ||
| ‘Alaja Krupnaja’ | 13.55 ± 0.36 a 1 | 159.43 ± 1.17 c | 165.20 ± 6.37 f | 4.50 ± 0.53 a | 190.38 ± 2.95 g | 0.24 ± 0.00 j | 59.67 ± 0.44 g | 8.90 ± 0.10 f |
| ‘Burka’ | 6.77 ± 0.64 cd | 130.97 ± 2.08 f | 144.71 ± 1.33 g | nd 2 | 158.36 ± 1.88 i | nd | 77.22 ± 0.88 d | 5.71 ± 0.01 j |
| ‘Businka’ | nd | 149.41 ± 3.72 d | 186.96 ± 0.42 cd | nd | 235.75 ± 4.32 de | nd | 44.30 ± 0.36 k | 12.91 ± 0.09 a |
| ‘Dodong’ | 6.68 ± 0.53 cd | 28.37 ± 0.84 n | 219.73 ± 2.96 a | nd | 252.27 ± 1.79 c | 1.70 ± 0.00 c | 38.35 ± 0.17 l | 12.66 ± 0.10 a |
| ‘Essenziani’ | 13.98 ± 1.34 a | 79.02 ± 4.18 jk | 90.51 ± 3.83 j | nd | 121.18 ± 5.06 k | 0.52 ± 0.00 h | 73.14 ± 1.14 e | 4.14 ± 0.13 k |
| ‘Fructo Lutea’ | 14.13 ± 0.13 a | 18.45 ± 0.47 o | 157.13 ± 0.57 f | nd | 196.31 ± 0.05 g | 2.20 ± 0.02 a | 97.14 ± 0.04 b | 3.89 ± 0.01 k |
| ‘Granatnaja’ | 8.07 ± 0.31 cd | 180.38 ± 1.15 a | 221.07 ± 1.79 a | 3.19 ± 0.10 b | 188.84 ± 1.40 g | 0.20 ± 0.00 k | 60.88 ± 0.35 g | 9.85 ± 0.02 de |
| ‘Kirsten Pink’ | 9.24 ± 0.01 bc | 56.87 ± 1.60 l | 67.35 ± 1.33 k | nd | 192.50 ± 0.42 g | 1.42 ± 0.01 d | 76.15 ± 0.04 d | 4.20 ± 0.04 k |
| ‘Konzentra’ | nd | 110.67 ± 0.74 h | 123.21 ± 0.68 h | nd | 261.78 ± 2.87 ab | 0.36 ± 0.00 i | 43.95 ± 0.03 k | 11.19 ± 0.09 c |
| ‘Krasnaja Krupnoplodnaja’ | 5.10 ± 0.04 d | 134.29 ± 1.49 ef | 160.95 ± 1.78 f | nd | 191.47 ± 2.18 g | 0.59 ± 0.00 g | 65.29 ± 0.31 f | 7.47 ± 0.05 h |
| ‘Likernaja’ | nd | 147.80 ± 0.44 d | 178.86 ± 0.33 de | nd | 216.06 ± 0.48 f | nd | 54.58 ± 1.05 h | 9.95 ± 0.17 de |
| ‘Miciurinskaja Desertnaja’ | nd | 161.05 ± 0.82 c | 174.33 ± 0.18 e | nd | 240.84 ± 1.77 d | 0.11 ± 0.00 m | 47.90 ± 0.87 j | 12.01 ± 0.28 b |
| ‘Nevezinskaja’ | 4.60 ± 0.35 d | 122.26 ± 1.76 g | 138.73 ± 1.19 g | nd | 254.26 ± 1.52 bc | 0.38 ± 0.00 i | 50.91 ± 0.26 i | 10.14 ± 0.04 d |
| ‘Pendula Variegata’ | 13.12 ± 0.15 ab | 72.24 ± 0.93 k | 87.00 ± 0.44 j | nd | 265.31 ± 3.80 a | 0.15 ± 0.00 l | 44.69 ± 0.27 k | 9.76 ± 0.06 e |
| ‘Red Tip’ | 5.52 ± 5.52 cd | 82.39 ± 0.20 j | 102.18 ± 0.70 i | nd | 231.53 ± 0.79 e | 0.91 ± 0.00 f | 60.19 ± 0.38 g | 6.90 ± 0.13 i |
| ‘Rosina Variegata’ | 7.14 ± 0.06 cd | 99.67 ± 3.70 i | 109.53 ± 2.05 i | nd | 103.92 ± 0.79 i | 1.07 ± 0.00 e | 102.37 ± 0.93 a | 3.10 ± 0.04 l |
| ‘Rubinovaja’ | 6.62 ± 0.33 cd | 187.28 ± 0.80 a | 207.83 ± 1.15 b | nd | 162.90 ± 0.61 hi | 0.21 ± 0.00 k | 63.62 ± 0.79 f | 8.85 ± 0.07 f |
| ‘Sorbinka’ | 5.85 ± 0.56 cd | 139.29 ± 8.17 e | 146.57 ± 3.26 g | nd | 210.87 ± 5.49 f | 0.60 ± 0.00 g | 50.65 ± 0.67 i | 9.81 ± 0.19 de |
| ‘Titan’ | 6.16 ± 0.24 cd | 169.18 ± 0.49 b | 188.33 ± 7.57 c | nd | 169.17 ± 1.05 h | 0.20 ± 0.00 k | 64.29 ± 0.04 f | 8.26 ± 0.12 g |
| ‘White Swan’ | 9.16 ± 0.35 bc | 48.10 ± 0.72 m | 53.37 ± 0.21 l | nd | 146.34 ± 0.23 j | 1.94 ± 0.02 b | 81.27 ± 1.18 c | 3.09 ± 0.03 l |
| Cultivar | Species |
|---|---|
| Alaja Krupnaja | Sorbus aucuparia |
| Burka | Sorbus aucuparia × Sorbaronia alpina [Sorbus aria × Aronia arbutifolia] |
| Businka | Sorbus aucuparia var. rossica |
| Dodong | Sorbus ulleungensis |
| Esseziani | Sorbus esseziani |
| Fructo Lutea | Sorbus aucuparia var. xanthocarpa |
| Granatnaja | Sorbus aucuparia × Crataegus sanguinea |
| Kirsten Pink | Sorbus × arnoldiana [Sorbus aucuparia × Sorbus discolor] |
| Konzentra | Sorbus aucuparia |
| Krasnaja Krupnoplodnaja | Sorbus aucuparia var. moravica |
| Likernaja | Sorbus aucuparia × Aronia melanocarpa |
| Miciurinskaja Desertnaja | [Sorbus aucuparia × Aronia melanocarpa] × Mespilus germanica |
| Nevezinskaja | Sorbus aucuparia |
| Pendula Variegata | Sorbus aucuparia |
| Red Tip | Sorbus × arnoldiana [Sorbus aucuparia × Sorbus discolor] |
| Rosina Variegata | Sorbus aucuparia var. moravica |
| Rubinovaja | Sorbus aucuparia × pollen from Pyrus species |
| Sorbinka | Sorbus aucuparia |
| Titan | Burka × mixture of pollen from Malus sp. and Pyrus sp. |
| White Swan | Sorbus × arnoldiana [Sorbus aucuparia × Sorbus discolor] |
| Compound | Tested Linear Range (mg/mL) | Calibration Curve Equation | Determination Coefficient |
|---|---|---|---|
| Neochlorogenic | 3.369–107.8 | y = 36,200x − 6330 | 0.9997 |
| Chlorogenic acid | 1.4654–93.734 | y = 61,500x – 32,800 | 0.9998 |
| Cryptochlorogenic acid | 0.977–125 | y = 43,800x − 8150 | 0.9997 |
| Rutin | 0.158–10.078 | y = 50,900x − 2670 | 0.9999 |
| Hyperoside | 0.170–9.600 | y = 61,600x − 4920 | 0.9998 |
| Isoquercitrin | 0.170–10.892 | y = 40,200x − 2510 | 0.9999 |
| Quercetin-malonylglucoside | 0.332–42.500 | y = 24,800x − 893 | 0.9999 |
| Isorhamnetin rutinoside | 0.488–15.625 | y = 36,500x + 1580 | 0.9999 |
| Astragalin | 0.168–10.754 | y = 35,200x − 2710 | 0.9999 |
| Cyanidin-3-galactoside | 3.54–88.46 | y = 25,800,000x | 0.9990 |
| Cyanidin-3-glucoside | 3.99–99.93 | y = 26,100,000x | 0.9988 |
| Cyanidin-3-arabinoside | 2.81–70.35 | y = 27,100,000x | 0.9992 |
| Peonidin-3-arabinoside | 0.25–6.14 | y = 27,200,000x | 0.9981 |
| Malvidin-3-arabinoside | 0.93–23.12 | y = 24,800,000x | 0.9979 |
| β-carotene | 3.07–98.5 | y = 21,100x − 3880 | 0.9997 |
| Xylose | 0.0625–2 | y = 1.91x + 5.30 | 0.9987 |
| Fructose | 0.625–10 | y = 1.77x + 5.18 | 0.9984 |
| Glucose | 0.25–4 | y = 1.76x + 5.20 | 0.9976 |
| Sucrose | 0.25–4 | y = 1.71x + 5.45 | 0.9993 |
| Sorbitol | 0.0625–1 | y = 1.74x + 5.31 | 0.9985 |
| Ascorbic acid | 0.1–0.5 | y = 19,100x + 41,000 | 0.9990 |
| Malic acid | 0.5–2.0 | y = 599x + 38.9 | 0.9999 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zymone, K.; Raudone, L.; Raudonis, R.; Marksa, M.; Ivanauskas, L.; Janulis, V. Phytochemical Profiling of Fruit Powders of Twenty Sorbus L. Cultivars. Molecules 2018, 23, 2593. https://doi.org/10.3390/molecules23102593
Zymone K, Raudone L, Raudonis R, Marksa M, Ivanauskas L, Janulis V. Phytochemical Profiling of Fruit Powders of Twenty Sorbus L. Cultivars. Molecules. 2018; 23(10):2593. https://doi.org/10.3390/molecules23102593
Chicago/Turabian StyleZymone, Kristina, Lina Raudone, Raimondas Raudonis, Mindaugas Marksa, Liudas Ivanauskas, and Valdimaras Janulis. 2018. "Phytochemical Profiling of Fruit Powders of Twenty Sorbus L. Cultivars" Molecules 23, no. 10: 2593. https://doi.org/10.3390/molecules23102593